Abstract
Background/Aims
We compared the long-term outcomes of balloon dilation versus botulinum toxin injection in Korean patients with primary achalasia and identified factors predicting remission.
Methods
We included 73 patients with achalasia newly diagnosed between January 1988 and January 2011. We ultimately enrolled 37 of 55 patients with primary achalasia through telephone interviews, who were observed for over 1 year. Short-term outcomes were evaluated from the medical records based on symptom relief after 1 month of treatment. Long-term outcomes were evaluated in a telephone interview using a questionnaire.
Results
Twenty-five patients were administered a botulinum toxin injection and 12 underwent balloon dilation. One month after the botulinum toxin injection, improvements were seen in chest pain (14 [56.0%] to 4 patients [16.0%]), regurgitation (16 [64.0%] to 4 [16.0%]), and dysphagia (25 [100.0%] to 5 [20.0%]). In the balloon dilation group, chest pain (8 [66.7%] to 1 [8.3%]), regurgitation (11 [91.7%] to 1 [8.3%]), and dysphagia (12 [100.0%] to 1 [8.3%]) had improved. A significant difference was observed in the mean remission duration between the botulinum toxin injection and balloon dilation groups (13 months [range, 1 to 70] vs. 29 months [range, 6 to 72], respectively; p = 0.036). Independent factors predicting long-term remission included treatment type (odds ratio [OR], 6.982; p = 0.036) and the difference in the lower esophageal sphincter pressure (OR, 7.198; p = 0.012).
Conclusions
Balloon dilation may be more efficacious than botulinum toxin for providing long-term remission in Korean patients with achalasia. Follow-up manometry may predict the long-term outcome.
Keywords: Achalasia, Balloon dilation, Botulinum toxins
INTRODUCTION
Achalasia is a primary esophageal motor disorder characterized by incomplete relaxation of the lower esophageal sphincter (LES) and the absence of esophageal peristalsis caused by degeneration of Auerbach's plexus [1,2,3]. The goal of therapy is to reduce LES pressure (LESP), resulting in improved esophageal emptying by gravity and improved symptoms, such as dysphagia, regurgitation, chest pain, and weight loss [4]. Pharmacological therapy, pneumatic balloon dilation (BD), and intrasphincteric botulinum toxin (Botox, Allergan, Irvine, CA, USA) injection are the medical treatment modalities used most commonly. Pharmacological therapy is ineffective. Pneumatic BD is used commonly and provides good symptomatic relief in 86% to 100% of cases [5,6].
Intrasphincteric Botox injection has been used as an alternative to pneumatic BD or laparoscopic Heller's myotomy with partial fundoplication. Botox, a potent inhibitor of acetylcholine release from nerve endings, relaxes the LES by decreasing unopposed cholinergic stimulation of the LES. Some studies have shown that 65% to 100% of patients respond to a single injection within 1 month, but the effect is sustained for 12 months in only 15% to 75% [6,7,8,9]. The treatment is very safe and effective in the short term for relieving symptoms and has few complications [4].
A few studies have compared the short-term outcomes of BD versus Botox injection in patients with primary achalasia. In a prospective study, Mikaeli et al. [6] reported that the 12-month remission rate was significantly higher after pneumatic dilation (53%) than after Botox injection (15%). Vaezi et al. [8] reported that 14/20 (70%) pneumatic dilation- and 7/22 (32%) Botox-treated patients were in symptomatic remission at 12 months.
LESP is measured after treatment to evaluate the efficacy of BD or intrasphincteric Botox injection by performing esophageal emptying scintigraphy to determine the barium height on an esophagram [10,11,12,13,14,15,16,17]. However, few studies have identified the predictors of long-term outcomes. Therefore, we compared the long-term outcomes for BD versus Botox injection in patients with primary achalasia and identified predictors of remission.
METHODS
Patients
Diagnoses were established using clinical, radiological, and manometric criteria. The clinical criteria included dysphagia, regurgitation, chest pain, and weight loss, and the radiological criteria included bird's beak appearance of the LES, decreased esophageal peristalsis, and delayed esophageal emptying. The manometric diagnostic criteria consisted of aperistalsis of the esophageal body, increased LESP, and incomplete relaxation on swallowing. Patients with secondary achalasia were excluded based on esophagogastroduodenoscopy (EGD). This study was approved by the Institutional Review Board of Soonchunhyang University Hospital, and informed consent was obtained from all patients.
