Summary
Remarkable advances have been made in recent years in our understanding of innate behavior and the underlying neural circuits. In particular, a wealth of neuromodulatory mechanisms have been uncovered that can alter the input-output relationship of a hereditary neural circuit. It is now clear that this inbuilt flexibility allows animals to modify their behavioral responses according to environmental cues, metabolic demands and physiological states. Here, we discuss recent insights into how modulation of neural circuits impacts innate behavior, with a special focus on how environmental cues and internal physiological states shape different aspects of feeding behavior in Drosophila.
Introduction
Innate behavior, programmed by genetically predetermined neural circuits, is robust and stereotyped. Although considered to be hardwired, innate behavior is also flexible and subject to modulation by internal states (e.g. satiety state) and external contexts of the stimuli (e.g. environmental cues) [1–6]. Dissecting the mechanisms whereby external and internal contexts of stimuli influence the behavioral outputs of a hardwired circuit might appear a daunting task. However, aided by powerful genetic tools, much progress has recently been made to address this fascinating question in genetic model organisms such as Caenorhabditis elegans and Drosophlia melanogaster (for review, see [1,2,4,7]). Here we will focus on recent advances in neuromodulation of Drosophila innate behavior.
Context-dependent modulation of innate behavior is particularly well described for fruit flies, which forage only when they are starved [8••], feed only when they verify that food is not spoilt [9••], and court vigorously only when they detect that a food source is nearby to sustain their progeny [10]. This inbuilt behavioral flexibility allows animals to mount appropriate behavioral responses to stimuli. At the circuit level, this flexibility is thought to be driven by information rerouting and neuromodulation [2]. The former involves reconfiguring information processing by alternative circuit pathways, and the latter refers to chemical neuronal communications that are neither simply excitatory nor inhibitory but serve to modulate the properties of existing synaptic connections [11].
Here we will first focus on how the context of an external stimulus influences innate behavior, using the example of how fruit odors suppress Drosophila's natural aversion to carbon dioxide. In the second part of this review, we will highlight the neuromodulatory mechanisms by which internal physiological states, particularly satiety levels, regulate appetitive behavior in Drosophila.
External context: how fruit odors suppress Drosophila's aversion to carbon dioxide
Carbon dioxide is a key component of the “stressed odors” released by agitated fruit flies, and is detected by the ab1C olfactory receptor neurons (ORNs) [12]. Activation of ab1C ORNs leads to robust aversive behavioral responses [12,13]. However, CO2 is also present in different contexts of the natural environment for Drosophila. For instance, CO2 is emitted from ripe fruits that are attractive to fruit flies [14]. Thus, CO2 in the context of fruit odors does not trigger aversion. Given that CO2 is detected primarily by a single class of ORNs, ab1C, how do fruit flies manage to avoid CO2 robustly in one context but tolerate the same compound in another?
Several recent studies have shed light on the circuit mechanisms that underlie this intriguing context-dependent behavior. Interestingly, as we describe below, these mechanisms appear to operate at every layer of the olfactory circuit, from the peripheral sensory organ, to the first information relay center, and further on into multiple higher brain regions (Figure 1).
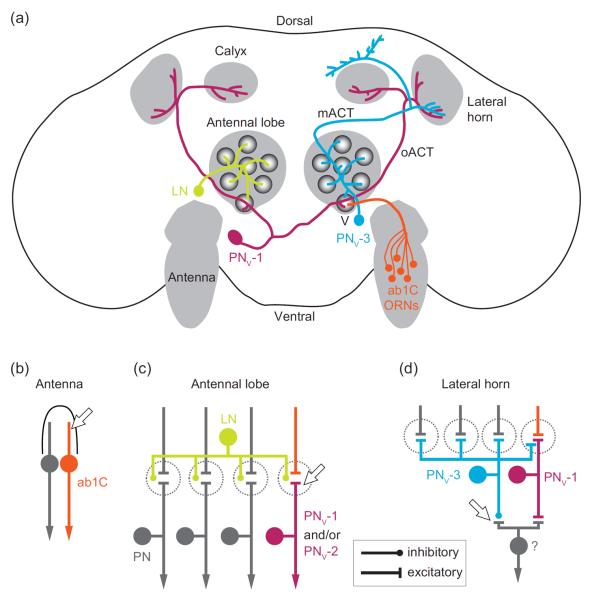
Figure 1.
