Abstract
Purpose
We evaluated serum procalcitonin (PCT) as a diagnostic marker of neonatal sepsis, and compared PCT levels with C-reactive protein (CRP) levels.
Methods
We retrospectively reviewed the medical records of 269 neonates with a suspected infection, admitted to Wonkwang University School of Medicine & Hospital between January 2011 and December 2012, for whom PCT and CRP values had been obtained. Neonates were categorized into 4 groups according to infection severity. CRP and PCT values were analyzed and compared, and their effectiveness as diagnostic markers was determined by using receiver operating characteristic (ROC) curve analysis. We also calculated the sensitivity, specificity, and positive, and negative predictive values.
Results
The mean PCT and CRP concentrations were respectively 56.27±81.89 and 71.14±37.17 mg/L in the "confirmed sepsis" group; 15.64±32.64 and 39.23±41.41 mg/L in the "suspected sepsis" group; 9.49±4.30 and 0.97±1.16 mg/L in the "mild infection" group; and 0.21±0.12 and 0.72±0.7 mg/L in the control group. High concentrations indicated greater severity of infection (P<0.001). Five of 18 patients with confirmed sepsis had low PCT levels (<1.0 mg/L) despite high CRP levels. In the ROC analysis, the area under the curve was 0.951 for CRP and 0.803 for PCT. The cutoff concentrations of 0.5 mg/L for PCT and 1.0 mg/L for CRP were optimal for diagnosing neonatal sepsis (sensitivity, 88.29% vs. 100%; specificity, 58.17% vs. 85.66%; positive predictive value, 13.2% vs. 33.3%; negative predictive value, 98.6% vs. 100%, respectively).
Conclusion
PCT is a highly effective early diagnostic marker of neonatal infection. However, it may not be as reliable as CRP.
Keywords: Sepsis, Procalcitonin, C-reactive protein
Introduction
Despite improvement in neonatal survival rates due to advanced neonatal treatment, neonatal infection-associated sepsis still has an important effect on neonatal morbidity and survival, as it affects 1-4 in 1,000 babies in developed countries1). Diagnosing sepsis is very difficult because its symptoms are vague and nonspecific. Therefore, antibiotics are administered before culture results are obtained to reduce morbidity and mortality rates-increasing antibiotic resistance and hospital charges. Thus, an accurate and rapid diagnostic test is necessary.
The most reliable test for diagnosing sepsis is a blood culture; however, false-positive results often occur due to contamination or no culture growth. Although neutrophil, total white blood cell (WBC), absolute neutrophil count (ANC), and platelet counts and blood culture are ordered to screen for suspected sepsis, these values are ineligible as infection markers due to insufficient sensitivity and specificity1). Thus, most hospitals commonly use C-reactive protein (CRP) levels as markers.
Recently, the effectiveness of procalcitonin (PCT) as an early diagnostic tool for neonatal sepsis has been reported. Research studies reported that PCT is more effective than CRP at follow-up, as PCT levels rise earlier and return to normal levels more rapidly than CRP levels2,3,4,5,6,7,8,9). However, some studies have shown a decrease in PCT specificity associated with an increase in uninfected preterm babies, hypoxia, neonatal respiratory distress syndrome (RDS), and hematologic failure10,11,12). Furthermore, one report suggested that PCT is only significant when used in combination with other tests, and it is not appropriate for diagnosing sepsis13). Here, we compared PCT with CRP levels for diagnosing neonatal sepsis.
Materials and methods
1. Subject and group classification
We targeted >4-day-old and <30-day-old patients with suspected bacterial infection who had undergone antibiotic injection after CRT and PCT tests and were hospitalized in the neonatal intensive care unit, nursery, pediatric wards from January 2011 to December 2012; we excluded patients who had major congenital anomalies and liver diseases.
We included 269 patients in the study and divided them into 4 groups based on the severity of infection14,15).