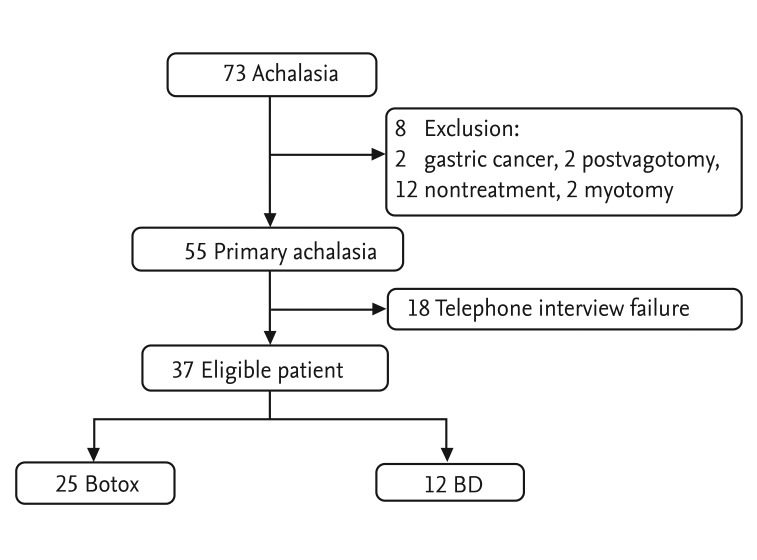
We enrolled 73 patients with achalasia newly diagnosed between January 1988 and January 2011. Exclusion criteria included gastric cancer (n = 2), postvagotomy (n = 2), no treatment (n = 12), and myotomy treatment (n = 2). With the exceptions of telephone interview failure (n = 18), contact failure due to the loss of a telephone number or address (n = 12), or refusal to interview (n = 6), we ultimately enrolled 37 of 55 patients with primary achalasia who were observed for 1 year: 25 underwent intrasphincteric Botox injection (Botox group) and 12 underwent BD group (Fig. 1). The median patient age was 56 years (range, 19 to 89). Twenty-two males and 15 females were enrolled. The median ages of the Botox and BD groups were 58 years (range, 39 to 89) and 38 years (range, 19 to 75), respectively. Thirty-seven patients (100%) had dysphagia, 22 (59.5%) had chest pain, and 27 (73.0%) had regurgitation. The median LESP values were 35.5 mmHg (range, 5.1 to 98.0) and 27.1 mmHg (range, 13.7 to 63.5) in the Botox and BD groups, respectively (Table 1).
Figure 1.
Patient enrollment and treatment modalities. Botox, group of patients who underwent intrasphincteric botulinum toxin injection as a first-line therapy. BD, group of patients who underwent balloon dilation as a first-line therapy.
Table 1.
Patient clinical characteristics
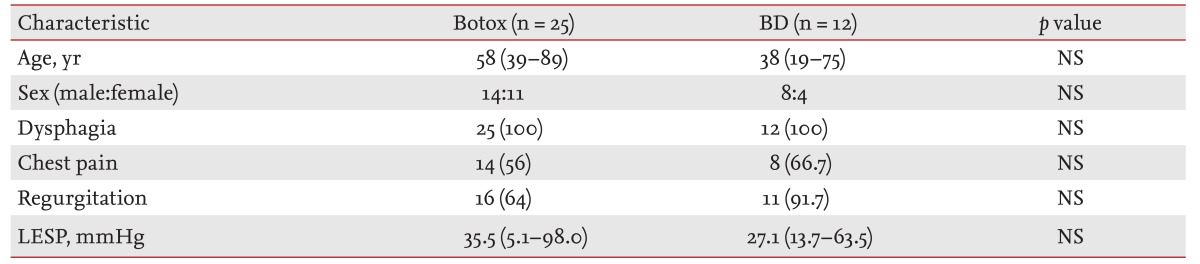
Values are presented as median (range) or number (%).
Botox, intrasphincteric botulinum toxin injection; BD, balloon dilation; NS, not significant; LESP, lower esophageal sphincter pressure.