Fruit odors suppress Drosophila's aversion to carbon dioxide by inhibiting propagation of CO2 information at multiple layers of the olfactory circuit. (a) Schematic of the fly olfactory circuit for CO2 information processing. CO2 is detected by ab1C olfactory receptor neurons (ORNs) in the antenna. In the antennal lobe, ab1C axons synapse with projection neurons (PNs) in the V glomerulus. GABAergic local neurons (LNs) innervate multiple glomeruli and suppress PN output from the V glomerulus via dendro-dendritic inhibition. Two types of PNs that innervate the V glomerulus (PNv) are highlighted: PNv-1 is excitatory and receives bilateral input from the V glomeruli. PNv-1 projects to the lateral horn and calyx of the mushroom body via the outer antennocerebral tract (oACT); PNv-3 is inhibitory and receives input from every glomerulus in the ipsilateral antennal lobe and projects to the lateral horn and other higher brain regions (not indicated) via the medial antennocerebral tract (mACT). Fruit odors inhibit the CO2 olfactory circuit at the antenna via lateral inhibition in a sensillum (b), at the antennal lobe via LN feed-forward inhibition (c), and at the lateral horn via parallel inhibition of an unidentified output neuron by PNv-3 (d). Arrows indicate sites of inhibition.
Direct modulation of CO2 response in the antenna
In fruit flies, the CO2-responsive ab1C ORNs are located in the primary olfactory organ, the antenna. Given that ORNs are the first neurons in the olfactory circuit, one effective means for flies to ignore CO2 would be to directly suppress the response of ab1C ORNs in the presence of fruit odors. Indeed, two complementary mechanisms by which fruit odors inhibit ab1C response to CO2 have been reported, one operating within ab1C and the other between ab1C and its neighboring ORN. The former mechanism acts on the CO2 receptor complex (Gr21a/Gr63a) localized on the outer dendrites of ab1C [15,16]. Interestingly, certain fruity odorants can directly interact with Gr21a/Gr63a to inhibit the response of ab1C to CO2, thus suppressing flies' behavioral aversion to CO2 [17].
In addition, a second, novel mechanism of inhibition occurs, driven by lateral inhibition between neighboring ORNs housed in the same sensillum (Figure 1b). Transient activation of any given ORN robustly inhibits the chronic olfactory response of its neighbor via ephaptic coupling [18••,19]. Notably, in the ab1 sensillum, ab1C is grouped with another ORN (ab1A) which responds strongly to fruit odors [20]. As a result, strong activation of ab1A by fruit odors may attenuate the response of ab1C to CO2, thereby making CO2 more tolerable in the presence of fruit odors [18••]. In this context, we consider ephaptic coupling as a means of neuromodulation.
Inhibition of CO2–activated output by interneurons in the antennal lobe
Upon ab1C activation, CO2 input is propagated by ab1C axons to a spherical neuropil structure called the V glomerulus in the antennal lobe [12,21]. In the V glomerulus, ab1C axon terminals form synapses with projection neurons (PNs), which are the main output neurons that relay CO2 information to higher brain regions (see below). Also innervating the V glomerulus are the inhibitory GABAergic local interneurons, which receive excitatory inputs from a wide variety of ORN types, including those that respond to fruit odors (Figure 1c) [22–25]. Interestingly, it has been suggested that these local interneurons can attenuate the response of PNs from the V glomerulus [26], likely by activating GABAA receptors on PN dendrites [27]. Therefore, activation of this GABAergic inhibitory pathway by fruit odors may further dampen CO2 signals by inhibiting PN outputs from the V glomerulus.
Inhibition of CO2–activated output by projection neurons in the higher brain regions
In addition to ab1C ORNs and their corresponding PNs, a third layer of regulation has been proposed recently. Remarkably, multiple types of PNs innervate the V glomerulus [28••,29]. These PNs differ in their sensitivity to CO2 and their axonal innervating patterns in higher brain regions. Among them, two PN types are largely responsible for flies' behavioral aversion to low (0.5%) and high (2%) concentrations of CO2. The response of PNv-1 to CO2 saturates at a low concentration (0.5%), whereas PNv-2 shows graded responses to different concentrations of CO2 (0.5% ~ 2%) [28••].
