Abstract.
Type 1 diabetes (T1D) is an organ-specific autoimmune disease caused by the autoimmune response against pancreatic β cells. T1D is often complicated with other autoimmune diseases, and anti-islet autoantibodies precede the clinical onset of disease. The most common coexisting organ-specific autoimmune disease in patients with T1D is autoimmune thyroid disease, and its frequency is estimated at > 90% among patients with T1D and autoimmune diseases. The prevalence of anti-thyroid antibodies in children with T1D at disease onset is about 20% and is particularly common in girls. Furthermore, patients with anti-thyroid antibodies are 18 times more likely to develop thyroid disease than patients without anti-thyroid antibodies. Therefore, for early detection of autoimmune thyroid disease in children with T1D, measurement of anti-thyroid antibodies and TSH at T1D onset and in yearly intervals after the age of 12 yr is recommended. Anti-islet autoantibodies are predictive and diagnostic markers for T1D. The most frequently detected autoantibodies in Japanese patients are GAD autoantibodies (~80%) followed by IA-2 autoantibodies (~60%), insulin autoantibodies (~55%) and ZnT8 autoantibodies (~50%). In a combined analysis, 94% of Japanese patients with T1D can be defined as having type 1A diabetes. Furthermore, autoantibodies to ZnT8 and IA-2 are associated with childhood-onset and acute-onset patients. Thus, it is important to develop a diagnostic strategy for patients with type 1A diabetes in consideration of the age or mode of disease onset.
Keywords: anti-islet autoantibodies, autoimmune thyroid disease, prediction, type 1 diabetes, Zinc transporter 8
Introduction
Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by the selective destruction of pancreatic β-cells. The histopathology of T1D is defined by a decreased β-cell mass with infiltration of mononuclear cells into the islets of Langerhans, which was described in 1901 by Opie (1). This lesion was later called ‘insulitis’, and it is the hallmark of T1D. In 1965, Gepts reported that insulitis was observed in 70% of patients with acute-onset T1D and concluded that this disease was caused by a β-cell-specific autoimmune process (2). Furthermore, in the 1970s, Nerup demonstrated cellular autoimmunity in patients with T1D using the leukocyte migration test and speculated that cellular hypersensitivity was the counterpart of lymphocytic infiltration in islets (3). Therefore, he speculated that cell-mediated immunity could play an important part in the pathogenesis of T1D. As a view suggesting that T1D is an autoimmune disease, there is some evidence that T1D is often complicated with other autoimmune diseases or that anti-islet autoantibodies precede the clinical onset of the disease. In this article, I focus on these two points and review the recent knowledge.
Type 1 Diabetes and Autoimmune Thyroid Disease
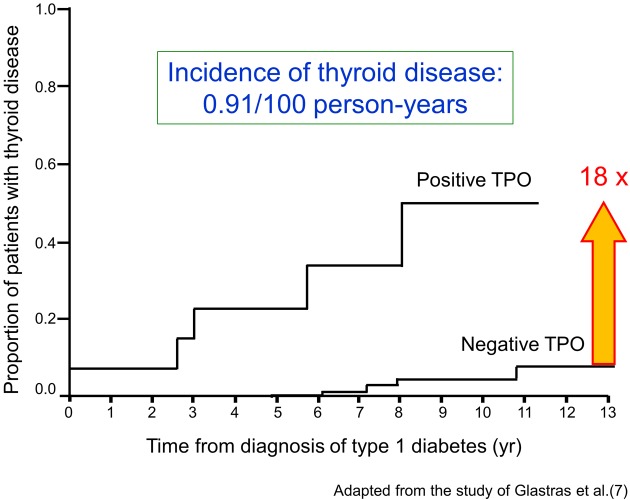
It is well known that T1D is frequently associated with other organ-specific autoimmune diseases, including autoimmune thyroid disease (AITD), pernicious anemia, and idiopathic Addison’s disease (4). Table 1 summarizes the prevalence of organ-specific autoimmune disease complicating T1D in Japanese and Caucasoid patients (5). In Japanese patients with T1D, the most common coexisting organ-specific autoimmune disease is AITD (> 90%). The prevalence of anti-thyroid autoantibodies in children with T1D at disease onset is about 20%, and anti-thyroid autoantibodies are particularly common in girls. Furthermore, it is reported that the prevalence of anti-thyroid antibodies increases with increasing age and that the presence of anti-thyroid antibodies at diagnosis of T1D predicts the development of future thyroid disease (6). Patients with anti-thyroid antibodies are 18 times more likely to develop thyroid disease than patients without anti-thyroid antibodies (7) (Fig.1). Therefore, for early detection of AITD in children with T1D, Glastras et al. suggested measurement of anti-thyroid antibodies and TSH at T1D onset and in yearly intervals after the age of 12 yr. Furthermore, the International Society for Pediatric and Adolescent Diabetes (ISPAD) Consensus Clinical Guidelines recommend the screening of thyroid function by analyzing circulating TSH at the diagnosis of diabetes and, thereafter, every 2nd yr in asymptomatic individuals without goiter and more frequent if goiter is present.