Methods
To evaluate the long-term treatment effect, we determined the patient's current symptoms through telephone interviews after reviewing their medical records. To obtain objective information, we conducted the interview using a predetermined questionnaire that included the status of symptom improvement after treatment, time of recurrence in case of recurrence, and information pertaining to the frequency and degree of dysphagia, regurgitation, and chest pain. These were classified using the Eckardt score [18]. The points for each clinical parameter were summed, and level 0 indicated a score < 2, level 1 a score of 2 to 4, level 2 a score of 5 to 6, and level 3 a score of ≥ 7. Eckardt levels of 0 to 1 indicated remission, whereas levels 2 to 3 indicated treatment failure. The rate of symptom improvement according to treatment method was based on the questionnaire, and we evaluated the short-term symptom improvement status compared with symptoms prior to the first treatment. Long-term symptom improvement was evaluated from the clinical medical records and a telephone interview.
Esophageal manometry
Esophageal manometry is perfusion manometry involving constant perfusion of water and measuring pressure through an EMC8 (Synthetics Medical, Stockholm, Sweden) measuring tube. This procedure was performed after we anesthetized the nasal cavity with lidocaine while the patient was sitting for 5 to 10 minutes to stabilize the reading. Using the station pull-through technique, we located the side pore in the stomach and measured LESP by subtracting the measurements at 1-cm intervals.
We performed high-resolution manometry (HRM) in eight patients who underwent BD using an HRM catheter (Sierra Scientific Instruments, Los Angeles, CA, USA), in which 36 columnar pressure sensors were located at 1-cm intervals. Each pressure sensor had a 2.5-mm-long detection device that detected pressure using pressure transmission technology (TactArray, Pressure Profile Systems, Los Angeles, CA, USA). The data measured by the pressure gauge were analyzed using ManoView software (Sierra Scientific Instruments).
Esophagogastroduodenoscopy
The EGD observations included the dilation status of the esophageal body, stasis of food in the esophagus, resistance of the LES, and contraction status of the LES with breathing when it was reversed inside the stomach. We also looked for tumors to distinguish secondary achalasia.
Balloon dilation
We performed EGD with sedation and the patients lying on their left sides. A guidewire was located in the stomach, and we inserted a balloon dilator (Rigiflex, Boston Scientific, Natick, MA, USA) into the stomach along the guidewire. We checked the location of the balloon using fluoroscopy or EGD. We then dilated the balloon using contrast medium until the curve in the unrelaxed LES was obvious. We attempted dilation for 15 to 60 seconds with 7 to 15 per square inch pressure using balloons with external diameters of 30, 35, and 40 mm. We performed EGD and esophageal manometry within 3 days after the operation as a tracking examination.
Intrasphincteric botulinum toxin injection
We inserted the EGD to the level of the LES after a 12-hour fast. We mixed 100 units Botox with 4 to 5 mL normal saline and injected 1 mL at four points around the LES using a syringe. We kept the patient nil per os the next day and looked for evidence of perforation on a chest radiograph. Afterwards, the patients were started on clear fluids and finally underwent follow-up EGD and esophageal manometry before discharge.
Statistical analysis
SPSS version 18.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. Quantitative variables are presented as medians and ranges. The Kaplan-Meier method was used to assess symptomatic recovery. Fisher exact test was used to compare the remission rate of each symptom between the two treatment methods. The Kaplan-Meier method and log-rank test were used for analysis of the treatment predictors in the univariate analyses, and the Cox regression method was used for the multivariate analysis. p values < 0.05 were considered significant.