Segregation of the CO2 processing circuit into multiple pathways may allow differential modulation of each individual pathway [29,30]. Indeed, in a higher brain region named the lateral horn, some higher order neurons that receive PNv-1 input can be inhibited by yet another PN type (PNv-3) [28••]. Unlike most PNs, PNv-3 is GABAergic and may belong to a reported parallel inhibitory pathway in the fly olfactory circuit [31•]. Notably, the dendrites of PNv-3 innervate multiple glomeruli, including those that are activated by fruit odors (Figure 1d). Thus, fruit odors activate PNv-3 to selectively inhibit the PNv-1 output neurons without affecting the PNv-2 pathway that mediates aversion to high concentrations of CO2 [28••]. As a result, fruit odors may selectively inhibit the behavioral aversion to low levels of CO2 present in ripe fruits [14], while the fly retains its ability to respond to high CO2 levels that may signal danger.
Much remains to be learned about how these neural substrates work in concert to modulate the CO2 olfactory circuit and to determine the contribution of each individual mechanism in shaping flies' behavioral response to CO2. Moreover, other factors, such as the fly's locomotive state, can also impact whether they find CO2 attractive or aversive [32•]. It will be of interest to know where locomotive information is integrated into the CO2 circuitry. A broader question is to determine whether similar mechanisms govern context-dependent responses to other stimuli. Importantly, though, the multiplicity of neural substrates highlighted here provides insight into the fundamental circuit logic that determines how environmental cues can alter the behavioral output of a hardwired neural circuit. In addition to these external cues, it has recently become clear that the internal states of the animal can also influence behavioral responses, as we explain below.
Internal context: how satiety state regulates Drosophila's feeding behavior
Feeding is a highly regulated behavior; many factors influence an animal's decision to eat, such as the aroma, palatability and nutritive value of food, as well as the satiety state and metabolic demands of the animal [33]. To integrate these diverse cues, animals produce a variety of signaling molecules. These molecules code for different internal states that modulate different aspects of feeding behavior, from sensory input to behavioral output and several processing stations in between (see below). In the following sections, we will highlight recent discoveries on the molecular and cellular mechanisms whereby starvation regulates feeding behavior. In particular, we will focus on how starvation promotes feeding by enhancing olfactory and gustatory sensitivity and by regulating activity of central neurons that express internal nutrient sensors in Drosophila (Figure 2a).
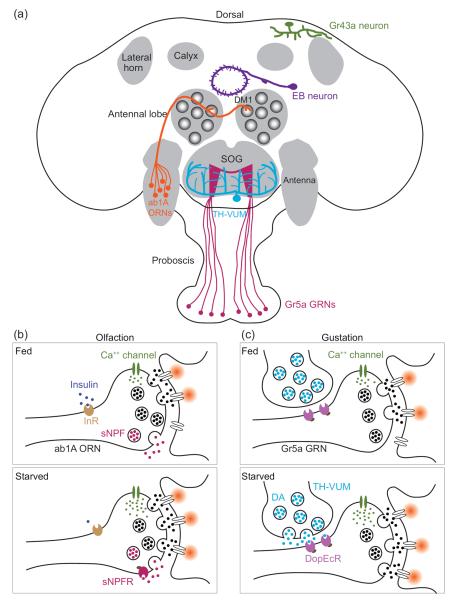
Figure 2.
Satiety state regulates feeding by a diverse array of neuromodulatory mechanisms. (a) Starvation regulates feeding in flies by enhancing olfactory and gustatory sensitivity and by regulating activity of central neurons that express internal nutrient sensors. These nutrient sensors include the fructose receptor, Gr43a, in the posterior superior lateral protocerebrum and a putative sodium-solute co-transporter, Cupcake, in some neurons in the ellipsoid body (EB). For simplicity, only one of the multiple, bilateral Gr43a- and Cupcake-neurons is shown. (b) By means of insulin and sNPF signaling, starvation enhances synaptic transmission between ORNs and PNs in several glomeruli. Among them, DM1 is crucial for flies' food searching behavior and receives input from ab1A ORNs that express Or42b receptor. Down regulation of insulin signaling promotes the expression of sNPF receptor (sNPFR) to enhance Ca2+ response at ORN synaptic terminals. We note that the precise subcellular localization of insulin receptor (InR) is unclear. For simplicity, InR is drawn near the synaptic terminal. (c) Starvation also promotes proboscis extension response (PER) in flies to facilitate feeding. Hunger elevates dopamine (DA) release from a class of interneurons in the SOG, named TH-VUM (tyrosine hydroxylase positive, ventral unpaired medial neurons), to activate a dopaminergic receptor (DopEcR) at the sugar-sensitive gustatory receptor neurons (Gr5a GRNs). Elevation of dopaminergic signaling enhances synaptic transmission from Gr5a GRNs to the central taste center, the subesophageal ganglion (SOG), to promote PER.