Table 1. The prevalence of autoimmune disease complicating type 1 diabetes.
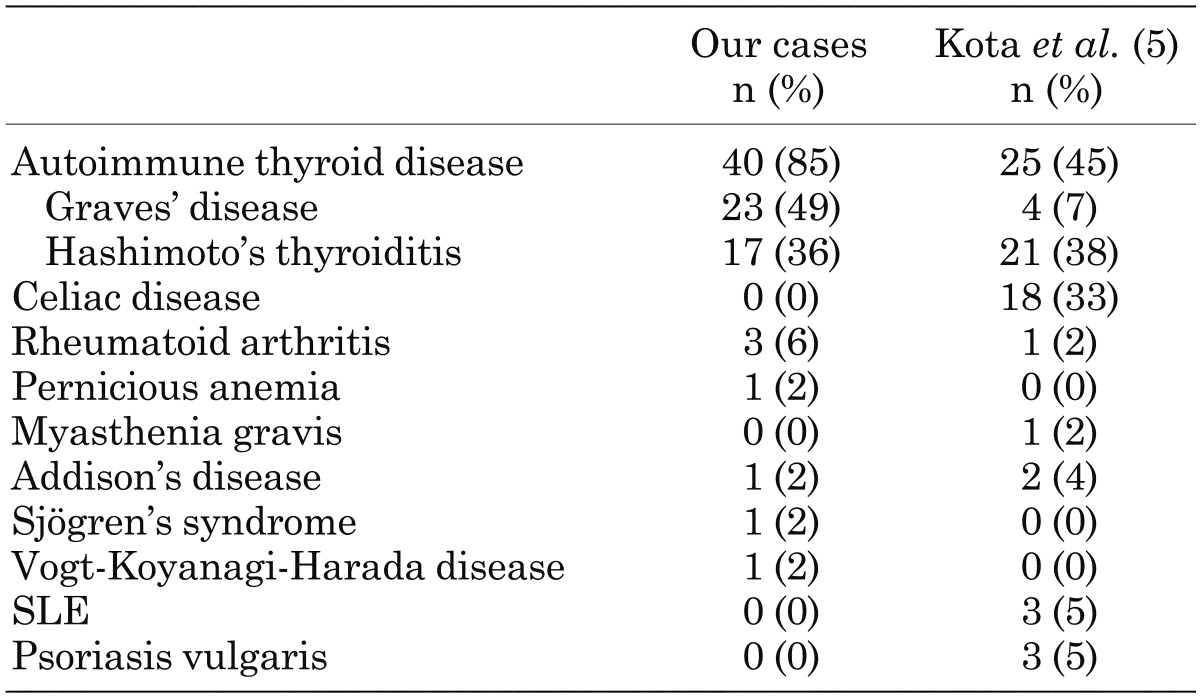
Fig. 1.
Risk for the development of autoimmune thyroid disease in children with type 1 diabetes. TPO, thyroid peroxidase antibodies. (Material from this publication has been used with the permission of American Diabetes Association from Glastras SJ, et al.: The role of autoimmunity at diagnosis of type 1 diabetes in the development of thyroid and celiac disease and microvascular complications. Diabetes Care 2005; 28(9): 2170-2175. Copyright and all rights reserved.)
To characterize the T1D patients complicated with AITD (autoimmune polyendocrine syndrome type 3 variant, APS3v), we have analyzed the clinical characteristics of patients with APS3v who were consecutively diagnosed at Nagasaki University Hospital (8). A remarkable female predominance (M:F=1:4.4), a slow and older age of onset of T1D and a higher prevalence of GAD autoantibodies were observed in APS3v patients compared with T1D patients without AITD. Furthermore, among the patients with T1D and Graves’ disease, 60% of patients developed Graves’ disease preceding the onset of T1D, and 30% developed Graves’ disease after the onset of T1D; there were also a few patients who developed T1D and Graves’ disease simultaneously (10%). The interval between the onsets of T1D and Graves’ disease was less than 10 yr in most cases but was close to 20 yr or more than 20 yr in some cases (Fig. 2).