RESULTS
Clinical progress
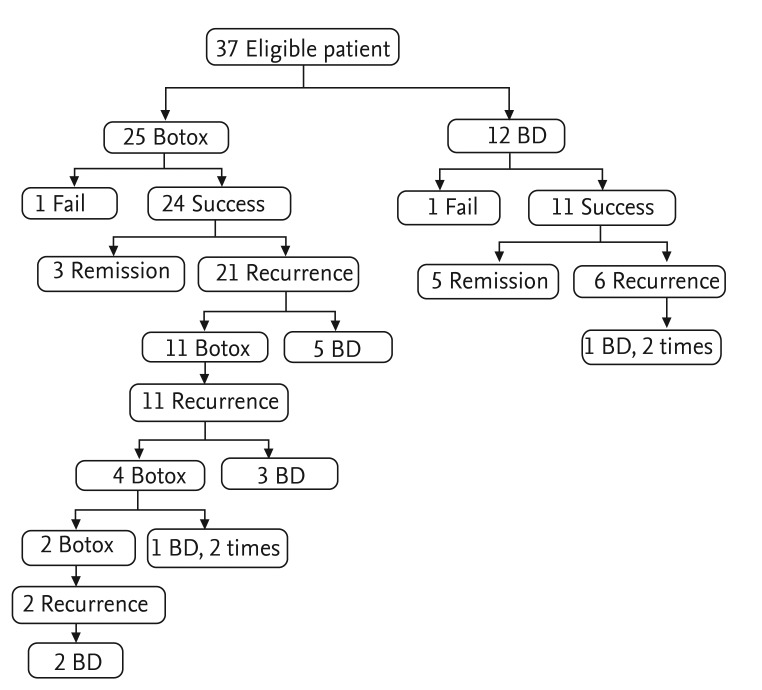
Thirty-seven of the 55 patients were contacted for telephone interviews; 25 had intrasphincteric Botox injections and 12 had undergone BD. Thirty-five patients showed symptom improvement after treatment, whereas two did not. Three of 24 in the Botox group who showed symptom improvement remained in remission, whereas 21 developed recurrent symptoms. Of these 21, 11 received an intrasphincteric Botox injection, and five underwent BD. Five of the 11 patients who showed improvement after BD as the first treatment continued in remission, and six had recurrent symptoms. One of the recurrent patients underwent two more rounds of BD (Fig. 2).
Figure 2.
Treatment modalities and follow-up. Twenty-five patients underwent intrasphincteric botulinum toxin injection (Botox), and 12 underwent balloon dilation (BD) as initial therapy.
No complications, such as esophageal perforation or bleeding requiring treatment, were observed. We performed a barium esophagogram, esophageal manometry, and EGD before and after BD or intrasphincteric Botox injection. If the patient had worsened subjective symptoms, the reoperation status was determined from these tests.
The median LESP was 32.2 mmHg (range, 5.1 to 98.0) before treatment and 22.5 mmHg (range, 0.8 to 48.0) after treatment. The difference was 18.4 mmHg (range, 0.0 to 65.0). During the median 61-month (range, 12 to 184) follow-up period, the median remission duration was 20 months (range, 1 to 72). The follow-up period was 56 months (range, 15 to 184) for the intrasphincteric Botox injection group and 33 months (range, 12 to 74) for the BD group.
Short-term symptom relief after the first treatment according to treatment method
The number of patients with chest pain in the Botox group decreased from 14 (56.0%) to 4 (16.0%), those with regurgitation decreased from 16 (64.0%) to 4 (16.0%), and those with dysphagia decreased from 25 (100.0%) to 5 (20.0%). The number of patients with chest pain in the BD group decreased from 8 (66.7%) to 1 (8.3%), regurgitation from 11 (91.7%) to 1 (8.3%), and dysphagia from 12 (100.0%) to 1 (8.3%) (Table 2).
Table 2.
Comparison of short-term symptom relief after intrasphincteric botulinum toxin injection and balloon dilation treatment
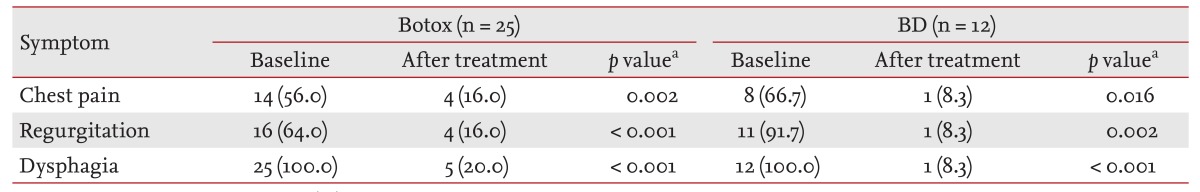
Values are presented as number (%).
Botox, group of patients who underwent intrasphincteric botulinum toxin injection as a first-line therapy; BD, group of patients who underwent balloon dilation as a first-line therapy.
aMcNemar test.