1) Group I: confirmed sepsis group
>3 positive sepsis-related clinical symptoms; >2 positive sepsis-related blood test results; CRP levels >6 mg/L; and positive blood culture test results
2) Group II: suspected sepsis group
>3 positive sepsis-related clinical symptoms; >2 positive sepsis-related blood test results; CRP levels >6 mg/L; negative blood culture test
3) Group III: mild infection group
>2 positive sepsis-related clinical symptoms; <2 positive sepsis-related blood tests; CRP levels < 6 mg/L; negative blood culture test results
4) Group IV: no infection group (control group)
All babies, excluding groups I, II, and III.
The criteria for sepsis-related clinical symptoms1) were divided into the following groups: (1) General: fever, temperature instability, and poor feeding; (2) gastrointestinal system: abdominal distension and hepatomegaly; (3) respiratory system: apnea, dyspnea, retraction, and cyanosis; (4) cardiovascular system: pallor, mottling, tachycardia, and bradycardia; (5) central nervous system: irritability, lethargy, abnormal Moro reflex, fontanelle bulging, seizure, and hypotonia; (6) hematologic system: jaundice, splenomegaly, and petechiae.
Sepsis-related blood tests included total WBC, ANC, CRP levels, and platelet counts. Total WBC and ANC were analyzed based on the criteria described by Manroe et al.16) and Rodwell et al.17) scoring system. Leukopenia was defined as <5,000/mm3, leukocytosis >21,000/mm3, normal ANC 1,800-5,400/mm3, thrombocytopenia18) <150,000/mm3, and CRP levels were >6 mg/L (which was our hospital's criterion for infection), and these were analyzed retrospectively.
2. Methods
We analyzed the results and divided patients into groups based on infection severity after investigating gestational age (GA), birth weight (BW), gender, CRP and PCT levels, blood culture, and clinical symptoms of the neonates with suspected bacterial infection.
Neonates with suspected bacterial infection were given antibiotics after blood sampling for total WBC, ANC, platelet count, CRP, PCT, and blood culture results.
Blood sampling was performed when bacterial infection was suspected. PCT was measured by enzyme-linked fluorescent assay using BRAHMS PCT (BioMerieux SA, Marcy l'Etoile, France) kit in the VIDAS equipment, and CRP levels were measured by immunoturbidimetric analysis using a CRP reagent kit (Sekisui Medical Co., Tokyo, Japan). To determine the diagnostic usefulness of PCT and CRP levels, we analyzed the receiver operating characteristic (ROC) curve and calculated the sensitivities, specificities, and positive and negative predictive values.
3. Statistics
For statistical analysis, characteristics of 4 patients group was assessed using analysis of variance and X2 test; PCT and CRP values using the Kruskal-Wallis test; and multiple comparison between groups using Mann-Whitney U test. Significance was defined as a P value <0.01.
Results
1. Patients' characteristics in each group
Of the 269 babies, 18 were included in the confirmed sepsis group, 56 in the suspected sepsis group, 81 in the mild infection group, and 114 in the control group. Patients' BWs were 2,370±1,090, 2,440±1,110, 2,430±3,840, and 2,140±960 g, respectively, and GAs were 34.8±6.92, 34.8±4.45, 32.8±5.07, and 33.4±4.64 weeks, respectively. BW and GA were not significantly different between the groups. There were more male (n=164) than female babies (n=105); however, the difference was not significant (Table 1).
Table 1.
Patient characteristics according to the groups
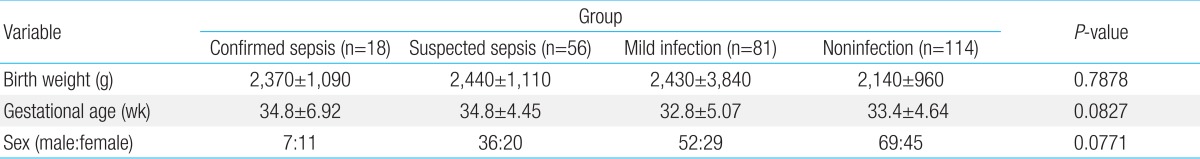
Values are presented as mean±standard deviation.
