Summary
Changes in temperature disrupt the fluidity of cellular membranes, which can negatively impact membrane integrity and cellular processes. Many ectotherms, including Drosophila melanogaster (Meigen), adjust the glycerophospholipid composition of their membranes to restore optimal fluidity when temperatures change, a type of trait plasticity termed homeoviscous adaptation.
Existing data suggest that plasticity in the relative abundances of the glycerophospholipids phosphatidylethanolamine (PE) and phosphatidylcholine (PC) underlies cellular adaptation to temporal variability in the thermal environment. For example, laboratory populations of D. melanogaster evolved in the presence of temporally variable temperatures have greater developmental plasticity of the ratio of PE to PC (PE/PC) and greater fecundity than do populations evolved at constant temperatures.
Here, we extend this work to natural populations of D. melanogaster by evaluating thermal plasticity of glycerophospholipid composition at different life stages, in genotypes isolated from Vermont, Indiana and North Carolina, USA. We also quantify the covariance between developmental and adult (reversible) plasticity, and between adult responses of the membrane to cool and warm thermal shifts.
As predicted by physiological models of homeoviscous adaptation, flies from all populations decrease PE/PC and the degree of lipid unsaturation in response to warm temperatures. Furthermore, these populations have diverged in their degree of membrane plasticity. Flies from the most variable thermal environment (Vermont, USA) decrease PE/PC to a greater extent than do other populations when developed at a warm temperature, a pattern that matches our previous observation in laboratory-evolved populations. We also find that developmental plasticity and adult plasticity of PE/PC covary across genotypes, but that adult responses to cool and warm thermal shifts do not.
When combined with our previous observations of laboratory-evolved populations, our findings implicate developmental plasticity of PE/PC as a mechanism of thermal adaptation in temporally variable environments. While little is known about the genetic bases of plastic responses to temperature, our observations suggest that both environmentally sensitive and environmentally specific alleles contribute to thermal adaptation of membranes, and that costs of plasticity may arise when the adult environment differs from that experienced during development.
Keywords: acclimation, cellular membranes, Drosophila melanogaster, homeoviscous adaptation, phenotypic plasticity, thermal adaptation
Introduction
Changes in temperature alter the fluidity of cell membranes through thermodynamic effects on the fatty acid hydrocarbons that comprise the membrane bilayer. In response to changes in temperature, cells dynamically remodel membrane composition. This plastic response maintains fluidity and reduces the deleterious effects of temperature on membranes, such as phase transitions, increased cellular permeability, and compromised function of membrane bound proteins (Sinensky 1974; Hazel 1995; Hochochka & Somero 2002; Montooth, Siebenthall & Clark 2006; Overgaard et al. 2008; Cooper et al. 2012). Changes in the relative abundance of particular glycerophospholipid (GPL) head groups (e.g., phosphatidylethanolamine (PE) and phosphatidylcholine (PC)) and/or lipid saturation enable homeostasis of membrane fluidity. Physiological models predict that relatively higher concentrations of PC and lipid saturation enable membrane homeostasis in warm environments, while higher concentrations of PE and of unsaturated lipids maintain membrane homeostasis in cool environments (Sinensky 1974; Hazel 1995). This homeoviscous adaptation describes both the adaptation of membrane GPL composition to the mean thermal environment experienced through evolutionary time (i.e., cellular specialization), and the acclimation of membrane GPL composition to the current environment (i.e., cellular generalization or plasticity).
Evolutionary models predict that environments that vary in time or space favor generalist strategies that maintain fitness across a range of environments (Levins 1968; Lynch & Gabriel 1987; Gilchrist 1995). Generalists with fixed phenotypes may have reduced fitness relative to specialists in their home environments due fitness tradeoffs across environments (Whitlock 1996; Reboud & Bell 1997; Kassen 2002). In contrast, generalists with plastic phenotypes can match their phenotypes to the current environment, and should be favored in variable environments when genetic variation for plasticity exists and the costs of plasticity are low relative to the benefits (Via & Lande 1985; van Tienderen 1991; Gomulkiewicz & Kirkpatrick 1992). Models of plasticity predict that environments that vary greatly across generations favor developmental responses, whereby the developmental environment determines the adult phenotype (Gabriel & Lynch 1992), while environments that vary greatly within generations favor reversible responses (e.g., within the adult life stage) (Gabriel et al. 2005). Evolutionary models generally assume genetic independence of developmental and reversible responses, but shared physiological mechanisms for traits like membrane remodeling could generate covariance between these types of plasticity. In holometabolous insects, the extent to which metamorphosis in the pupal stage decouples larval and adult responses to temperature remains unknown. Such decoupling could enable selection to act independently on thermal physiologies across life stages (Moran 1994).
The holometabolous Drosophilid flies inhabit a wide range of thermal environments from the relatively constant tropics to the highly seasonal temperate latitudes (David & Capy 1988; Hoffmann, Sørensen & Loeschcke 2003; Cooper, Czarnoleski & Angilletta 2010). Yet, we know relatively little about how membrane composition and plasticity have diverged between species or among populations. Species of Drosophila from different latitudes in Japan have diverged in two GPL components (monoenoic and dienoic fatty acids), but not in overall levels of unsaturation (Ohtsu, Kimura & Katagiri 1998). Drosophila melanogaster (Meigen) changes the PE to PC ratio (PE/PC) in response to developmental temperature as predicted by physiological models of homeoviscous adaptation (Overgaard et al. 2008), but the contribution of this GPL plasticity to surviving short, cold bouts of thermal stress is less clear (Overgaard et al. 2005; Overgaard et al. 2006; MacMillan, Guglielmo & Sinclair 2009). Laboratory evolution experiments using D. melanogaster found no evidence for GPL specialization to constant 16° and 25°C environments, but demonstrated that temporal variation in temperature across generations selectively maintained greater developmental plasticity of PE/PC (Cooper et al. 2012). Populations with greater plasticity of PE/PC also have higher fecundity across a range of temperatures, suggesting that this cellular response impacts components of fitness (Condon et al. in press). Yet, the extent to which natural populations have diverged in membrane plasticity, whether this plasticity covaries across life stages, and the evolutionary genetic basis for this important physiological adaptation remain unknown.