The symptom-free period after the first treatment
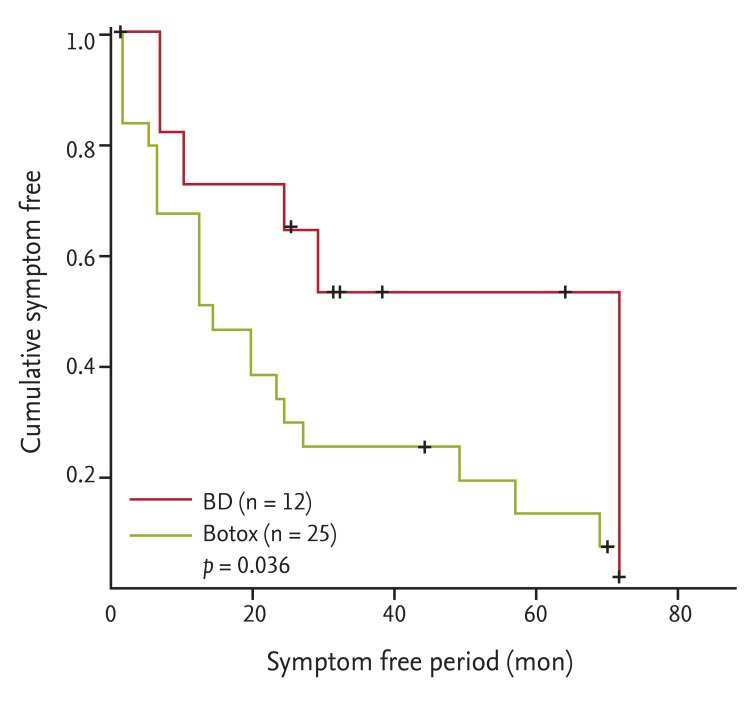
The median time to the symptom-free period in the 35 patients who showed symptom improvement after the first treatment was 20 months. The symptom-free period was 13 months (range, 1 to 70) in the Botox group and 29 months (range, 6 to 72) in the BD group. The symptom-free period was significantly longer in the BD group (p = 0.036) than then Botox group (Fig. 3).
Figure 3.
Kaplan-Meier curves for the symptom-fee period in patients undergoing intrasphincteric botulinum toxin injection (Botox) or balloon dilation (BD). A significant difference in the symptom-free period was observed between the Botox and BD groups (13 months [range, 1 to 70] vs. 29 months [range, 6 to 72], respectively; p = 0.036).
Predictors of long-term outcome
Univariate analyses of age, sex, reflux, chest pain, and LESP after treatment were conducted, and the differences in LESP values before and after treatment were used to evaluate the effect of each factor on long-term outcome. The symptom-free period was significantly longer (p = 0.015) when the difference in the LESP before and after treatment was > 19 mmHg. The treatment method was also a significant predictor. Patients undergoing BD had a significantly longer symptom-free period (p = 0.036) than those who received Botox (Table 3).
