To the Editor,
The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib is active in patients with metastatic lung adenocarcinoma carrying mutations in the EGFR gene. Although erlotinib has been found to improve markedly progression-free survival and quality of life, most patients who show an initial response eventually develop disease progression. Possible mechanisms of acquired or secondary resistance to erlotinib include second mutations in the EGFR gene such as T790M, activation of an alternative pathway including Met or HER2 amplification, histological transformation to small cell lung cancer (SCLC) and epithelial to mesenchymal transition [1]. However, acquired resistance due to histological transformation into a large cell neuroendocrine carcinoma (LCNEC) is uncommon. Herein we describe a patient who developed a LCNEC at the time of acquired resistance to erlotinib during treatment for primary lung adenocarcinoma.
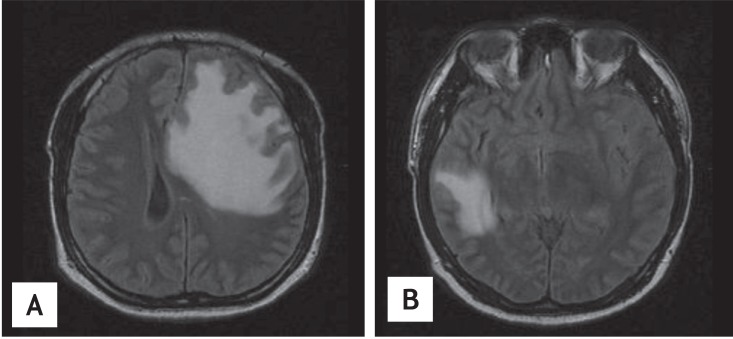
A 33-year-old male was admitted to the Department of Neurosurgery because of headache and diplopia. Brain magnetic resonance imaging (MRI) revealed multiple metastatic masses in the left frontal and right temporal lobes and the left cerebellum of the brain. The largest mass in the left frontal lobe measured 3.4 cm and caused midline shifting of the brain (Fig. 1).
Figure 1.
Magnetic resonance imaging of multiple metastatic masses in the brain. (A) T2-weighted image of the left frontal lobe mass. (B) T2-weighted image of the right temporal lobe.
A left frontal craniotomy was performed to remove the mass in the left frontal lobe. The specimen was positive for cytokeratin-7 (CK-7), thyroid transcription factor-1 (TTF-1), leukocyte common antigen, CK-20, and vimentin, indicating a well-differentiated adenocarcinoma (Fig. 2). The patient was subsequently referred to the Department of Medical Oncology.
Figure 2.
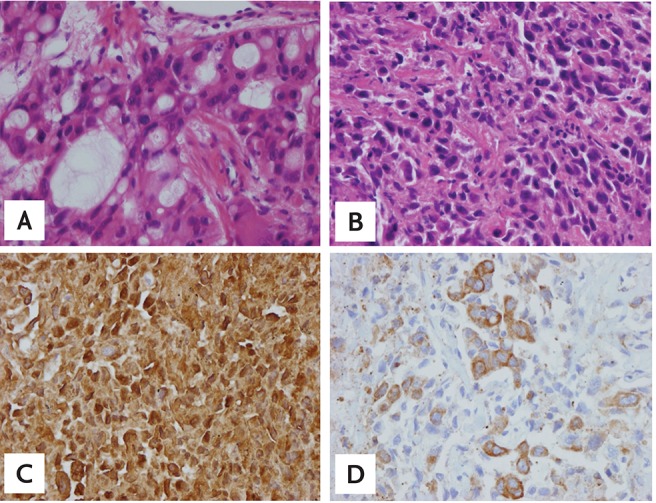
(A) Adenocarcinoma from brain tissue after craniotomy at the time of initial diagnosis (H&E, ×400). (B) Large-cell neuroendocrine carcinoma (LCNEC) from rebiopsied sample (H&E, ×400). (C) LCNEC from rebiopsied sample (chromogranin, ×400). (D) LCNEC from rebiopsied sample (neuron specific enolase, ×400).