Here, we investigate patterns of cellular membrane adaptation to temperature among populations and across life stages of D. melanogaster. We characterize membrane composition and plasticity in populations of D. melanogaster from Vermont (VT), Indiana (IN), and North Carolina (NC), USA estimated to experience both different mean temperatures and different amounts of temporal variation in temperature (see Appendix S1 and Table S1 in Supporting Information), which should favor GPL specialization and plasticity, respectively. We tested 1) whether flies that experience the lowest mean temperatures (VT) have greater PE/PC and greater lipid unsaturation regardless of thermal treatment, 2) given our previous results in the laboratory, whether flies from the most variable thermal environment (VT) more strongly decrease PE/PC when developed at a warm temperature, and 3) whether plasticity of PE/PC covaries across life stages, as would be expected if there was a shared genetic basis of this trait throughout ontogeny.
Methods
Populations of D. melanogaster
In the fall of 2011, we sampled genotypes from East Calais, Vermont, USA; Bloomington, Indiana, USA; and Raleigh, North Carolina, USA. These populations reside at different latitudes and, as a result, experience different mean temperatures and variances in temperature, which we verified using a degree-day model (see Table S1) (Cooper, Czarnoleski & Angilletta 2010; Nilsson-Ortman et al. 2012). Captured females were placed into individual vials and allowed to lay eggs. For the following two generations, one virgin female was paired with one male sibling to establish isofemale genetic lines. These lines have since been maintained at very small densities in culture vials to minimize genetic variation within lines with standard Bloomington Drosophila cornmeal-yeast medium at 20.5°C on a 12:12 light cycle.
Experimental treatments
We quantified the developmental and adult plasticity of membrane GPL composition in 13, 14, and 12 isofemale lines from VT, IN, and NC, respectively. During the experiment, 0–3 day old mated females from each line were placed into yeasted food vials for conditioning. Three days later, twenty females from these vials were split between two yeasted vials and allowed to lay eggs for 24 hours at 20.5°C. Eggs from each line were then placed into 16° and 26°C constant environments and allowed to develop to adults. To evaluate adult responses, subsets of adults from each line were shifted on the first day of adult emergence from their respective developmental environments to the opposite thermal environment for 72 hours (i.e., larvae developed at 16°C were shifted upon adult emergence to 26°C, and vice versa). In total, we exposed a median of 4 replicates of each genotype from each population to four treatments: 1) development at 16°C and maintained at 16°C, 2) development at 26°C and maintained at 26°C, 3) development at 16°C with an adult shift to 26°C, and 4) development at 26°C with an adult shift to 16°C.
Extraction, separation and quantification of membrane GPL composition
We used a lipid-profiling assay that we previously developed to quantify the GPL composition of D. melanogaster membranes (Hammad et al. 2011; Cooper et al. 2012). We weighed replicate groups of twenty 2–5 day old male flies from each treatment and extracted their GPLs in ice-cold chloroform-methanol (2:1) (Folch, Lees & Stanley 1957; Kostal & Simek 1998). We centrifuged each homogenate at 5,000 g for 10 minutes at 4°C and extracted the supernatant. After this process was repeated, the supernatants were combined with 0.2 ml of 0.9% NaCl, vortexed and centrifuged again. We then dried the lower organic phase under nitrogen and stored samples at -80°C. Whole-fly extracts represent the entire pool of available GPLs, but preclude the measurement of tissue heterogeneity or other membrane structures (e.g., lipid rafts). Nevertheless, PE and PC comprise the bulk of Drosophila membranes (Jones et al. 1992), and both PE/PC and lipid saturation measured from whole-fly extracts change in response to temperature in D. melanogaster (Overgaard et al. 2008; Cooper et al. 2012).
Lipids were separated and quantified by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS). Internal PE and PC standards were used to account for biased detection of GPLs with different head groups (Hammad et al. 2011). Dried GPL samples were reconstituted in 1 mL methanol, further diluted by a factor of fifty in methanol, and the internal standards (1,2-dimyristoyl-sn-glycero-3- phosphoethanolamine and 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine) were added to a final concentration of 200 pg/µl. We analyzed our samples using a Dionex 3000 Ultimate LC system (Dionex, Sunnyvale, CA) interfaced to a QTRAP 4000 triple quadrupole instrument (ABI Sciex, Foster City, CA). A 1-µL aliquot of the reconstituted lipid extract was injected onto a Kinetex C18 column (100 mm × 2.1 mm, 2.6 µm particle size) maintained at 25°C. Mobile phase A consisted of 10 mM ammonium acetate in H20:methanol (10%:90%, v:v). Mobile phase B consisted of 10 mM ammonium acetate in isopropanol:methanol (50%:50%, v:v). The gradient conditions were 30% B to 65% B from 3–10 minutes followed by 3 minutes at 100% B.
We converted analyte peak areas into mole fraction percentages and calculated the percent abundance of each PE and PC GPL in the total pool of PE and PC (Overgaard et al. 2008; Cooper et al. 2012). Percentages were corrected using the peak areas of the internal standards in each sample. For each sample, we then calculated PE/PC as the proportion of GPLs containing PE divided by the proportion of GPLs containing PC. We assigned the degree of unsaturation of each GPL as the sum of the number of double bonds across its two acyl chains, and calculated the proportion of monounsaturated (one double bond) and polyunsaturated (>1 double bond) GPLs in each sample.