Table 3.
Univariate analysis of factors affecting long-term clinical remission
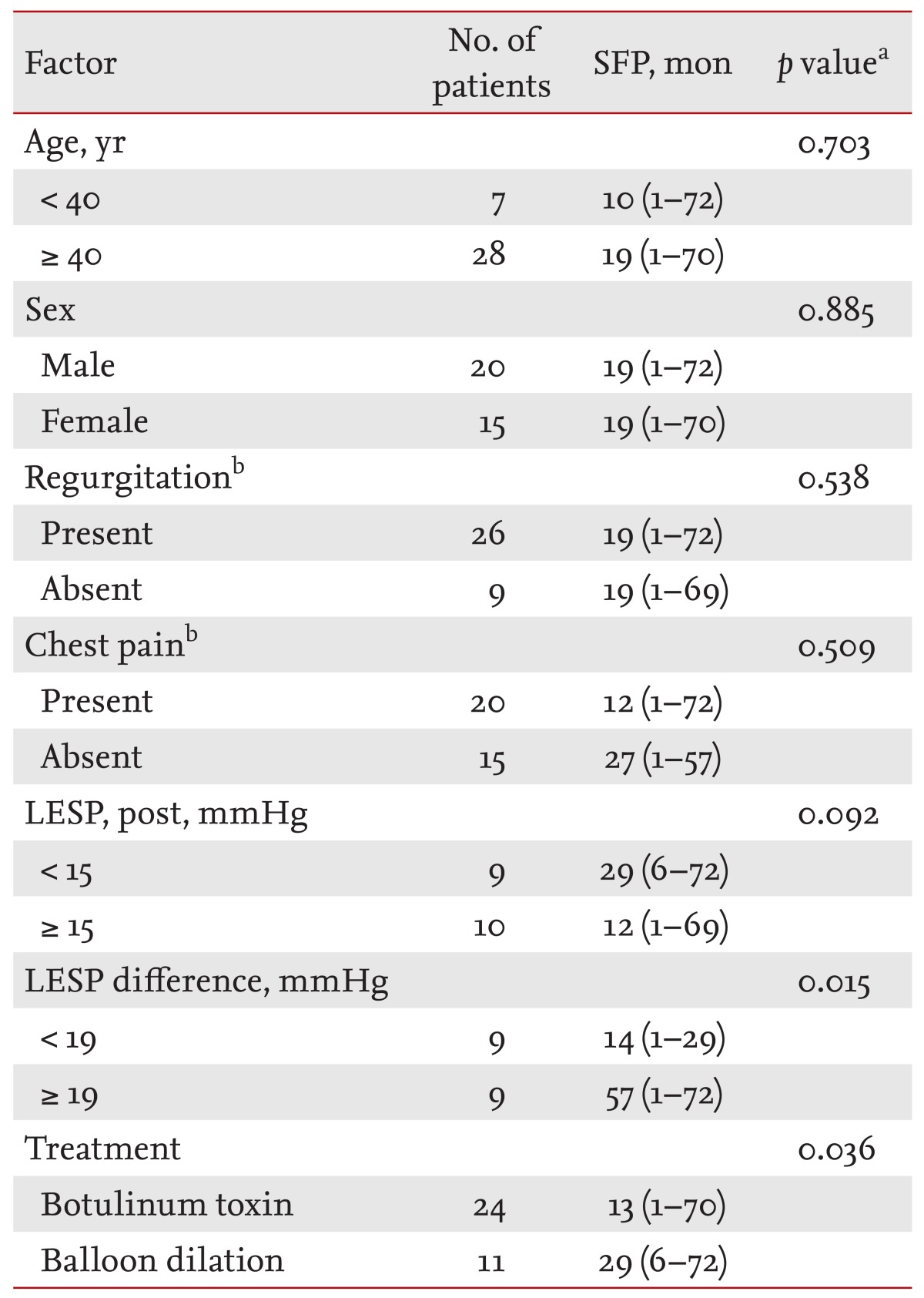
Values are presented as median (range).
SFP, symptom-free period; LESP, lower esophageal sphincter pressure.
aLog rank test.
bRegurgitation and chest pain mean "before treatment."
The symptom-free period was significantly longer when the difference in LESP before and after treatment was > 19 mmHg, according to the Cox multivariate regression analysis for age, sex, difference in LESP, and treatment type (p = 0.012). BD also resulted in a significantly longer symptom-free period than that of intrasphincteric Botox injection after correcting for other variables, such as age and sex (p = 0.036) (Table 4).
Table 4.
Multivariate analysis of factors affecting clinical remission
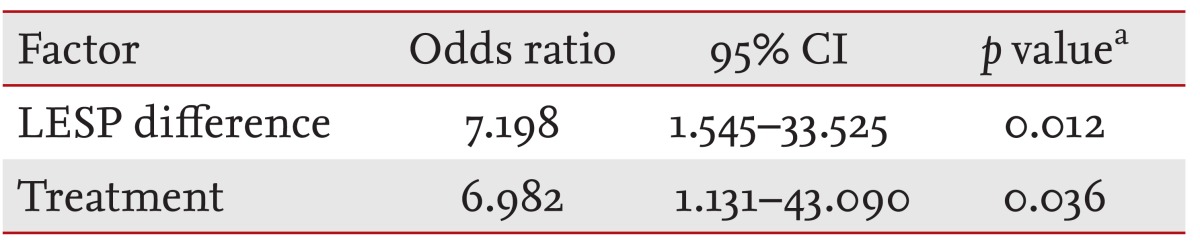
Age- and sex-adjusted values. Treatments were intrasphincteric botulinum toxin injection and balloon dilation. Balloon dilation resulted in a significantly longer symptom- free period than that of intrasphincteric botulinum toxin injection after correcting for other variables, such as age and sex (p = 0.036).
CI, confidence interval; LESP, lower esophageal sphincter pressure.
aCox regression test.