2. PCT and CRP levels in each group
PCT levels were significantly high according to infection severity: 56.27±81.89 mg/L, confirmed sepsis group; 15.64±32.64 mg/L, suspected sepsis group; 9.49±4.30 mg/L, mild infection group; and 0.21±0.12 mg/L, control group (P<0.001). CRP values showed similar results (71.14±37.17, 39.23±41.41, 0.97±1.16, and 0.72± 0.7 mg/L, respectively, in each group, P<0.001) (Table 2).
Table 2.
Mean PCT and CRP levels, according to group
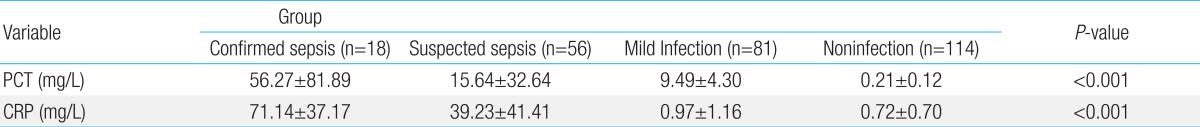
Values are presented as mean±standard deviation.
PCT, Procalcitonin; CRP, C-reactive protein
3. Causative microorganisms in the confirmed sepsis group
Bacteria were cultured in 18 cases. Enterococcus faecium and Streptococcus were the most common microbes on blood culture (4 cases, 22%) (Table 3).
Table 3.
Procalcitonin and C-reactive protein levels, and associated causative microorganism, in patients with confirmed sepsis
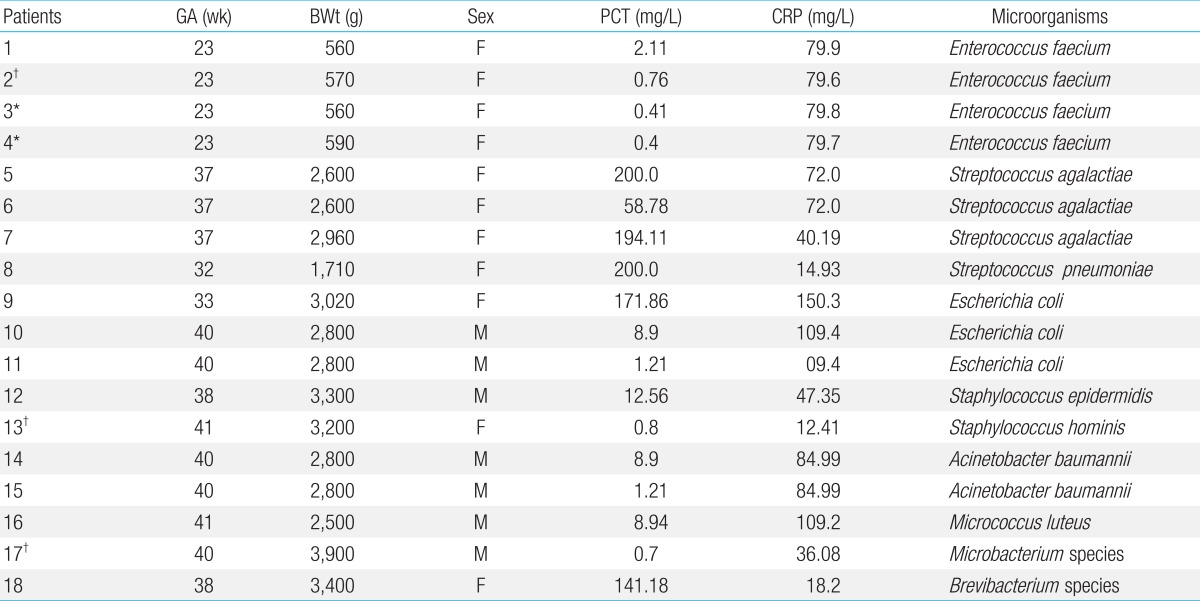
BW, birth weight; GA, gestational age; PCT, procalcitonin; CRP, C-reactive protein.