Statistical analyses
We used mixed model analyses of variance to test our hypotheses using the nlme library in the software package R version 2.12.1 (R Core Team 2008). We evaluated the fixed effects of population, temperature treatment, LC-MS/MS block, their interactions, and the random effect of isofemale line on the membrane GPL traits, PE/PC and Saturation. LC-MS/MS block accounts for technical effects of changing the LC column. Samples from all lines were randomized across blocks, with every population represented in each block, and samples from all treatments for any included line were represented within the same block. Because the LC column can only be used for ~ 100 samples, not all lines can be represented within a single block. This causes sampling effects between blocks that can result in population by block interaction effects (Table 1). Because of this, we used a high level of biological replication for each line that resulted in in 46, 57, and 35 measures of each trait in each of the four treatments for VT, IN, and NC populations, respectively, for a total of 552 samples for lipid quantification. This level of replication (median of 4 biological replicates per line per treatment) exceeds that of other recent lipid profiling studies using MS/MS in Drosophila (e.g., Scheitz et al. 2013). A significant effect of treatment indicates that the trait is plastic in response to temperature, while a significant treatment by population interaction indicates that populations differ in the degree of plasticity. To test for specialization of membrane GPL traits across populations (e.g., population differences in PE/PC regardless of treatment), we evaluated the fixed effects of population, LC-MS/MS block, their interactions, and the random effect of line on membrane traits within temperature treatments.
Table 1.
Mixed model ANOVAs evaluating the effect of developmental temperature on membrane traits. The random effect of genetic line is included in each model.
| Treatments compared |
Dependent variable |
Fixed effect | num df | den df | F | P-value |
|---|---|---|---|---|---|---|
| Developmental | PE/PC | Treatment | 1 | 204 | 210.96 | <0.001 |
| plasticity: | Population | 2 | 36 | 1.82 | 0.177 | |
| 16°C /16°C1 v. | Block | 5 | 204 | 25.83 | <0.001 | |
| 26°C / 26°C | Population×Treatment | 2 | 204 | 3.27 | 0.040 | |
| Population×Block | 10 | 204 | 3.35 | 0.001 | ||
| Treatment×Block | 5 | 204 | 1.61 | 0.158 | ||
| Population×Treatment ×Block | 10 | 204 | 0.69 | 0.735 | ||
| Saturation | Treatment | 1 | 204 | 90.85 | <0.001 | |
| Population | 2 | 36 | 0.45 | 0.640 | ||
| Block | 5 | 204 | 16.96 | <0.001 | ||
| Population×Treatment | 2 | 204 | 0.75 | 0.472 | ||
| Population×Block | 10 | 204 | 1.54 | 0.127 | ||
| Treatment×Block | 5 | 204 | 1.22 | 0.302 | ||
| Population×Treatment ×Block | 10 | 204 | 0.44 | 0.926 |
Larval-to-adult development temperature / adult temperature after eclosion
To quantify covariance between plasticity traits across life stages, we calculated correlations between isofemale line means for each trait. These correlations approximate the combined additive and non-additive components of the genetic correlation. The degree of developmental plasticity (ΔPE/PC and ΔSaturation) was calculated for each genetic line as the difference between the mean trait values between flies developed at 16°C and at 26°C, and not including adults that were shifted to different temperatures. The degree of adult plasticity was calculated in two ways: (1) the difference between adults developed at 16°C, and adults developed at 16°C that were then shifted to 26°C for 72 hours (upward thermal shift) and (2) the difference between adults developed at 26°C that were then shifted to 16°C for 72 hours, and adults developed at 26°C (downward thermal shift). We used paired t-tests to compare PE/PC after development and following adult shifts for each genetic line to test whether reversible plasticity in adults recovers the developmental trait values when adult environments shift.
Results
Patterns of GPL plasticity support physiological models
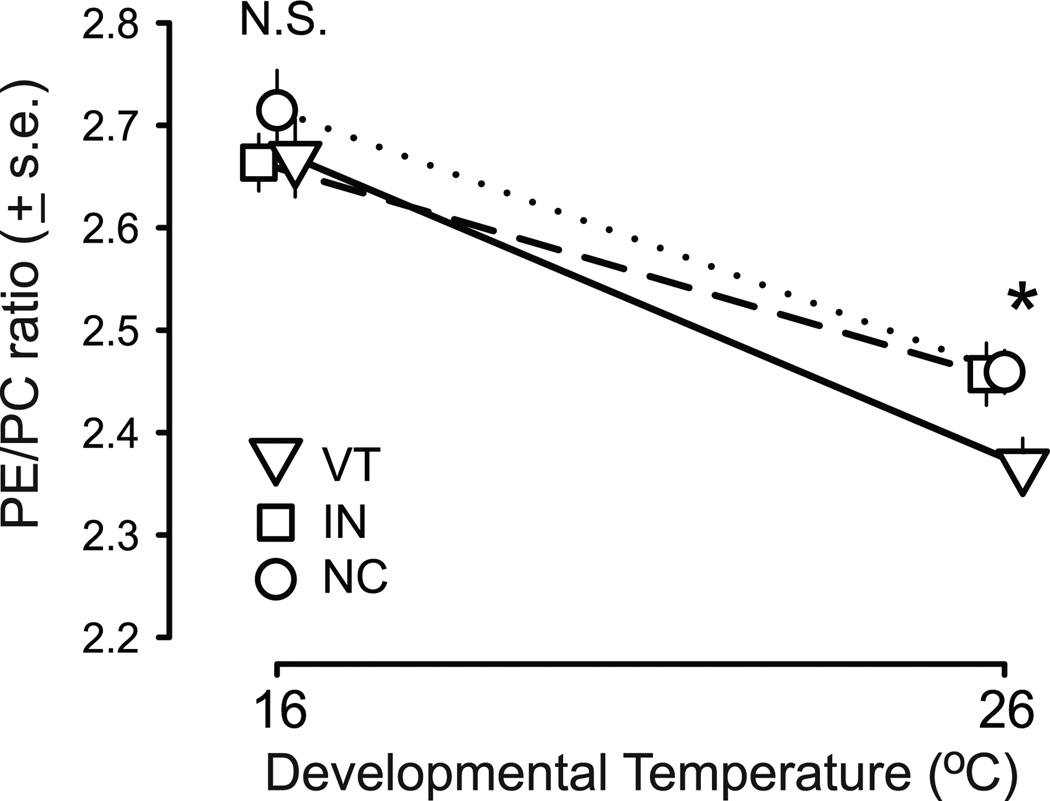
Membrane GPL composition responded strongly to both developmental and adult temperature treatments in all populations. Both PE/PC and lipid unsaturation were significantly increased at 16°C relative to 26°C treatments in all populations (Table 1; Table 2; Fig. 1; Fig. S1), consistent with the predictions of homeoviscous adaptation. While populations did not differ in the degree of developmental plasticity of lipid unsaturation (Table 1; Fig. S1), populations did differ in the degree of developmental plasticity of PE/PC, as indicated by a significant population by treatment interaction (Table 1; Fig. 1). There was not a significant population by treatment by block interaction (Table 1), indicating that this population by treatment interaction was robust to any technical effects of block. The significant population by treatment interaction was driven by differences among populations after development at 26°C. Following development at 16°C, PE/PC did not differ significantly among the three populations (Fig. 1; F2,36 = 0.68, P = 0.513). However, when developed at 26°C, flies from the three populations differed significantly in PE/PC (F2,36 = 3.46, P = 0.04) with VT having lower PE/PC than IN or NC flies (Fig. 1).