DISCUSSION
The rates of therapeutic success from intrasphincteric Botox injection were 48%, 24%, and 8.7% after 1, 2, and 5 years, respectively, whereas the rates for BD were 66.7%, 58.3%, and 25%. Bansal et al. [19] also compared BD and intrasphincteric Botox injection and found respective success rates of 89% and 38% after 1 year. Gutschow et al. [20] found that symptoms recurred or continued in 6.3% of patients after 35 months of observations following BD, whereas they recurred or continued in 71.4% 9 months after intrasphincteric Botox injection. Other studies have shown that 65% to 100% of patients respond to a single injection within 1 month, and on further follow-up, this effect was sustained for 12 months in 15% to 75% [5,6,7,8,9]. The results of our study were similar.
Perforation is a complication of BD and intrasphincteric Botox injection in 1% to 4% of cases [4,19,21]. However, no perforation occurred in our series.
Many studies have examined predictors of primary achalasia treatment. Alderliesten et al. [13] reported that the rate of recurrence is higher in younger patients and in those with a LESP > 10 mmHg 3 months after treatment. Nam et al. [10] reported that the remission rate was high if the difference in the LESP between before and after BD was > 13 mmHg. In addition, useful predictors have included sex, esophageal emptying scintigraphy, esophageal diameter on esophagography, length of retained barium, normalization of the reversed stomach-esophagus pressure, and occurrence of different simultaneous contraction configurations [8,11,12,14,15,16,17,22,23].
In this study, the remission duration was significantly longer if the difference in the LESP between before and after treatment was > 19 mmHg. BD was a more useful treatment than intrasphincteric Botox injection.
Selection bias was a potential limitation of this study due to its nonrandomized nature. The group undergoing intrasphincteric Botox injection showed selection bias due to age. We depended on the patients' memory of subjective symptoms and the recurrence period, which is a limitation of retrospective studies. The number of patients included was small, and 18 patients were excluded because they were unavailable for telephone interviews. Therefore, the study was impractical for a statistical interpretation. In addition, eight patients undergoing BD recently underwent HRM. The pressure on HRM appeared to be lower than that using conventional manometry. Hence, the examination we used could have affected the numerical pressure value. It was possible that the LESP appeared low in the group of patients who underwent BD.
Many patients remained symptomatic > 5 years after treatment. BD and intrasphincteric Botox injection do not restore destroyed nerves but alter the LES. Laparoscopic Heller's myotomy with partial fundoplication is another treatment option. A recent 2-year randomized prospective study showed that the efficacy of BD is similar to that of Heller's myotomy and Dor's fundoplication for treating achalasia [24]. Japanese and European researchers successfully conducted a peroral endoscopic myotomy (POEM), which is a submucosal myotomy using an endoscope, without complications [25,26]. A successful case of POEM was reported here. The number of effective treatments available for achalasia is increasing. Therefore, an evaluation of POEM is needed. However, this method does not treat the root problem; i.e., destruction of the LES, and it is also necessary to treat reflux. The root solution would be to restore the destroyed splanchnic nerve plexus. Neural stem cell transplantation is a future alternative treatment [27]. Many studies currently in progress are evaluating cerebral cortex neural stem cells and should assess the survival and side effects of transplanted stem cells.
In conclusion, BD had a better long-term outcome than that of intrasphincteric Botox injection. The symptom-free period was significantly longer if the difference in LESP was > 19 mmHg, suggesting the usefulness of manometric follow-up after treatment. A future prospective randomized study is needed to identify the predictors of long-term remission in patients with primary achalasia.
KEY MESSAGE
In Korean patients with achalasia, the short-term symptom relief after intrasphincteric botulinum toxin injection and balloon dilation treatment are similar.
Ballon dilatation had a better long-term outcome than that of intrasphincteric Botox injection in the treatment of achalasia.