Starvation modulates feeding by elevating olfactory input
In mammals, up-regulation of Neuropeptide Y (NPY) signaling in the hypothalamus increases food intake [34]. Interestingly, in addition to hypothalamic neurons, NPY is also expressed in the olfactory epithelium of a variety of vertebrates [35,36], suggesting that NPY signaling may regulate olfactory sensitivity to modulate food searching behavior.
Indeed, in flies, there is direct evidence supporting this hypothesis. Two fly homologs of mammalian NPY, neuropeptide F (NPF) and short neuropeptide F (sNPF), have been implicated in regulating feeding behavior in Drosophila [37–39]. Like its mammalian counterpart, fly sNPF is expressed in olfactory tissues [40]. Interestingly, sNPF and its receptor, sNPFR1, are both expressed in a subset of ORNs. Among them, DM1 ORNs are both necessary and sufficient to promote food searching behavior in Drosophila [20]. By down-regulating insulin signaling, starvation increases the expression of sNPFR1 at ORN axon terminals, thus strengthening ORN-PN synaptic transmission in DM1 (Figure 2b). Consequently, starvation enhances DM1 response to fruit odors, which triggers a more robust food searching behavior in hungry flies [8••]. These studies illustrate the profound impact of elevated olfactory input on the feeding behavior in adult flies.
A similar logic applies to Drosophila larvae, where the presence of fruity odorants, such as pentyl acetate, promotes feeding in larvae [41•]. Mechanistically, appetitive odorants appear to promote feeding by activating NPF receptors that are expressed in a subclass of dopaminergic interneurons in the lateral horn (DL2-LH neurons) [41•]. Similarly, another study shows food odors excite NPF neurons which are necessary to drive attraction to food odors in flies [42].
Taken together, these findings reveal a direct link between heightened olfactory activity and enhanced appetitive behavior. Remarkably, a recent study shows that endocannabinoid signaling promotes food intake by increasing odor detection in mice [43•], suggesting that the link between olfaction and feeding may be evolutionarily conserved.
Starvation modulates feeding by enhancing gustatory sensitivity
Hungry flies show heightened sensitivity to sugar and are more prone to extend their proboscis when they encounter food. Here we will describe several recent studies that address how starvation alters gustatory sensitivity by means of metabolic hormone and dopaminergic signaling mechanisms.
Upon starvation, flies release adipokinetic hormone (AKH, the fly equivalent of glucagon) in the hemolymph to signal hunger [44]. Like insulin, AKH is implicated in regulating flies' feeding behavior. Interestingly, the AKH receptor is expressed in certain gustatory receptor neurons that respond to sugar (Gr5a GRNs) [45]. Activation of the Gr5a GRNs promotes proboscis extension response (PER) in fruit flies to facilitate food intake [46–48••]. Therefore, these findings suggest that starvation may increase the sensitivity of sugar-sensitive GRNs to promote feeding in hungry flies.
Additionally, starvation heightens gustatory sensitivity by dopaminergic signaling. At the level of sensory input, as measured by Ca2+ imaging, short-term starvation (~6 hr) enhances the response of the Gr5a GRNs in the subesophageal ganglion (SOG), the primary taste center of the fly brain. Mechanistically, starvation causes dopamine release in the SOG (see below), which activates dopamine receptors (DopEcR) at Gr5a presynaptic terminals to facilitate Ca2+ influx (Figure 2c) [48••]. Interestingly, DopEcR is dispensable for enhanced PER in the flies that are starved for more than 24 hrs [48••], suggesting that multiple neuromodulatory mechanisms acting at different time scales are involved.
Indeed, a group of dopaminergic neurons in the SOG, named TH-VUM (tyrosine hydroxylase positive, ventral unpaired medial neurons), were shown to enhance PER after 24-hr starvation [49•]. TH-VUM neurons are interneurons that likely release dopamine to activate DopEcR at Gr5a axon terminals as mentioned earlier (Figure 2c). In addition, TH-VUM may innervate yet another group of neurons in the SOG that express dopamine-2 receptor (D2R) to regulate PER. TH-VUM neurons are unusual in that their basal spike activity scales with the duration of starvation up to 24 hrs. Notably, upregulating TH-VUM activity enhances proboscis extension probability when flies are presented with low concentrations of sucrose, suggesting that TH-VUM sets the behavioral threshold for PER [49•].