Table 2.
Mixed model ANOVAs evaluating the effect of adult temperature shifts on membrane traits. The random effect of genetic line is included in each model.
| Treatments compared |
Dependent variable |
Fixed effect | num df | den df | F | P-value |
|---|---|---|---|---|---|---|
| Adult plasticity | PE/PC | Treatment | 1 | 204 | 48.73 | <0.001 |
| 26°C / 26°C1 v. | Population | 2 | 36 | 3.12 | 0.056 | |
| 26°C / 16°C | Block | 5 | 204 | 25.63 | <0.001 | |
| Population×Treatment | 2 | 204 | 0.06 | 0.940 | ||
| Population×Block | 10 | 204 | 1.62 | 0.102 | ||
| Treatment×Block | 5 | 204 | 0.72 | 0.606 | ||
| Population×Treatment × Block | 10 | 204 | 0.82 | 0.607 | ||
| Saturation | Treatment | 1 | 204 | 50.25 | <0.001 | |
| Population | 2 | 36 | 0.14 | 0.869 | ||
| Block | 5 | 204 | 13.53 | <0.001 | ||
| Population×Treatment | 2 | 204 | 0.42 | 0.657 | ||
| Population×Block | 10 | 204 | 1.35 | 0.206 | ||
| Treatment×Block | 5 | 204 | 0.52 | 0.763 | ||
| Population×Treatment ×Block | 10 | 204 | 0.91 | 0.521 | ||
| Adult plasticity: | PE/PC | Treatment | 1 | 204 | 80.21 | <0.001 |
| 16°C / 16°C v. | Population | 2 | 36 | 0.44 | 0.649 | |
| 16°C / 26°C | Block | 5 | 204 | 19.88 | <0.001 | |
| Population×Treatment | 2 | 204 | 1.06 | 0.347 | ||
| Population×Block | 10 | 204 | 1.64 | 0.097 | ||
| Treatment×Block | 5 | 204 | 0.66 | 0.653 | ||
| Population×Treatment ×Block | 10 | 204 | 0.96 | 0.477 | ||
| Saturation | Treatment | 1 | 204 | 2.29 | 0.132 | |
| Population | 2 | 36 | 1.31 | 0.282 | ||
| Block | 5 | 204 | 18.60 | <0.001 | ||
| Population×Treatment | 2 | 204 | 2.02 | 0.13 | ||
| Population×Block | 10 | 204 | 1.52 | 0.136 | ||
| Treatment×Block | 5 | 204 | 5.12 | <0.001 | ||
| Population×Treatment×Block | 10 | 204 | 0.46 | 0.912 |
Larval-to-adult development temperature / adult temperature after eclosion
Figure 1.
Flies from all populations have a strong plastic response of PE/PC to developmental temperature, but flies from the most variable thermal environment (VT) respond more strongly to warm developmental conditions. Following development at 16°C, flies from the three populations did not differ significantly in the mean PE/PC (F2,36 = 0.679, P = 0.5134), but when developed at 26°C, flies from the three populations differed significantly in PE/PC (F2,36 = 3.461, P = 0.04), with VT having lower PE/PC than IN or NC flies.
In contrast to the population divergence we observed in developmental plasticity of PE/PC, there were no significant population by treatment interactions for GPL composition at the adult stage (Table 2). Rather, across the three populations the degree of plasticity differed depending on the direction of the adult shift (i.e., whether adults were shifted to cooler versus warmer temperatures relative to their developmental temperature). Adults changed PE/PC more strongly following a shift to 26°C, relative to the shift to 16°C (tpaired = 5.21, df = 2, P = 0.035), while the change in lipid unsaturation was greater following shifts to 16°C, relative to the shift to 26°C (tpaired = 11.72, df = 2, P = 0.007)(Table S2).
Plasticity of PE/PC diverges rather than other membrane GPL traits
Populations did not differ in the plasticity of GPL traits other than PE/PC (Tables 1 and 2), and there was no evidence of physiological specialization of membrane GPL composition across populations. Specialization to the mean thermal environment would predict increased PE/PC or lipid unsaturation in the colder VT population regardless of developmental environment. Populations did not differ in PE/PC after development at 16°C (Fig. 1); and at 26°C the greater response of VT flies resulted in a lower ratio of PE/PC relative to other populations (see results above), a result in the opposite direction of that predicted for specialization of GPL composition. Similarly, we found no evidence of greater lipid unsaturation in populations from cooler mean thermal environments. Population had no significant effect on lipid unsaturation during development at 16°C (F2,36 = 1.21, P = 0.311) or 26°C (F2,36 = 0.05, P = 0.956).