Independent factors predicting long-term remission included treatment type and the difference in the lower esophageal sphincter pressure. The symptom-free period was significantly longer if the difference in LESP was > 19 mmHg, suggesting the usefulness of manometric follow-up after treatment.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cohen S, Fisher R, Tuch A. The site of denervation in achalasia. Gut. 1972;13:556–558. doi: 10.1136/gut.13.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38. doi: 10.1186/1750-1172-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS. Endoscopic treatment of primary esophageal motility disorders. Korean J Gastrointest Endosc. 2011;42:341–348. [Google Scholar]
- 5.Cox J, Buckton GK, Bennett JR. Balloon dilatation in achalasia: a new dilator. Gut. 1986;27:986–989. doi: 10.1136/gut.27.8.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikaeli J, Fazel A, Montazeri G, Yaghoobi M, Malekzadeh R. Randomized controlled trial comparing botulinum toxin injection to pneumatic dilatation for the treatment of achalasia. Aliment Pharmacol Ther. 2001;15:1389–1396. doi: 10.1046/j.1365-2036.2001.01065.x. [DOI] [PubMed] [Google Scholar]
- 7.Annese V, Basciani M, Perri F, et al. Controlled trial of botulinum toxin injection versus placebo and pneumatic dilation in achalasia. Gastroenterology. 1996;111:1418–1424. doi: 10.1016/s0016-5085(96)70002-1. [DOI] [PubMed] [Google Scholar]
- 8.Vaezi MF, Richter JE, Wilcox CM, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44:231–239. doi: 10.1136/gut.44.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghoshal UC, Chaudhuri S, Pal BB, Dhar K, Ray G, Banerjee PK. Randomized controlled trial of intrasphincteric botulinum toxin A injection versus balloon dilatation in treatment of achalasia cardia. Dis Esophagus. 2001;14:227–231. doi: 10.1046/j.1442-2050.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Nam SM, Kim JS, Kim SG, Jung HC, Song IS. Long-term efficacy of pneumatic dilatation in primary achalasia. Korean J Gastrointest Endosc. 2002;25:63–69. [Google Scholar]
- 11.Ghoshal UC, Rangan M. A review of factors predicting outcome of pneumatic dilation in patients with achalasia cardia. J Neurogastroenterol Motil. 2011;17:9–13. doi: 10.5056/jnm.2011.17.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sym SJ, Jung HY, Jo CL, et al. Predictors of outcome in patients with primary achalasia treated by pneumatic dilation. Korean J Gastrointest Endosc. 2002;25:187–191. [Google Scholar]
- 13.Alderliesten J, Conchillo JM, Leeuwenburgh I, Steyerberg EW, Kuipers EJ. Predictors for outcome of failure of balloon dilatation in patients with achalasia. Gut. 2011;60:10–16. doi: 10.1136/gut.2010.211409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaezi MF. Quantitative methods to determine efficacy of treatment in achalasia. Gastrointest Endosc Clin N Am. 2001;11:409–424. [PubMed] [Google Scholar]
- 15.Shen YY, Shiau YC, Sun SS, Kao CH. Using radionuclide esophageal emptying test to evaluate pneumatic dilatation effects for achalasia. Hepatogastroenterology. 2001;48:1061–1063. [PubMed] [Google Scholar]
- 16.Bittinger M, Wienbeck M. Pneumatic dilation in achalasia. Can J Gastroenterol. 2001;15:195–199. doi: 10.1155/2001/593657. [DOI] [PubMed] [Google Scholar]
- 17.Song CS, Yu YI, Park SK, et al. Predictors of successful balloon dilation in patients with achalasia: especially in patients who showed peristalsis after dilation. Korean J Med. 1998;54:666–674. [Google Scholar]
- 18.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629–633. doi: 10.1136/gut.2003.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal R, Nostrant TT, Scheiman JM, et al. Intrasphincteric botulinum toxin versus pneumatic balloon dilation for treatment of primary achalasia. J Clin Gastroenterol. 2003;36:209–214. doi: 10.1097/00004836-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Gutschow CA, Tox U, Leers J, Schafer H, Prenzel KL, Holscher AH. Botox, dilation, or myotomy? Clinical outcome of interventional and surgical therapies for achalasia. Langenbecks Arch Surg. 2010;395:1093–1099. doi: 10.1007/s00423-010-0711-5. [DOI] [PubMed] [Google Scholar]
- 21.Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15:3969–3975. doi: 10.3748/wjg.15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon JT, Jung IS, Kim YS, Cho SH, Park H, Lee SI. Correlation between clinical symptoms and radiologic findings before and after pneumatic balloon dilatation for achalasia. Korean J Gastroenterol. 2008;52:16–20. [PubMed] [Google Scholar]
- 23.Song CW, Jeen YT, Um SH, Kim CD, Ryu HS, Hyun JH. Predictors of symptom improvement and recurrence in patients with achalasia treated by pneumatic balloon dilation. Korean J Gastroenterol. 1997;29:279–288. [Google Scholar]
- 24.Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 25.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 26.von Renteln D, Inoue H, Minami H, et al. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411–417. doi: 10.1038/ajg.2011.388. [DOI] [PubMed] [Google Scholar]
- 27.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–1824. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]