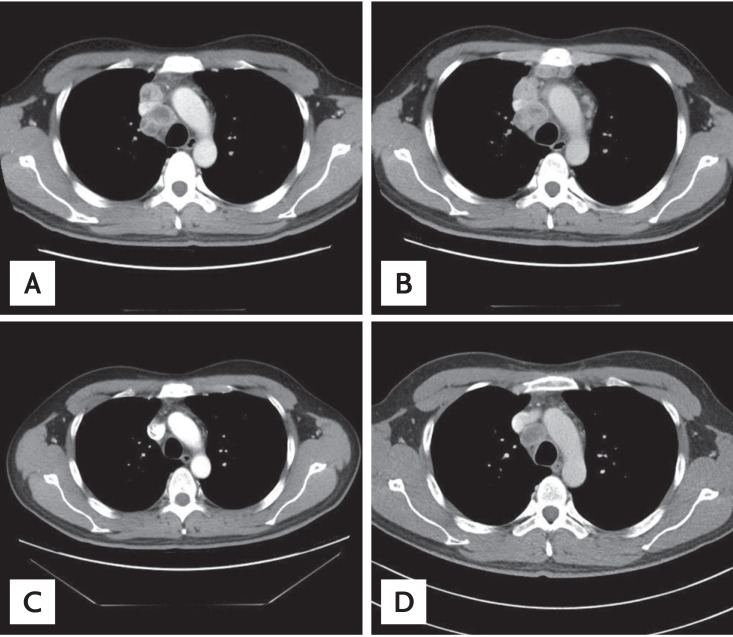
Chest computed tomography (CT) showed multiple nodules in both lung parenchyma and enlarged lymph nodes in both mediastinal and right hilar and right supraclavicular areas (Fig. 3). Abdominal CT showed a heterogeneously enhanced mass, measuring 3.6 × 4.1 cm in the lower portion of the left kidney, along with multiple enlarged lymph nodes near the left renal vein and a low density mass 1.4 cm in size near the right kidney. A bone scan showed sacral metastasis.
Figure 3.
Chest computed tomography images of paratracheal lymph nodes. (A) Paratracheal lymph node enlarged at the time of initial diagnosis. (B) Disease progression after two cycles of pemetrexed-cisplatin as first-line chemotherapy. (C) Partial response after 2 months of erlotinib. (D) Image before rebiopsy, showing the paratracheal lymph node had increased in size despite erlotinib treatment.
The patient was diagnosed with metastatic lung adenocarcinoma. Analysis of the EGFR gene showed a deletion in exon 19.
The patient underwent CyberKnife surgery treatment of the left cerebellar hemisphere, right temporal lobe and left precentral gyrus, followed by two cycles of pemetrexed-cisplatin as first-line chemotherapy. However, follow-up CT showed progressive disease (Fig. 3).
Second-line treatment with erlotinib for 2 months showed a partial response in the lung nodules, mediastinal lymph nodes, and kidney masses. Four months after erlotinib was started, the patient experienced pain in the hip and right leg. MRI of the L-spine and sacrum revealed a 3.8 × 7.8 × 4.4 cm lobular mass involving the right sacral body crossing the midline with extraosseous extension into the spinal canal and obliteration of the right side S1 nerve root. The patient was treated with palliative radiotherapy to the sacrum (total, 4,200 cGy in 14 fractions). Erlotinib was continued during palliative radiotherapy. After 10 months of treatment with erlotinib, the right lower paratracheal lymph node increased in size from 1 to 3 cm (Fig. 3).
A rebiopsy of the right paratracheal lymph node using mediastinoscopy showed two different types of cells: adenocarcinoma cells, as shown on initial pathology, and LCNEC cells, comprising 50% of the microscopic field. The LCNEC cells were positive for chromogranin, CK-7, neuron specific enolase, and TTF-1 and negative for CD56a (Fig. 2). The EGFR gene in the second biopsy specimen was not analyzed due to patient refusal. Despite receiving three cycles of etoposide-cisplatin chemotherapy, along with maintenance erlotinib to prevent disease flare up from erlotinib discontinuation, the disease progressed (Fig. 3).
After 1 month observation period, the patient visited our emergency room with fever and dyspnea. Chest CT showed enlarged mediastinal lymph nodes invading the trachea and superior vena cava (SVC), consistent with SVC syndrome. Palliative emergency radiation treatment of the mediastinal mass (3,000 cGy in 10 fractions) was started immediately. Follow-up chest CT showed that several parenchymal nodules in both lungs had increased in size, while others had decreased and the size of the mediastinal lymph nodes had decreased. However, the SVC remained obstructed and salvage chemotherapy with topotecan was started. Topotecan chemotherapy was discontinued after one cycle due to worsening of the patient's general condition and tumor lysis syndrome. After 5 days of intensive care unit care, the patient died.