*PCT<0.5 mg/L. †PCT<1 mg/L.
Of the 18 patients with confirmed sepsis, 2 patients (patient 8, 18) had slightly increased CRP values to 14.93 and 18.2 mg/L, respectively; however, their PCT values increased considerably to 200 and 141.18 mg/L, respectively. Therefore, PCT seems to be more reliable for diagnosing neonatal sepsis than CRP in 2 cases. However, despite increased CRP values in 5 cases, their PCT values remained very low, and were <0.5 mg/L (as the cutoff value) in 2 cases (patient 3 and 4) and <1 mg/L (as the cutoff value) in 3 cases (patient 2, 13, and 17), for detecting neonatal sepsis. Therefore, PCT may have a lower accuracy as a diagnostic marker of neonatal sepsis than CRP (Table 3).
4. ROC curve
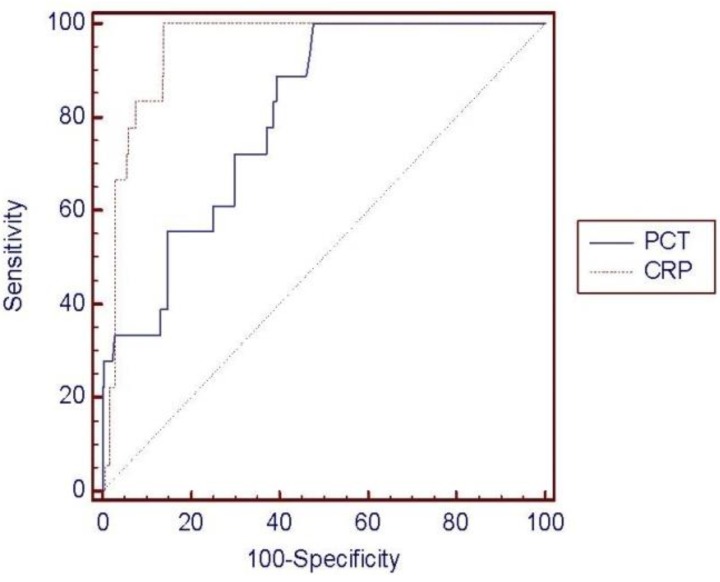
ROC curve analysis was performed to determine the diagnostic usefulness of PCT compared with CRP for detecting neonatal sepsis. In the ROC curve, area under the curve (AUC) was higher for CRP (0.951, 95% confidence interval [CI], 0.918-0.973) than for PCT (0.803, 95% CI, 0.751-0.849), showing that CRP is more useful; however, PCT levels also showed a high result (P<0.0015) (Fig. 1). PCT sensitivity and specificity were 88.89% and 58.17%, respectively, with a diagnostic threshold of 0.5 mg/L, and 72.22% and 69.32%, respectively with a threshold of 1 mg/L. The sensitivity and specificity of CRP were 100% and 78.09%, respectively, with a diagnostic threshold of 6 ng/L, and 100% and 85.66% with a threshold of 10 mg/L (Table 4). Therefore, PCT seems very useful for diagnosing neonatal sepsis with high sensitivity and specificity; however, the sensitivity and specificity of CRP were much higher. Moreover, the most sensitive diagnostic cutoff values were 0.5 mg/L for PCT and 10 mg/L for CRP. The positive predictive rate (0.5 mg/L, 13.2%; 1 mg/L, 14.4%) of PCT was very low, whereas the negative predictive rate (0.5 mg/L, 98.6%; 1 mg/L, 97.2%) showed a similar high result to CRP (6 and 10 mg/L, 100%) (Table 4).
Fig. 1.
Comparison of the receiver operating characteristic (ROC) curves of procalcitonin (PCT) and C-reactive protein (CRP). The area under the curve was 0.803 for PCT and 0.951 for CRP. The difference between areas was significant (0.148; 95% confidence interval, 0.056-0.239; P=0.0015).