Plasticity of PE/PC covaries across, but not within, life stages
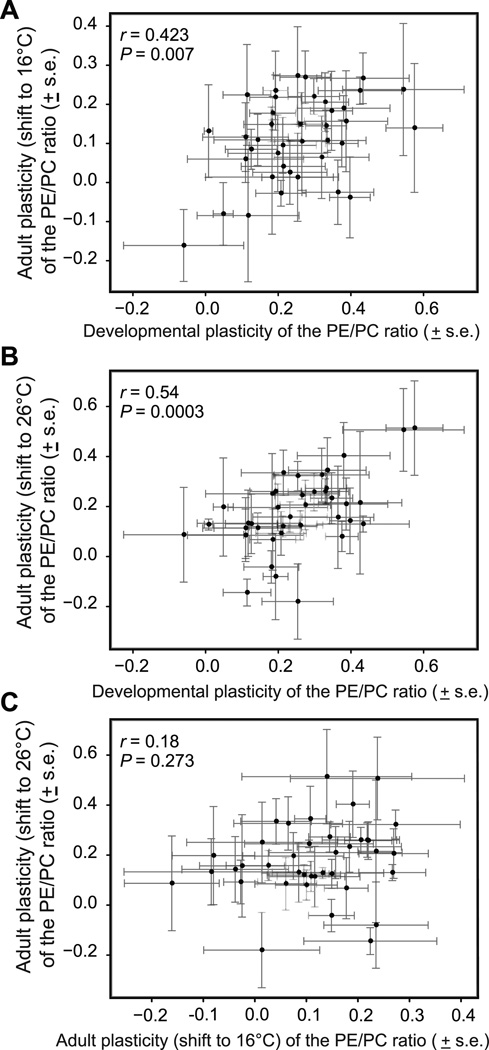
We used the correlation among the isofemale line means for ΔPE/PC to estimate the covariance between developmental and adult plasticity, and between plastic responses of adults to downward and upward thermal shifts. The degree of developmental plasticity of PE/PC was significantly and positively correlated with the degree of adult plasticity (Fig. 2A,B). However, the plastic responses of adults to downward and to upward thermal shifts were not significantly correlated (Fig. 2C).
Figure 2.
Developmental and adult responses of PE/PC to temperature covary across but not within life stages. A) Developmental plasticity is significantly and positively correlated with the response of adults shifted to 16°C, and B) with the response of adults shifted to 26°C. C) Adult responses to upward and downward thermal shifts do not significantly covary with one another. Plasticity is calculated as the difference between line means as: developmental plasticity = dev16°C - dev26°C, adult plasticity (shift to 16°C) = adult16°C - dev26°C, and adult plasticity (shift to 26°C) = dev16°C - adult26°C. Points represent mean plasticity +/− s.e. for each genetic line.
Adult plasticity of PE/PC fails to recover developmental trait values
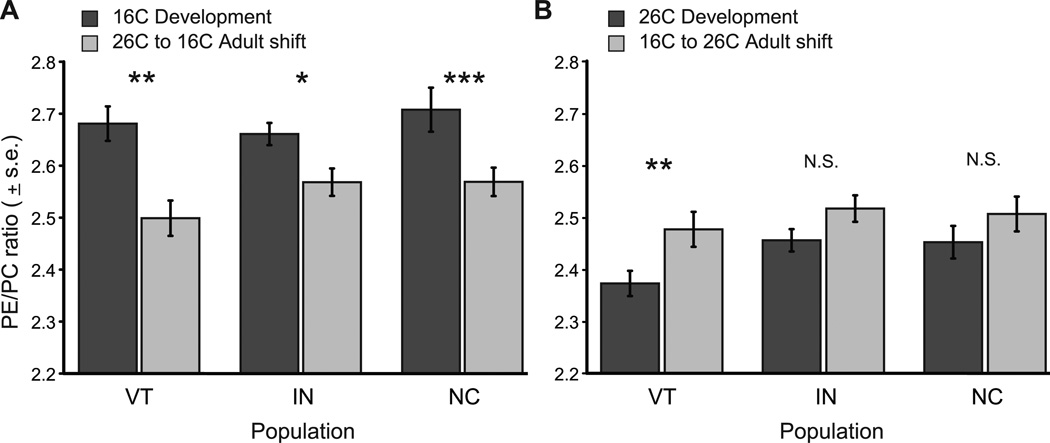
We compared adult-shifted and developmental trait values of each isofemale line at particular temperatures to determine the degree to which adult plasticity recovered developmental trait values. Across all populations, adults changed PE/PC more strongly when shifted to 26°C, relative to the shift to 16°C (Table S2). As a result of this reduced adult response to cooler temperature, no populations recovered their 16°C developmental values when adults developed at 26°C were shifted to 16°C, as evidenced by significant differences in the developmental and adult PE/PC ratios at 16°C (Fig. 3A). In contrast, when shifted to a warmer temperature, adult flies from IN and NC recovered their 26°C developmental trait values, but genotypes from VT did not (Fig. 3B). This lack of recovery of the developmental value of PE/PC in VT flies indicates a potential cost of greater developmental plasticity experienced by adults when their thermal environments shift from cooler to warmer temperatures.
Figure 3.