Regulation of feeding behavior and food preference by internal nutrient sensors
Finally, we consider internal nutrient sensors as a novel mechanism by which satiety state regulates feeding. Although not strictly neuromodulators, internal nutrient sensors may play a key role in modulating circuit function by engaging existing neuromodulatory pathways [50].
In addition to metabolic hormones, satiety state is also encoded by hemolymph sugar level (i.e. blood sugar) [51••,52•]. Gr43a, a fructose receptor expressed in peripheral GRNs, is also expressed in a small cluster of the central neurons located in the posterior superior lateral protocerebrum (Figure 2a). These Gr43a-positive neurons can thus report hemolymph fructose level directly and, strikingly, their activation assigns opposing valence to feeding experience in a satiety state-dependent manner. That is, Gr43a neurons suppress feeding in satiated flies and promote feeding in hungry flies [51••].
In addition to Gr43a, another internal sugar sensor has been identified in a genetic screen. A mutant line was found to be insensitive to nutritive sugars, which are usually preferred by hungry flies in a taste-blind assay. The affected gene, named cupcake, encodes a putative sodium/solute co-transporter, which may function to transport glucose into a subset of brain neurons in the ellipsoid body (Figure 2a) [52•]. These neurons may thus function as an internal nutrient sensor to promote feeding in hungry flies.
In addition, an internal sensor for essential amino acids (EAAs) has been identified [50]. Flies tend to avoid food sources that are EAA-deficient. This behavioral avoidance is mediated by activation of the central neurons which express an amino acid sensor, GCN2, a kinase that promotes dopamine release from these neurons [50]. This finding illustrates how metabolic demands shape food preference via an internal nutrient sensor to promote a balanced diet by recruiting an existing dopaminergic neuromodulatory pathway.
Conclusions
To conclude, the multitude of signaling molecules featured in this review provide a glimpse of how satiety state and metabolic demands regulate aspects of feeding behavior but raise an equally exciting new set of questions for future investigation. For example, do other metabolic hormones, such as the recently identified fly homolog of leptin [53], also regulate the olfactory/gustatory sensitivity in Drosophila? What is the circuit mechanism that affords Gr43a central neurons the ability to encode opposing valence in a satiety-dependent manner? Is innate olfactory or gustatory aversion also regulated by satiety state? Why does Drosophila employ multiple internal nutrient sensors? Do they act on different time scales?
The neuromodulatory mechanisms featured in this review will serve as a foundation to delineate broader principles by which neural circuits incorporate internal state to shape innate behavior. The study of neural circuit function will benefit tremendously from a growing number of available high-resolution anatomical wiring diagrams [54–57]. Finally, we note that several neuromodulators important for regulating innate behavior, including dopamine, sNPF and NPF, have also been implicated in regulating appetitive olfactory learning [58–61], suggesting a shared neuromodulatory mechanism for innate and learned behaviors. Thus, a better understanding of how innate behavior is modulated at the circuit level may well provide new insights into the neuromodulatory mechanisms that impact complex behavior, such as learning.
Highlights
Innate behavior is flexible and subject to modulation by stimulus context.
Fruit odors modulate Drosophila's innate aversion to CO2 by multiple mechanisms, operating at every known station of the CO2 olfactory circuit.
Satiety state regulates feeding behavior by altering olfactory and gustatory sensitivity.
Satiety state is communicated by a variety of metabolic cues, which regulate neuromodulator signaling to influence neural circuit function.
Acknowledgments
We thank G.M. Thomas (Temple University) and S.M. Kim for comments on the manuscript. Work in our laboratories is supported by the University of California San Diego Start-up Fund (C-Y.S.) and the National Institutes of Health Grants DC009597 and DK092640 (J.W.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–65. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 3.Riffell JA. Olfactory ecology and the processing of complex mixtures. Curr. Opin. Neurobiol. 2012;22:236–42. doi: 10.1016/j.conb.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta P. The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Curr. Opin. Neurobiol. 2013;23:68–75. doi: 10.1016/j.conb.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat. Rev. Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012;13:225–39. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 7.Sasakura H, Mori I. Behavioral plasticity, learning, and memory in C. elegans. Curr. Opin. Neurobiol. 2013;23:92–9. doi: 10.1016/j.conb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–44. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study demonstrates that insulin acts as a satiety signal that negatively regulates the expression of a neuropeptide (sNPF) receptor at ORN axon terminals. Through an autocrine mechanism, activation of the sNPF receptor increases olfactory sensitivity to food odor, thereby promoting appetitive behavior in hungry flies. This study identifies the molecular and cellular mechanisms that link starvation to heightened olfactory sensitivity to enhanced food-searching behavior.