Fig. 2.
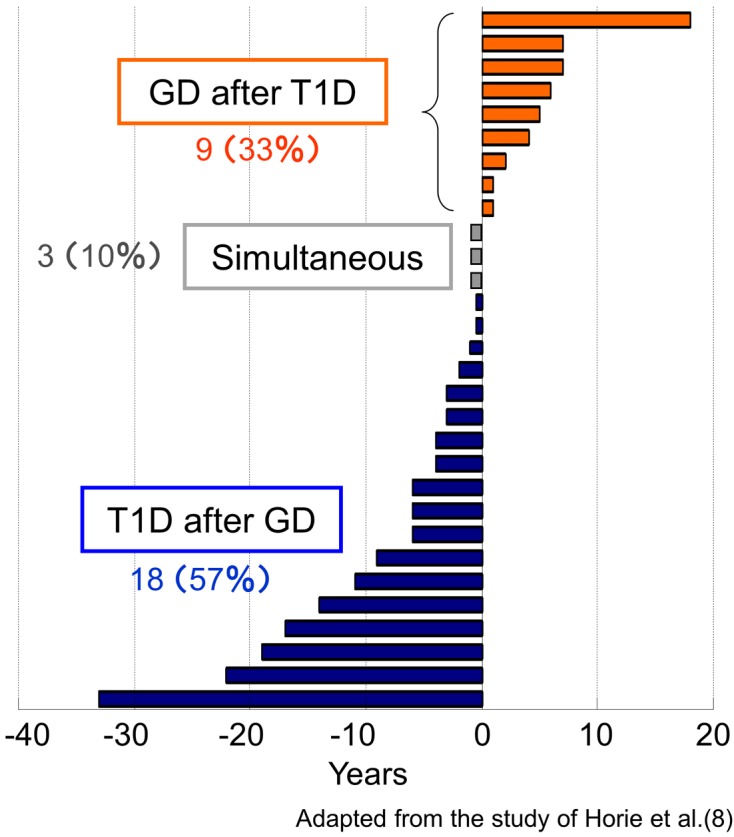
The interval from the onset of type 1 diabetes to the onset of Graves’ disease in APS3v patients. T1D, type 1 diabetes; GD, Graves’ disease; APS3v, autoimmune polyendocrine syndrome type 3 variant.
Anti-islet Autoantibodies in Type 1 Diabetes
Japanese T1D can be divided into three subtypes, i.e., the fulminant form, acute-onset form and slow-onset form (slowly-progressive form) (9). Among patients with them, those with slowly-progressive T1D are generally indistinguishable from type 2 diabetes if anti-islet autoantibodies are not examined.
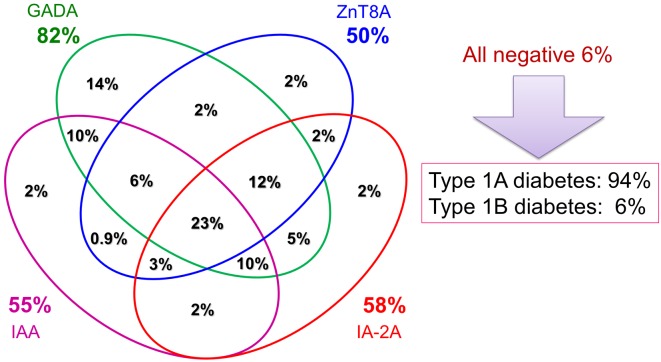
In 1974, Bottazzo and MacCuish firstly described the presence of anti-islet autoantibodies (islet cell antibodies, ICA) in patients with autoimmune polyendocrine syndrome by an indirect immunofluorescence technique (10, 11). In the 1990s, many investigators tried to find target autoantigens against ICA, and glutamic acid decarboxylase (GAD), insulinoma-associated antigen-2 (IA-2) and, more recently, zinc transporter 8 (ZnT8) were identified (12,13,14). Previous studies have reported that anti-islet autoantibodies were detected in > 90% of Caucasian patients with T1D (14, 15). In a radioligand binding assay using an in vitro transcribed/translated 35S-labeled protein, we identified GAD autoantibodies in 82% patients with Japanese T1D at disease onset (16). The next most frequently identified anti-islet autoantibodies in Japanese T1D were IA-2 autoantibodies (58%) followed by insulin autoantibodies (IAA) (55%) and ZnT8 autoantibodies (50%) (Fig. 3). Furthermore, the prevalence of autoantibodies to ZnT8 and IA-2 was inversely related to the onset age and significantly higher in childhood-onset patients compared with adult-onset patients (Table 2). Thus, autoantibodies to ZnT8 and IA-2 identify heterogeneity in the age of diabetes onset and are good markers of childhood-onset T1D.