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value of procalcitonin and C-reactive protein level for the diagnosis of neonatal sepsis
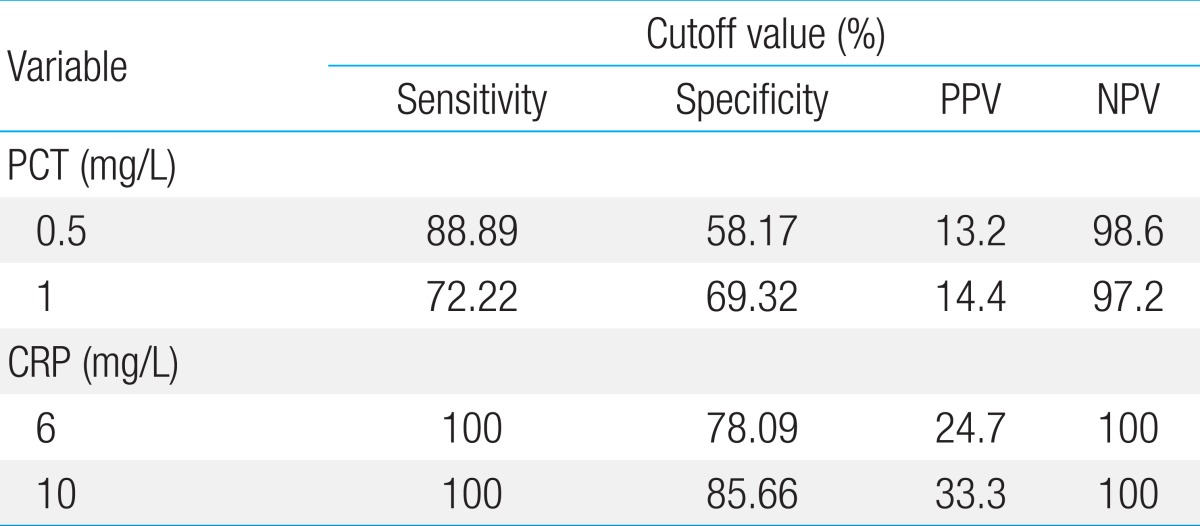
CI, confidence interval; PCT, procalcitonin; CRP, C-reactive protein; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Although blood culture results are important for diagnosing neonatal sepsis, it has a low rate of culture, making it difficult to diagnose neonatal sepsis early and resulting in unnecessary or delayed treatment. Therefore, a rapid test with the best degree of sensitivity, reliability, and predictability is required for the early diagnosis and treatment of neonatal sepsis.
The diagnostic markers of neonatal sepsis include total WBC and differential counts; an immature-to-total neutrophil ratio, ≥0.2; neutropenia; thrombocytopenia; and levels of CRP, PCT, haptoglobin, fibrinogen, and cytokines (interleukin [IL] 6, IL-8, and tumor necrosis factor-α), etc., with the bacterial culture providing a definitive diagnosis1). Among these markers, CRP is most commonly used in all hospitals during follow-up and diagnosis. Although CRP levels can be obtained easily and rapidly through an automatic method, and has a high sensitivity, its specificity is low, making it difficult to diagnose sepsis19).
Comparatively, PCT is more specific than other markers and is detected faster than CRP. Therefore, PCT is believed to be a new marker of infection; however, its use as the only marker for early diagnosis of sepsis is controversial19).
PCT, a precursor of calcitonin, is a peptide composed of 116 amino acids and a glycoprotein with a molecular weight of 14.5 KDa. In ordinary times, it is synthesized in the C cells of the thyroid gland and becomes the mature hormone calcitonin formed by 32 amino acids through proteolysis20). Although it exists in extremely low concentrations in healthy persons, its levels increase in the presence of bacterial infections, such as sepsis, meningitis, and urethritis, and it rises rapidly in severe sepsis or septic shock21). At that time, high serum PCT levels are found because macrophages and monocytes, and the C cells of the thyroid produce it through induction by bacterial endotoxin. Dandona et al.22) found that PCT is first detected 4 hours after injection of a small quantity of endotoxin in healthy people due to inflammation; its levels increase rapidly after 6-8 hours, reach a plateau, and then return to normal levels after 24 hours.