- 9.Stensmyr MC, Dweck HKM, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]; •• The authors performed a series of elegant and comprehensive experiments to demonstrate that fruit flies have a dedicated class of ORNs for the detection of geosmin, a volatile chemical emitted by harmful microbes. Flies avoid feeding or laying eggs on food contaminated by geosmin. The Or56a-expressing ORNs, which are highly sensitive and specifically tuned to geosmin, are both necessary and sufficient for the robust innate aversion to geosmin.
- 10.Grosjean Y, Rytz R, Farine J-P, Abuin L, Cortot J, Jefferis GSXE, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–40. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 11.Katz PS. What are we talking about? Modes of neuronal communication. In: Katz P, editor. Beyond Neurotransmission: Neuromodulation and its Importance for Information Processing. 1999. pp. 1–28. [Google Scholar]
- 12.Suh GSB, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–9. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 13.Suh GSB, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr. Biol. 2007;17:905–8. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 14.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol. 2006;209:2739–48. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 15.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 16.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3574–8. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–81. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 18.Su C-Y, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper demonstrates that ORNs housed in the same sensillum inhibit each other by ephaptic coupling, a form of non-synaptic neuronal interaction. The authors showed that lateral inhibition between grouped ORNs can be observed broadly among insect sensilla and has functional impacts on the behavioral response to odor mixtures in Drosophila.
- 19.Van der Goes van Naters W. Inhibition among olfactory receptor neurons. Front. Hum. Neurosci. 2013;7:690. doi: 10.3389/fnhum.2013.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–23. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott K, Brady R, Cravchik a, Morozov P, Rzhetsky a, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–73. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 22.Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenböck G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–74. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 23.Chou Y-H, Spletter ML, Yaksi E, Leong JCS, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci. 2010;13:439–49. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki Y, Rybak J, Wicher D, Sachse S, Hansson BS. Physiological and morphological characterization of local interneurons in the Drosophila antennal lobe. J. Neurophysiol. 2010;104:1007–19. doi: 10.1152/jn.00249.2010. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005;25:9069–79. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–50. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Das S, Sadanandappa MK, Dervan A, Larkin A, Lee JA, Sudhakaran IP, Priya R, Heidari R, Holohan EE, Pimentel A, et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E646–54. doi: 10.1073/pnas.1106411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin H-H, Chu L-A, Fu T-F, Dickson BJ, Chiang A-S. Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science. 2013;340:1338–41. doi: 10.1126/science.1236693. [DOI] [PubMed] [Google Scholar]; •• The authors use elegant genetic approaches to delineate the olfactory neural circuit for CO2 detection. They found that multiple types of PNs innervate the V glomerulus, which receives CO2-responsive ORN (ab1C) input. These PNs differ in their sensitivity to ab1C input and brain projection pattern. Interestingly, the PNs mediating behavioral aversion at low concentrations of CO2 input are subject to inhibition by a class of GABAergic PNs that receive input from many glomeruli, implying that aversion to low levels of CO2 could be suppressed by activity in other glomeruli.
- 29.Bräcker LB, Siju KP, Varela N, Aso Y, Zhang M, Hein I, Vasconcelos ML, Grunwald Kadow IC. Essential role of the mushroom body in context-dependent CO2 avoidance in Drosophila. Curr. Biol. 2013;23:1228–34. doi: 10.1016/j.cub.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Su C-Y, Carlson JR. Neuroscience. Circuit logic of avoidance and attraction. Science. 2013;340:1295–7. doi: 10.1126/science.1240139. [DOI] [PubMed] [Google Scholar]
- 31.Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, Luo L. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79:917–31. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors describe the functional implication of GABAergic PNs that receive input from multiple glomeruli in the antennal lobe and project to multiple brain regions via the medial antennocerebral tract (mACT).