Fig. 3.
Combined analysis of anti-islet autoantibodies in Japanese patients with type 1 diabetes at disease onset. GADA, GAD autoantibodies; IAA, insulin autoantibodies; ZnT8A, ZnT8 autoantibodies; IA-2A, IA-2 autoantibodies.
Table 2. Combined analysis of anti-islet autoantibodies in childhood- and adult-onset patients with type 1 diabetes.
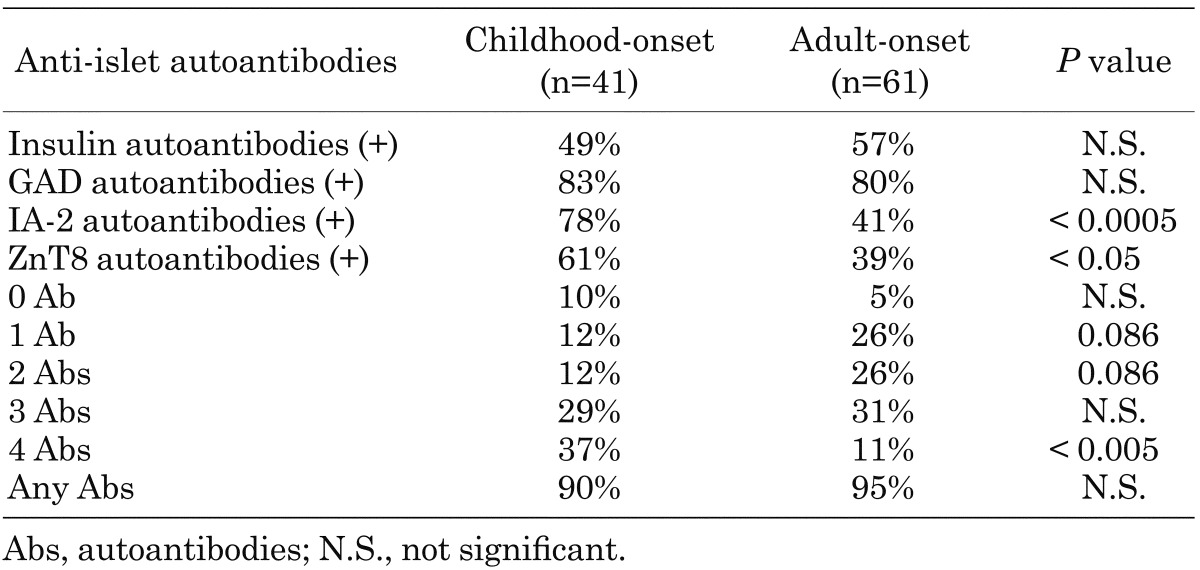
Measurement of a combination of autoantibody markers has been suggested as a useful tool for determining type 1A diabetes. In a combined analysis, 94% of Japanese patients have at least one of these autoantibodies and are defined as having type 1A (autoimmune-mediated) diabetes (16) (Fig. 3). However, the clinical utility of ZnT8 autoantibodies is limited over testing autoantibodies to GAD, IA-2 and insulin in childhood-onset patients. In our cohort, 90% of the childhood-onset patients had autoantibodies to GAD and/or IA-2, but inclusion of autoantibodies to insulin and/or ZnT8 did not increase the sensitivity for identifying type 1A diabetes. In contrast, inclusion of the ZnT8 autoantibodies reduced the number of autoantibody-negative subjects in the adult-onset patients from 8% to 5%, and 40% of patients who were negative for autoantibodies to GAD, IA-2, and insulin were positive for ZnT8 autoantibodies. Such a broader autoantibody response in adult-onset patients suggests that different pathogenic mechanisms may be involved between adult-onset and childhood-onset T1D.