PCT levels show a physiologic increase 24-48 hours after birth, and it is normalized after 3 days 12). Other than infection, PCT levels increase in premature infants, hypoxia, RDS, and hemodynamic instability, decreasing its specificity in early-onset sepsis10,11,12).
In 1993, Assicot et al.23) were the first to report increased PCT levels after severe bacterial infection, using a monoclonal immunoradiometric assay. Thereafter, many PCT studies have been reported, but few have found meaningful results for the early diagnosis of bacterial infection. Although CRP level is widely used as an indicator of acute infection, increased CRP levels after the beginning of inflammation is slower than that in PCT levels. This difference can be attributed to CRP starting to increase 4-6 hours later than PCT, after the beginning of inflammation, and reaching its peak about 36 hours later21). Further, although PCT decreases 24-48 hours later after antibiotics are initiated and its level returns to normal 5 days after, CRP levels remains high for at least 24-48 hours, and then decline 5 days later despite treatment with antibiotics24). Therefore, PCT is known as a more useful indicator for diagnosing early-onset sepsis in newborns because it increases earlier within 12 hours of life than either CRP or IL-6 levels, and it may also be useful for follow-up examination.
However, Blommendahl et al.19) suggested that PCT was not a better marker than CRP levels because PCT was affected by perinatal factors within 48 h of birth. A study by Chiesa et al. 24) on the perinatal influence on PCT suggests that the prenatal antibiotic therapy may be associated with false-negative PCT results in those with early neonatal sepsis. Furthermore, Martin-Denavit et al.25) mentioned that prenatal antibiotics administered to neonates 1 day after birth increases PCT level to a slightly higher concentration than normal.
We divided patients into 4 groups, who were suspected bacterial infection after 4 days to below 30 days of birth, according to their clinical states. PCT levels were higher in patients with more severe clinical symptoms such as that observed for CRP. Additionally, we performed ROC curve analysis to determine the diagnostic usefulness of PCT compared with CRP for detecting neonatal sepsis. In the ROC curve, the AUC for CRP was 0.951 and that for PCT was 0.803; thus, both markers had a great diagnostic value; however, CRP levels showed more usefulness. To check diagnosis accuracy, we used the following guidelines based on the AUC level: noninformative (0.5), less accurate (0.5<AUC≤0.7), moderately accurate (0.7<AUC≤0.9), and highly accurate (0.9<AUC<1)26). Thus, based on our results, CRP is a highly accurate marker, whereas PCT is moderately accurate for the diagnosis of neonatal sepsis. Moreover, PCT levels remained normal to very low in 5 patients, while CRP levels increased (Table 3). Of these 5 cases, PCT values were <0.5 mg/L of the cutoff value for neonatal sepsis in 2 patients and <1 mg/L of cutoff value (mildly increased) in 3 patients. Therefore, PCT showed lower accuracy than CRP.
Our results suggest that the best cutoff value for diagnosing neonatal bacterial infection is 0.5 mg/L for PCT and 10 mg/L for CRP. PCT had a sensitivity of 88.79% and specificity of 58.17%, with a cutoff value 0.5 mg/L, while CRP had a sensitivity of 100% and specificity of 52.66%. Although the sensitivity and specificity was slightly lower for PCT, they are considered sufficiently high for a diagnostic marker. Previous studies have reported the sensitivity and specificity of PCT as follows: Sakha et al.27), 66.7% and 50%; Boo et al.28), 88.9% and 65.2%; Chiesa et al.24), 92.6% and 97.5%; and Hatherill et al.3), 92.6% and 97.5%, respectively. In a domestic study, the sensitivity, specificity, positive, and negative predictive values for PCT were 66.7%, 94.4%, 88.9%, and 81.0%, respectively, suggesting that PCT is a better clinical marker than CRP-although it is associated with a more expensive cost29).