Acquired or secondary resistance to EGFR TKIs is associated with histological transformation into SCLC and changes in the molecular genetics of lung adenocarcinoma [2]. Therefore, a rebiopsy is warranted when disease progression is observed during treatment with an EGFR TKI. The mechanism of histological transformation remains unclear. A female patient diagnosed initially with adenocarcinoma subsequently developed SCLC upon disease progression, with the SCLC cells harboring an 18 bp deletion in exon 19 of the EGFR gene [3]. An analysis of the molecular genetic and histological features of 37 patients with progressive disease after durable response to EGFR TKI showed the EGFR T790M mutation in 18 patients (49%) and Met amplification and PIK3CA mutations in two patients (5%) each [2]. In five patients (14%), histological transformation to SCLC was confirmed based on expression of neuroendocrine markers, with the original EGFR mutation maintained. One of the five patients with SCLC transformation also had a mutation in PIK3CA, with four showing resistance during treatment with an EGFR TKI.
Two cases of histological transformation to neuroendocrine carcinoma during or after treatment with an EGFR TKI were reported recently [4,5]. For example, one patient with an adenocarcinoma showed transformation to combined SCLC and NSCLC with neuroendocrine morphology after 12 months of gefitinib treatment [4]. The patient showed disease progression after four cycles of pemetrexed-cisplatin combination chemotherapy and erlotinib treatment for 12 months, with an EGFR exon 19 deletion observed in a tissue specimen obtained at the time of progression. Therefore, whether histological transformation reflects acquired resistance to EGFR TKI remains unclear. Another patient showed transformation from an adenocarcinoma to EGFR-positive LCNEC after treatment with an EGFR TKI. Following surgery, this patient received radiation treatment concurrent with combination chemotherapy of vinorelbine and cisplatin for disease progression and adjuvant chemotherapy for stage IIIA lung adenocarcinoma with an EGFR mutation. This patient was treated with gefitinib for 24 months and then changed to erlotinib. Since the rebiopsy was obtained when the patient developed resistance to erlotinib, the time of phenotypic switch to LCNEC could not be determined [5].
The timing of histological transformation may require obtaining biopsy samples when the patient shows resistance to each type of systemic treatment. However, repeated biopsies are difficult in clinical practice.
Our patient presented with a combined tumor, consisting of adenocarcinoma and LCNEC components when he developed resistance to erlotinib. The pathological results were confirmed by the histological features of the tumor and with immunohistochemistry of neuroendocrine markers. Molecular genetic analysis of the rebiopsied tissue was not performed and changes in molecular genetic and epigenetic profiles occurring at the time of transformation into LCNEC were not determined because the patient refused further study.
Differential diagnosis of SCLC and LCNEC is difficult in some cases because they have different morphologies, such as organoid nesting, trabecular, rosette-like, and palisading patterns, with neuroendocrine differentiation confirmed immunohistochemically. LCNEC cells are larger than small resting lymphocytes. Compared with SCLC cells, the nucleoli in LCNEC cells are more prominent and their nuclear-cytoplasmic ratio is lower.
Our findings suggest that, during treatment with the EGFR TKI erlotinib, the adenocarcinoma had partially transformed to LCNEC or was undergoing histological transformation. Initial pathological diagnosis was confirmed since the tissue obtained with craniotomy showed no evidence of the LCNEC component. Moreover, rebiopsy of the paratracheal lymph node showed the tumor had a marked initial response to erlotinib but became enlarged again at the time of resistance to EGFR TKI.
The mechanisms of the LCNEC transformation during treatment with EGFR TKI and the development of acquired resistance require further investigations.
In conclusion, we described a patient with metastatic lung adenocarcinoma who experienced a histological transformation to combined LCNEC and adenocarcinoma of the lung during treatment with erlotinib.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31:1070–1080. doi: 10.1200/JCO.2012.43.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zakowski MF, Ladanyi M, Kris MG Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome Group. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355:213–215. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 4.Popat S, Wotherspoon A, Nutting CM, Gonzalez D, Nicholson AG, O'Brien M. Transformation to "high grade" neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;80:1–4. doi: 10.1016/j.lungcan.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa S, Morikawa N, Kimura Y, Nagano Y, Murakami K, Tabata T. Large-cell neuroendocrine carcinoma with epidermal growth factor receptor mutation: possible transformation of lung adenocarcinoma. Respirology. 2012;17:1275–1277. doi: 10.1111/j.1440-1843.2012.02258.x. [DOI] [PubMed] [Google Scholar]