Anti-islet Autoantibodies and Specificity of β Cell Destruction
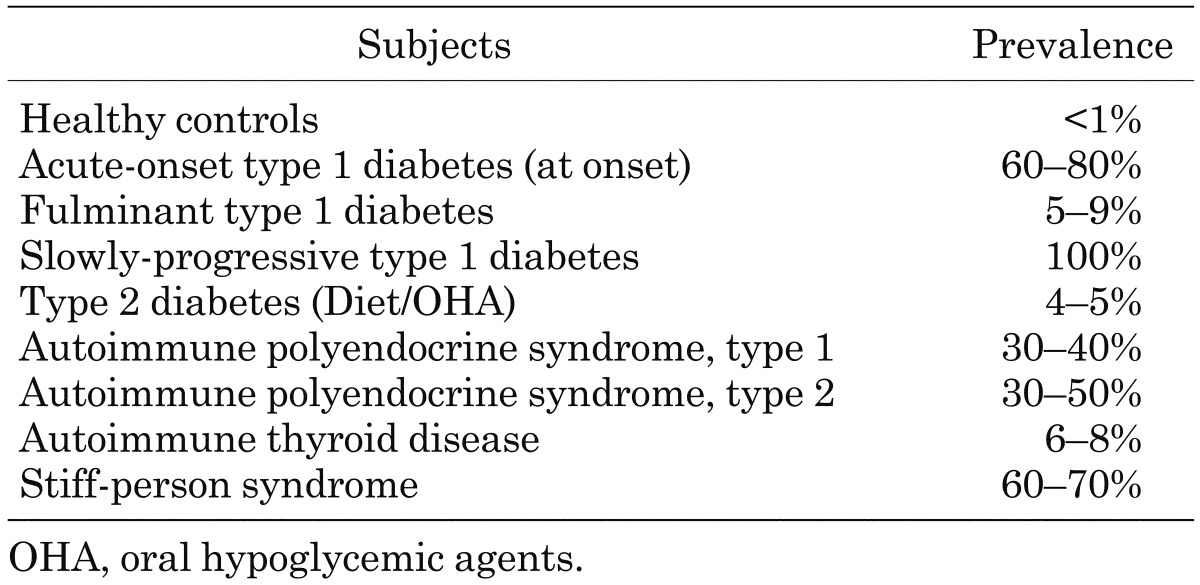
It is generally accepted that T1D is a T cell-mediated autoimmune disease and that circulating autoantibodies to various islet cell antigens are induced following the destruction of pancreatic β cells. Therefore, anti-islet autoantibodies are used as a predictive marker for the development of T1D. However, associations between the autoantibody positivity and the specificity of β cell destruction are variable depending on the target autoantigens. Table 3 summarizes the disease specificity of GAD autoantibodies. GAD autoantibodies were originally identified in patients with stiff-person syndrome regardless of the coexistence of T1D (17). Furthermore, GAD autoantibodies can be detected in other diseases such as APS1, AITD, or type 2 diabetes. We and others have previously reported the association between anti-thyroid autoimmunity and anti-islet autoantibodies, especially autoantibodies to GAD. Patients with T1D and AITD (i.e., APS3) show higher levels of GAD autoantibodies compared with patients with T1D alone in both cross-sectional and longitudinal observations (18). Because high levels of GAD autoantibodies are observed in insulin-deficient patients as in our case, production of GAD autoantibodies may not associated with the residual β cell antigens. Furthermore, it has been reported that GAD is not only expressed in β cells but also in the thyroid gland. In contrast, it is suggested that autoantibodies to IA-2 and ZnT8 are more specific markers of autoimmune-mediated β cell destruction.
Table 3. Disease specificity of GAD autoantibodies.
Conclusion
In this article, I reviewed the recent knowledge regarding the autoimmune diseases associated with T1D and anti-islet autoantibodies. Although the underlying mechanisms with respect to the development of multiple autoimmune diseases within the same person are largely unknown, recent progress including the identification of several loci with associations to more than one autoimmune disease (19) suggests that common genetic factors or immunological processes are present among the different autoimmune diseases. As the most common coexisting organ-specific autoimmune disease associated with Japanese T1D is autoimmune thyroid disease, children with T1D, or with a family history of T1D, should be aware of the tendency to develop additional autoimmune disorders, especially autoimmune thyroid disease.
The clinical utilities of anti-islet autoantibodies in patients with diabetes include diagnosis (type 1A or type 1B), prediction (progressor or non-progressor) and understanding of pathophysiology (insulitis-specific or nonspecific phenomenon) (Fig. 4). It is especially necessary to pay attention to the interpretation of GAD autoantibodies. The development of a high-throughput assay to detect epitope-specific or immunoglobulin isotype-specific autoantibodies should warrant accurate diagnosis and prediction of autoimmune disorders.