In our study, negative predictive values of PCT were 98.6% with a 0.5 mg/L cutoff value and 97.2% with a 1 mg/L cutoff value. Without sepsis, normal cases from sepsis cases could be distinguished and the results were the same as those with CRP. Ballot et al.13) reported a high negative predictability of 95% and Guibourdenche et al.30) emphasized its significance in PCT tests.
As stated above, although many reports have suggested that PCT is a more useful marker than CRP, our study highlights the risk of evaluating PCT levels as the only test for neonatal sepsis. This is because the sensitivity and specificity of PCT are slightly lower than those of CRP. Moreover, 5 of 18 patients with confirmed sepsis had PCT levels within the normal to very low level despite having high CRP levels.
Ballot et al.13) suggested that the PCT test alone was not sufficient to confirm neonatal sepsis, because of its slightly lower sensitivity, specificity, positive predictability, and AUC. Lapillonne et al.10) discovered that premature birth increased PCT concentrations without any bacterial infection; thus, it had no specificity despite of its diagnostic value. Furthermore, Monneret et al.12) found that high PCT levels observed on the second day after birth (enough to suspect bacterial infection) return to the physiologically normal range (<0.5 mg/L) on the fourth day, similar to other healthy babies. Their conclusion was as follows: "It is common in RDS and condition related to temporal hypoxia during labor so high PCT level doesn't mean the bacterial infection all the time."
Based on our analysis, PCT may not be a sufficiently reliable diagnostic marker of neonatal sepsis compared with CRP. Therefore, we conclude that PCT can be used as a diagnostic marker in combination with other tests for the diagnosis of neonatal sepsis.
Acknowledgments
This study was supported by Wonkwang University in 2014. We thank Dr Cho of the Department of Laboratory Medicine for his contribution to the data collection.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Stoll BJ. Infection of the neonatal infant. In: Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 629–648. [Google Scholar]
- 2.Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Procalcitonin and C-reactive protein levels in neonatal infections. Acta Paediatr. 1997;86:209–212. doi: 10.1111/j.1651-2227.1997.tb08870.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatherill M, Tibby SM, Sykes K, Turner C, Murdoch IA. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child. 1999;81:417–421. doi: 10.1136/adc.81.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazzalwar R, Pina-Rodrigues E, Puppala BL, Angst DB, Schweig L. Procalcitonin as a screening test for late-onset sepsis in preterm very low birth weight infants. J Perinatol. 2005;25:397–402. doi: 10.1038/sj.jp.7211296. [DOI] [PubMed] [Google Scholar]
- 5.Turner D, Hammerman C, Rudensky B, Schlesinger Y, Goia C, Schimmel MS. Procalcitonin in preterm infants during the first few days of life: introducing an age related nomogram. Arch Dis Child Fetal Neonatal Ed. 2006;91:F283–F286. doi: 10.1136/adc.2005.085449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köksal N, Harmanci R, Cetinkaya M, Hacimustafaoglu M. Role of procalcitonin and CRP in diagnosis and follow-up of neonatal sepsis. Turk J Pediatr. 2007;49:21–29. [PubMed] [Google Scholar]
- 7.Adib M, Bakhshiani Z, Navaei F, Saheb Fosoul F, Fouladi S, Kazemzadeh H. Procalcitonin: a reliable marker for the diagnosis of neonatal sepsis. Iran J Basic Med Sci. 2012;15:777–782. [PMC free article] [PubMed] [Google Scholar]
- 8.Naher BS, Mannan MA, Noor K, Shahiddullah M. Role of serum procalcitonin and C-reactive protein in the diagnosis of neonatal sepsis. Bangladesh Med Res Counc Bull. 2011;37:40–46. doi: 10.3329/bmrcb.v37i2.8432. [DOI] [PubMed] [Google Scholar]