Adult plasticity does not always recover developmental values of PE/PC. A) Across all populations, flies developed at 26°C and shifted to 16°C for 72 hours as adults do not recover the 16°C developmental value of PE/PC. B) Flies from IN and NC do recover their 26°C developmental values of PE/PC when shifted as adults to 26°C, while adults from VT do not. Dark bars represent developmental values and light bars represent adult values of PE/PC after being thermally shifted. Data are means +/− s.e. of all genetic lines within each population. *P < 0.05, **P < 0.01, ***P < 0.0001 for paired t-tests
Discussion
Remodeling the GPL composition of membranes enables ectotherms to maintain membrane fluidity and ultimately fitness when temperatures change (Sinensky 1974; Hazel 1995; Hochochka & Somero 2002; Condon et al. in press). Consistent with previous observations of laboratory lines (Overgaard et al. 2008; Cooper et al. 2012), we found that North American D. melanogaster decreased PE/PC and lipid unsaturation when developed at 26°C and when adults were shifted to 26°C, relative to exposure to 16°C. While populations shared similar developmental responses of GPL saturation and similar adult responses in all GPL traits, flies from the most temporally variable thermal environment (VT) more strongly remodeled PE/PC in response to warm developmental temperatures, relative to other populations. This pattern recapitulates observations from replicated laboratory-evolved populations, where high variance in temperature across generations favored greater developmental response of PE/PC to warm temperatures (Cooper et al. 2012). These populations with greater membrane plasticity also had greater female fecundity than did populations with lower PE/PC plasticity when developed in a warm environment (Condon et al. in press). Finally, the magnitude of developmental PE/PC plasticity that we observed in natural populations was the same as that of laboratory populations evolved in the presence of thermal variability, but was greater than that of populations evolved at constant temperatures (Cooper et al. 2012). This suggests that thermal variability maintained membrane plasticity, while evolution in constant thermal environments relaxed selection on plasticity. This result supports the predictions of evolutionary models of genes that are conditionally expressed (Van Dyken & Wade 2009), and suggests that alleles for membrane plasticity may be costly during bouts of thermal stasis (sensu van Tienderen 1991). While we cannot make conclusions about the specific magnitude of temporal variation in temperature in nature that generates a selective advantage for PE/PC plasticity, we can conclude that when the multiple populations of D. melanogaster that we have measured diverge in this plastic trait they do so via the same mechanism – populations from more thermally variable environments more strongly decrease PE/PC in response to warm developmental environments.
Development temperature more strongly affected PE/PC than did adult thermal shifts, with the consequence that thermally shifted adults were not able to recover developmental values of PE/PC. The stronger developmental, but similar adult plasticity of VT flies resulted in VT flies being further from their 26°C developmental PE/PC value following shifts to 26°C as adults. If developmental values reside near the physiological optimum, this would indicate a greater cost of matching cellular physiology to the developmental environment for VT flies when adult environments change (i.e., a cost of developmental plasticity realized by adults). Our interpretation of this putative cost assumes that the 3-day exposure of adults was a long enough period of time for a complete membrane response. While more experiments are needed to determine the timing of membrane turnover in D. melanogaster, theory predicts that selection should favor the evolution of reversible responses so long as the response time does not exceed the rate of environmental change (Gabriel et al. 2005). Three days represents an extensive amount of time for adaptive membrane turnover when we consider that adult flies experience thermal variation on much finer timescales. Yet, the way in which D. melanogaster experiences variation in the thermal environment differs across life stages. Larvae develop in fermenting fruit with limited capacity to buffer thermal variation, while flight enables adults to behavioral avoid thermal extremes within their lifetime (Feder, Blair & Figueras 1997; Huey, Hertz & Sinervo 2003; Dillon et al. 2009). If adults can behaviorally buffer the thermal environment, then adult flies may never experience a cost of decreased performance when temperatures shift (Angilletta et al. 2006).
Despite the fact that the natural populations we sampled experience different mean thermal environments, we found no evidence for specialization of membrane GPL composition. While not consistent with predictions of homeoviscous adaptation, this result also matches observations from laboratory-evolved populations, where evolution in constant environments did not lead to specialization of membrane GPL composition (Cooper et al. 2012). Drosophila species that inhabit different latitudes in Japan have diverged in the percentage of monoenoic and dienoic acids in their membranes, but in a way that maintains a similar degree of lipid saturation across species (Ohtsu, Kimura & Katagiri 1998). Thus, it remains unclear whether this species divergence is adaptive or a result of compensatory evolution to maintain the overall degree of GPL saturation. The populations that we sampled may have specialized GPL composition in ways that our assay does not capture. For example, cholesterol affects membrane dynamics (Robertson & Hazel 1995; Robertson & Hazel 1997; Shreve, Yi & Lee 2007), and higher order membrane structures (e.g., lipid rafts) can change in response to environmental temperature (Zehmer & Hazel 2003). Alternatively, effective developmental plasticity in a species like D. melanogaster with a relatively short life span, combined with behavioral thermoregulation in adults (Dillon et al. 2009), may decrease selection pressure to specialize membrane composition across different mean thermal environments.
Across genotypes, we observed a positive correlation between developmental and adult responses of PE/PC to temperature. This correlation could arise through correlated selection (Agrawal & Stinchcombe 2009) on distinct genes and mechanisms that underlie developmental and adult plasticity, although theory suggests that distinct types of environmental heterogeneity (across- versus within-generation variance) favor these two types of plasticity (Gabriel & Lynch 1992; Gabriel et al. 2005). Alternatively, the covariance across life stages may reflect shared genes and mechanisms that affect PE/PC responses in larvae and adults. In contrast to this covariance across life stages, we found no correlation between the magnitudes of adult change in PE/PC to upward and downward thermal shifts. This indicates a degree of genetic decoupling of membrane remodeling in response to warm versus cool temperatures within the adult life stage. Our data provide some evidence for regulation of different GPL membrane components in response to upward and downward thermal shifts in adults; PE/PC responded more strongly to upward thermal shifts, while the degree of lipid saturation responded more strongly to downward thermal shifts. Distinct biochemical pathways underlie PE/PC modification and lipid desaturation (Miller, Yates & Geer 1993; Dobrosotskaya et al. 2002; Montooth, Siebenthall & Clark 2006), providing potential mechanisms by which adult responses to different thermal shifts may be genetically and physiologically independent.