Fig. 4.
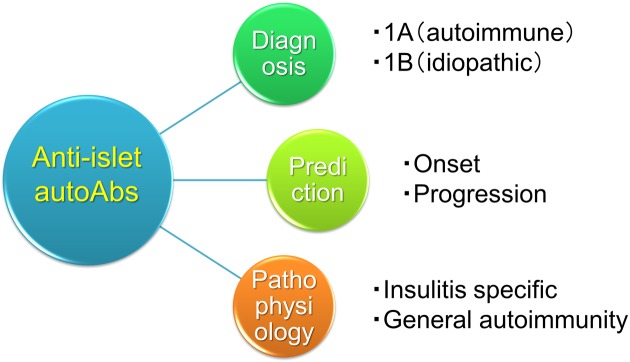
Clinical utilities of anti-islet autoantibodies in patients with diabetes.
Acknowledgments
This study was partly supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Opie EL. On the Relation of chronic interstitial pancreatitis to the islands of Langerhans and to diabetes melutus. J Exp Med 1901;5: 397–428. doi: 10.1084/jem.5.4.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14: 619–33. [DOI] [PubMed] [Google Scholar]
- 3.Nerup J, Andersen OO, Bendixen G, Egeberg J, Poulsen JE. Anti-pancreatic cellular hypersensitivity in diabetes mellitus. Diabetes 1971;20: 424–7. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki E, Gill RG, Eisenbarth GS. Type 1 diabetes mellitus. In: Eisenbarth GS, ed. Molecular mechanisms of endocrine and organ specific autoimmunity. Austin, Texas: R.G. Landes Company; 1999:149-82. [Google Scholar]
- 5.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr 2012;6: 70–6. doi: 10.1016/j.dsx.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Kordonouri O, Klinghammer A, Lang EB, Grüters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care 2002;25: 1346–50. doi: 10.2337/diacare.25.8.1346 [DOI] [PubMed] [Google Scholar]
- 7.Glastras SJ, Craig ME, Verge CF, Chan AK, Cusumano JM, Donaghue KC. The role of autoimmunity at diagnosis of type 1 diabetes in the development of thyroid and celiac disease and microvascular complications. Diabetes Care 2005;28: 2170–5. doi: 10.2337/diacare.28.9.2170 [DOI] [PubMed] [Google Scholar]
- 8.Horie I, Kawasaki E, Ando T, Kuwahara H, Abiru N, Usa T, et al. Clinical and genetic characteristics of autoimmune polyglandular syndrome type 3 variant in the Japanese population. J Clin Endocrinol Metab 2012;97: E1043–50. doi: 10.1210/jc.2011-3109 [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia 2006;49: 828–36. doi: 10.1007/s00125-006-0213-8 [DOI] [PubMed] [Google Scholar]
- 10.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974;2: 1279–83. doi: 10.1016/S0140-6736(74)90140-8 [DOI] [PubMed] [Google Scholar]
- 11.MacCuish AC, Irvine WJ, Barnes EW, Duncan LJP. Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet 1974;2: 1529–31. doi: 10.1016/S0140-6736(74)90281-5 [DOI] [PubMed] [Google Scholar]
- 12.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347: 151–6. doi: 10.1038/347151a0 [DOI] [PubMed] [Google Scholar]
- 13.Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 1994;13: 505–14. doi: 10.1089/dna.1994.13.505 [DOI] [PubMed] [Google Scholar]
- 14.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104: 17040–5. doi: 10.1073/pnas.0705894104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45: 926–33. doi: 10.2337/diab.45.7.926 [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki E, Nakamura K, Kuriya G, Satoh T, Kobayashi M, Kuwahara H, et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin Immunol 2011;138: 146–53. doi: 10.1016/j.clim.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 17.Solimena M, Folli F, Denis-Donini S, Comi GC, Pozza G, De Camilli P, et al. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med 1988;318: 1012–20. doi: 10.1056/NEJM198804213181602 [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki E, Takino H, Yano M, Uotani S, Matsumoto K, Takao Y, et al. Autoantibodies to glutamic acid decarboxylase in patients with IDDM and autoimmune thyroid disease. Diabetes 1994;43: 80–6. doi: 10.2337/diab.43.1.80 [DOI] [PubMed] [Google Scholar]
- 19.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447: 661–78. doi: 10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]