- 9.Sucilathangam G, Amuthavalli K, Velvizhi G, Ashihabegum GA, Jeyamurugan T, Palaniappan N. Early diagnostic markers for neonatal sepsis: comparing procalcitonin and C-reactive protein. J Clin Diag Res. 2012;6:627–631. [Google Scholar]
- 10.Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet. 1998;351:1211–1212. doi: 10.1016/S0140-6736(05)79165-0. [DOI] [PubMed] [Google Scholar]
- 11.Nylen ES, Jeng J, Jordan MH, Snider RH, Thompson KA, Lewis MS, et al. Late pulmonary sequela following burns: persistence of hyperprocalcitonemia using a 1-57 amino acid N-terminal flanking peptide assay. Respir Med. 1995;89:41–46. doi: 10.1016/0954-6111(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 12.Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Increased serum procalcitonin levels are not specific to sepsis in neonates. Clin Infect Dis. 1998;27:1559–1561. doi: 10.1086/517758. [DOI] [PubMed] [Google Scholar]
- 13.Ballot DE, Perovic O, Galpin J, Cooper PA. Serum procalcitonin as an early marker of neonatal sepsis. S Afr Med J. 2004;94:851–854. [PubMed] [Google Scholar]
- 14.Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, et al. Effects of melatonin treatment in septic newborns. Pediatr Res. 2001;50:756–760. doi: 10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Töllner U. Early diagnosis of septicemia in the newborn. Clinical studies and sepsis score. Eur J Pediatr. 1982;138:331–337. doi: 10.1007/BF00442511. [DOI] [PubMed] [Google Scholar]
- 16.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 17.Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis using a hematologic scoring system. J Pediatr. 1988;112:761–767. doi: 10.1016/s0022-3476(88)80699-1. [DOI] [PubMed] [Google Scholar]
- 18.Placzek MM, Whitelaw A. Early and late neonatal septicaemia. Arch Dis Child. 1983;58:728–731. doi: 10.1136/adc.58.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blommendahl J, Janas M, Laine S, Miettinen A, Ashorn P. Comparison of procalcitonin with CRP and differential white blood cell count for diagnosis of culture-proven neonatal sepsis. Scand J Infect Dis. 2002;34:620–622. doi: 10.1080/00365540210147723. [DOI] [PubMed] [Google Scholar]
- 20.Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem. 2001;38(Pt 5):483–493. doi: 10.1177/000456320103800505. [DOI] [PubMed] [Google Scholar]
- 21.Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002;323:17–29. doi: 10.1016/s0009-8981(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 22.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 23.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiesa C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26:664–672. doi: 10.1086/514576. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Denavit T, Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, et al. Usefulness of procalcitonin in neonates at risk for infection. Clin Chem. 1999;45:440–441. [PubMed] [Google Scholar]
- 26.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 27.Sakha K, Husseini MB, Seyyedsadri N. The role of the procalcitonin in diagnosis of neonatal sepsis and correlation between procalcitonin and C-reactive protein in these patients. Pak J Biol Sci. 2008;11:1785–1790. doi: 10.3923/pjbs.2008.1785.1790. [DOI] [PubMed] [Google Scholar]
- 28.Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi-quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Med J. 2008;49:204–208. [PubMed] [Google Scholar]
- 29.Kim EK, Lee BS, Lee JA, Jo HS, Park JD, Kim BI, et al. Clinical availability of serum procalcitonin level in the diagnosis of neonatal bacterial infection. J Korean Soc Neonatol. 2001;8:211–221. [Google Scholar]
- 30.Guibourdenche J, Bedu A, Petzold L, Marchand M, Mariani-Kurdjian P, Hurtaud-Roux MF, et al. Biochemical markers of neonatal sepsis: value of procalcitonin in the emergency setting. Ann Clin Biochem. 2002;39(Pt 2):130–135. doi: 10.1258/0004563021901874. [DOI] [PubMed] [Google Scholar]