- 32.Wasserman S, Salomon A, Frye MA. Drosophila tracks carbon dioxide in flight. Curr. Biol. 2013;23:301–6. doi: 10.1016/j.cub.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors devised an automated behavioral assay to monitor Drosophila's behavioral responses to odors when flies are in flight. They found that flying flies are attracted to CO2 and this in-flight attraction is mediated by central octopaminergic neurons, which may receive CO2 input from other primary sensory neurons besides ab1C ORNs.
- 33.Dethier VG. The hungry fly: A physiological study of the behavior associated with feeding. Harvard University Press: 1976. [Google Scholar]
- 34.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3940–3. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–4. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 36.Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide Y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum) J. Neurosci. 2006;26:7707–17. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K-S, You K-H, Choo J-K, Han Y-M, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J. Biol. Chem. 2004;279:50781–9. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- 38.Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–61. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 39.Nässel DR, Winther AME. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson M a, Diesner M, Schachtner J, Nässel DR. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J. Comp. Neurol. 2010;518:3359–80. doi: 10.1002/cne.22405. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Pu Y, Shen P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013;3:820–30. doi: 10.1016/j.celrep.2013.02.003. [DOI] [PubMed] [Google Scholar]; • The authors uncovered the neuromodulatory mechanism by which appetitive odorants promote feeding in Drosophila larvae. Briefly, olfactory input from appetitive odorants activates NPF receptors that are expressed in a subset of dopaminergic neurons in the lateral horn to promote feeding.
- 42.Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J. Neurosci. 2013;33:15693–704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, Ruehle S, Remmers F, Desprez T, Matias I, et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014;17:407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]; • This study shows that endocannabinoid signaling may promote food intake by enhancing odor detection in mice, demonstrating a direct link between olfactory sensitivity and feeding behavior.
- 44.Kim S, Rulifson E. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 45.Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 2008;211:3103–10. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–91. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–79. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–95. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors developed an innovative and elegant genetic approach, named TANGO-map, to label neurons in which dopaminergic signaling is elevated during starvation. They found that starvation lead to a higher level of dopaminergic signaling in sugar-sensing gustatory receptor neurons (Gr5a GRNs) and that expression of a dopamine receptor at the axon terminals of Gr5a GRNs is necessary for the enhanced sensory and behavioral PER response in hungry flies.
- 49.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–50. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper shows that starvation-modulated PER requires a class of dopaminergic neurons in the SOG, a brain region that processes taste information. Interestingly, the basal spike activity of these dopaminergic neurons, which scales with starvation time, set the threshold for PER so that starved flies are more prone to extend their proboscis when they encounter food.
- 50.Bjordal M, Arquier N, Kniazeff J, Pin JP, Léopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–21. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–25. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• These authors identified the first internal sugar sensor in the Drosophila brain. The sensor, Gr43a, is specifically tuned to fructose, the level of which rises rapidly upon feeding. Notably, activation of GR43a-expressing brain neurons promotes feeding in hungry flies but suppresses feeding in satiated flies, suggesting that these central neurons integrate satiety information to assign valence for feeding experience.
- 52.Dus M, Ai M, Suh G. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat. Neurosci. 2013;16:526–8. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Using a genetic screen for mutant flies that fail to discriminate between nutritive and non-metabolizable sugars, the authors identified a novel gene, named cupcake, which likely functions as an internal nutrient sensor. cupcake is expressed in a subset of the neurons in the ellipsoid body, implicating a new brain region that also regulates feeding behavior in Drosophila.
- 53.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–37. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takemura S, Bharioke A, Lu Z, Nern A, Vitaladevuni S, Rivlin PK, Katz WT, Olbris DJ, Plaza SM, Winston P, et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature. 2013;500:175–81. doi: 10.1038/nature12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiang A-S, Lin C-Y, Chuang C-C, Chang H-M, Hsieh C-H, Yeh C-W, Shih C-T, Wu J-J, Wang G-T, Chen Y-C, et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 57.Bargmann CI, Marder E. From the connectome to brain function. Nat. Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Plaçais P-Y, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–6. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 59.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–27. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perisse E, Burke C, Huetteroth W, Waddell S. Shocking revelations and saccharin sweetness in the study of Drosophila olfactory memory. Curr. Biol. 2013;23:R752–63. doi: 10.1016/j.cub.2013.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knapek S, Kahsai L, Winther AME, Tanimoto H, Nässel DR. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. J. Neurosci. 2013;33:5340–5. doi: 10.1523/JNEUROSCI.2287-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]