We previously found support for the prediction that temporal variability in temperature favors alleles with greater thermal sensitivity (Cooper et al. 2012). We find similar support from natural populations. Selection favoring alleles specific to different mean environments would result in population specialization. The fact that we observed greater developmental plasticity in the absence of specialization suggests that divergence in developmental plasticity of PE/PC occurs via alleles with greater environmental sensitivity (sensu Via 1993). Models of conditional gene expression predict that genotypes expressed in specific environments experience less effective selection than do those expressed more ubiquitously (Kawecki 1994; Whitlock 1996; Van Dyken & Wade 2009). Thus, plasticity may more likely diverge among populations via environmentally sensitive genotypes that affect phenotypes across a range of environments. Nevertheless, responses of the membrane to upward and downward thermal shifts in adults were not correlated, suggesting environment-specific responses in this life stage.
While membrane remodeling is a complex phenotype controlled by many genes, the quantitative divergence that we observed may result from changes at a small number of loci that modify membrane responses to temperature. In Drosophila, the sterol regulatory element binding protein (SREBP) regulates the expression of enzymes that synthesize PE and may directly respond to alterations in membrane fluidity Dobrosotskaya et al. 2002; Rawson 2003). A number of genes regulated by SREBP, as well as lipid desaturases, change in expression in response to temperature during development and during adult thermal shifts in D. melanogaster (Montooth, Siebenthall & Clark 2006), providing a set of candidate genes that enable a mechanistic approach to understanding how cellular plasticity evolves in response to environmental heterogeneity.
Supplementary Material
Acknowledgments
We would like to thank P. White and S. Mentzer for assistance in the laboratory. M. Angilletta Jr., C. Lively, B. Lockwood, C. Meiklejohn, R. Wilson and anonymous reviewers provided comments that greatly improved our manuscript. B.S.C. was supported by the Indiana University Genetics, Cellular and Molecular Sciences Training Grant T32-GM007757 funded by the National Institutes of Health, and by a Student Research Award from the American Society of Naturalists. This research was supported by funds from a National Science Foundation CAREER award IOS-1149178 and from Indiana University to K.L.M. A portion of the LC-MS/MS assays was supported by the METACyt initiative funded by a grant from the Eli Lilly Endowment.
Footnotes
Data Accessibility
Data associated with this archive are deposited in the Dryad Data Archive doi:10.5061/dryad.96pr7
Supporting Information
Additional supporting information may be found in the online version of this article.
Appendix S1: Supplementary methods and results for degree-day model
Table S1. Summary of weather data and degree-day model estimates for each population
Table S2. Magnitude of cellular responses to adult thermal shifts
Figure S1. Developmental reaction norms for lipid saturation
References
- Agrawal AF, Stinchcombe JR. How much do genetic covariances alter the rate of adaptation? Proceedings of the Royal Society B-Biological Sciences. 2009;276:1183–1191. doi: 10.1098/rspb.2008.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta MJ, Bennett AF, Guderley H, Navas CA, Seebacher F, Wilson RS. Coadaptation: a unifying principle in evolutionary thermal biology. Physiological and Biochemical Zoology. 2006;79:282–294. doi: 10.1086/499990. [DOI] [PubMed] [Google Scholar]
- Condon CH, Cooper BS, Yeaman S, Angilletta MJ. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution. doi: 10.1111/evo.12296. (in press) [DOI] [PubMed] [Google Scholar]
- Cooper BS, Czarnoleski M, Angilletta MJ. Acclimation of thermal physiology in natural populations of Drosophila melanogaster : a test of an optimality model. Journal of Evolutionary Biology. 2010;23:2346–2355. doi: 10.1111/j.1420-9101.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- Cooper BS, Hammad LA, Fisher NP, Karty JA, Montooth KL. In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution. 2012 doi: 10.1111/j.1558-5646.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- Cooper BS, Hammad LA, Montooth KL. Data from: Thermal adaptation of cellular membranes in natural populations of Drosophila melanogaster. Dryad Digital Repository. 2014 doi: 10.1111/1365-2435.12264. http://doi.org/10.5061/dryad.96pr7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JR, Capy P. Genetic variation of Drosophila melanogaster natural populations. Trends in Genetics. 1988;4:106–111. doi: 10.1016/0168-9525(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Wang G, Garrity PA, Huey RB. Thermal preference in Drosophila. Journal of Thermal Biology. 2009;34:109–119. doi: 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Feder ME, Blair N, Figueras H. Natural thermal stress and heat-shock protein expression in Drosophila larvae and pupae. Functional Ecology. 1997;11:90–100. [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Gabriel W, Luttbeg B, Sih A, Tollrian R. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. American Naturalist. 2005;166:339–353. doi: 10.1086/432558. [DOI] [PubMed] [Google Scholar]
- Gabriel W, Lynch M. The selective advantage of reaction norms for environmental tolerance. Journal of Evolutionary Biology. 1992;5:41–59. [Google Scholar]
- Gilchrist GW. Specialists and generalists in changing environment. I. Fitness landscapes of thermal sensitivity. American Naturalist. 1995;146:252–270. [Google Scholar]
- Gomulkiewicz R, Kirkpatrick M. Quantitative genetics and the evolution of reaction norms. Evolution. 1992;46:390–411. doi: 10.1111/j.1558-5646.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Hammad LA, Cooper BS, Fisher NP, Montooth KL, Karty JA. Profiling and quantification of Drosophila melanogaster lipids using liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2011;25:2959–2968. doi: 10.1002/rcm.5187. [DOI] [PubMed] [Google Scholar]
- Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annual Review of Physiology. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Hochochka PW, Somero GN. Biochemical Adaptation. Oxford: Oxford University Press; 2002. . [Google Scholar]
- Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. Journal of Thermal Biology. 2003;28:175–216. [Google Scholar]
- Huey RB, Hertz PE, Sinervo B. Behavioral drive versus Behavioral inertia in evolution: A null model approach. American Naturalist. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- Jones HE, Harwood JL, Bowen ID, Griffiths G. Lipid Composition of Subcellular Membranes from Larvae and Prepupae of Drosophila melanogaster. Lipids. 1992;27:984–987. doi: 10.1007/BF02535576. [DOI] [PubMed] [Google Scholar]
- Kassen R. The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology. 2002;15:173–190. [Google Scholar]
- Kawecki TJ. Accumulation of Deleterious Mutations and the Evolutionary Cost of Being a Generalist. The American Naturalist. 1994;144:833–838. [Google Scholar]
- Kostal V, Simek P. Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 1998;168:453–460. [Google Scholar]
- Levins R. Evolution in Changing Environments: Some Theoretical Explorations. Princeton: Princeton University Press; 1968. [Google Scholar]
- Lynch MJ, Gabriel W. Environmental tolerance. American Naturalist. 1987;129:283–303. [Google Scholar]
- MacMillan HA, Guglielmo CG, Sinclair BJ. Membrane remodeling and glucose in Drosophila melanogaster : A test of rapid cold-hardening and chilling tolerance hypotheses. Journal of Insect Physiology. 2009;55:243–249. doi: 10.1016/j.jinsphys.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Miller RR, Yates JW, Geer BW. Dietary ethanol stimulates the activity of phosphatidylcholine specific phospholipase D and the formation of phosphatidylethanol in Drosophila melaongaster larvae. Insect Biochemistry and Molecular Biology. 1993;23:749–755. doi: 10.1016/0965-1748(93)90049-x. [DOI] [PubMed] [Google Scholar]
- Montooth KL, Siebenthall KT, Clark AG. Membrane lipid physiology and toxin catabolism underlie ethanol and acetic acid tolerance in Drosophila melanogaster. Journal of Experimental Biology. 2006;209:3837–3850. doi: 10.1242/jeb.02448. [DOI] [PubMed] [Google Scholar]
- Moran NA. Adaptation and constraint in the complex life cycles of animals. Annual Review of Ecology and Systematics. 1994;25:573–600. [Google Scholar]
- Nilsson-Ortman V, Stoks R, De Block M, Johansson F. Generalists and specialists along a latitudinal transect: patterns of thermal adaptation in six species of damselflies. Ecology. 2012;93:1340–1352. doi: 10.1890/11-1910.1. [DOI] [PubMed] [Google Scholar]
- Ohtsu T, Kimura MT, Katagiri C. How Drosophila species acquire cold tolerance: qualitative changes of phospholipids. European Journal of Biochemistry. 1998;252:608–611. doi: 10.1046/j.1432-1327.1998.2520608.x. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sorensen JG, Petersen SO, Loeschcke V, Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. Journal of Insect Physiology. 2005;51:1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sorensen JG, Petersen SO, Loeschcke V, Holmstrup M. Reorganization of membrane lipids during fast and slow cold hardening in Drosophila melanogaster. Physiological Entomology. 2006;31:328–335. [Google Scholar]
- Overgaard J, Tomcala A, Sorensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. Journal of Insect Physiology. 2008;54:619–629. doi: 10.1016/j.jinsphys.2007.12.011. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Rawson RB. The SREBP pathway — insights from insigs and insects. Nature Reviews Molecular Cell Biology. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- Reboud X, Bell G. Experimental evolution in Chlamydomonas 3. Evolution of specialist and generalist types in environments that vary in space and time. Heredity. 1997;78:507–514. [Google Scholar]
- Robertson JC, Hazel JR. Cholesterol Content of Trout Plasma-Membranes Varies with Acclimation Temperature. American Journal of Physiology- Regulatory Integrative and Comparative Physiology. 1995;38:R1113–R1119. doi: 10.1152/ajpregu.1995.269.5.R1113. [DOI] [PubMed] [Google Scholar]
- Robertson JC, Hazel JR. Membrane constraints to physiological function at different temperatures: Does cholesterol stabilize membranes at elevated temperatures? In: Wood CM, McDonald DG, editors. Society for Experimental Biology Seminar Series; Global warming: Implications for freshwater and marine fish. 1997. pp. 25–49. [Google Scholar]
- Scheitz CJF, Guo Y, Early AM, Harshman LG, Clark AG. Heritability and inter-populational differences in lipid profiles of Drosophila melanogaster. PLOS ONE. 2013;8:e72726. doi: 10.1371/journal.pone.0072726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve SM, Yi S-X, Lee RE., Jr Increased dietary cholesterol enhances cold tolerance in Drosophila melanogaster. Cryoletters. 2007;28:33–37. [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation – homeostatic process that regulates viscosity of membrane lipids in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken JD, Wade MJ. The genetic signature of conditional expression. Genetics. 2009;184:557–570. doi: 10.1534/genetics.109.110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tienderen PH. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution. 1991;45:1317–1331. doi: 10.1111/j.1558-5646.1991.tb02638.x. [DOI] [PubMed] [Google Scholar]
- Via S. Adaptive phenotypic plasticity – target of byproduct of selection in a variable environment. American Naturalist. 1993;142:352–365. doi: 10.1086/285542. [DOI] [PubMed] [Google Scholar]
- Via S, Lande R. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution. 1985;39:505–522. doi: 10.1111/j.1558-5646.1985.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Whitlock MC. The Red Queen Beats the Jack-Of-All-Trades: The Limitations on the Evolution of Phenotypic Plasticity and Niche Breadth. The American Naturalist. 1996;148:S65–S77. [Google Scholar]
- Zehmer JK, Hazel JR. Plasma membrane rafts of rainbow trout are subject to thermal acclimation. Journal of Experimental Biology. 2003;206:1657–1667. doi: 10.1242/jeb.00346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.