Abstract
The synthesis of α-aminooxy trisaccharide moiety [α-d-Gal-(1,4)-β-d-Gal-(1,4)-β-d-Glc-α-aminooxy], related to the cell surface globotriaosylceramide (Gb3) receptor of the B subunit of the AB5 Shiga toxin of Shigella dysenteriae, has been synthesized for the first time in 11 steps with a 15% overall isolated yield. A highlight of this work entails utilizing chemically compatible synthetic transformations, including those related to glycosylation, incorporative of the succinimidyl moiety as a precursor to the aminooxy Gb3 derivative. The fully deprotected trisaccharide aminooxy compound was reacted with a carbonyl compound leading to oxime formation in quantitative yield underscoring the importance for future glyco-conjugations.
Keywords: aminooxy sugar, Gb3 trisaccharide, Shiga toxin, glycosylation
Human oligosaccharides and glycoconjugates[1–5] are well-known entities that play critical roles in maintaining homeostatic biological processes,[6,7] however those that come from foreign sources (e.g. bacterial-based) or that are aberrant in nature have attracted a great deal of attention from the synthetic and biological communities for a sustained period of time due to their strong relationship in diseased states. Within the aforementioned classifications, glycosphingolipids[8–12] are known to be naturally occurring bioactive molecules most often embedded in the membrane of all animal cells and even in some plant cells.[13–16] Physiological functions of glycosphingolipids include mediating intercellular interactions as well as modulating the activity of some proteins in the plasma membrane. Accumulation of glycospingolipids in the lysome can result in glycosphingolipid storage diseases such as Gaucher’s disease[17,18] Fabry disease[19,20] urinary-tract infections[21] as well as a host of others complications including pediatric neurodegenerative disease[22]
One detrimental glycolipid effect caused by Shiga toxin(s) is the loss of millions of human lives each year. In 1897 Kiyoshi Shiga first described the bacterial origin of dysentery caused by the bacillus Shigella dysenteriae. The Shiga toxins have two subunits: 1) unit A and 2) unit B and belong to the AB5 toxin[23,24] family of antigens. The cytotoxic enzyme unit A, an N-glycosidase which catalyzes rRNA depurination activity leading to cell death, is non-covalently linked with a non-toxic homopentameric cell-adhesion carrier B5 unit. Each of these units have three binding sites for a specific glycolipid called globotriaosyl (Gb3), known as a Pk-trisaccharide.[21,25] The B5 unit mediates uptake and intracellular transport of the toxin through specific binding to the sugar domain of the Gb3 in the plasma membrane of cells. Gb3 also serves as a cell-surface receptor for Shiga-like toxins (SLTs).[26–30] Not surprisingly, SLT vaccine development has been an important area of study from the early 1990’s.[31] Encouragingly, there are Shiga toxin vaccine candidates currently being pursued. One strategy utilizes live attenuated[32] Shiga-toxin producing E. coli and another uses the carrier protein KLH conjugated to Shiga-toxin[33]. In both of the aforementioned accounts, the vaccines have been able to produce active and functional IgG antibodies capable of neutralizing Shiga-toxin, however a successful verdict is still pending.
In noting the importance of these carbohydrate-protein binding interactions, numerous syntheses of Gb3 have been reported by prominent, synthetic carbohydrate groups.[34–46] Those strategies have incorporated linkers at the anomeric position including the ceramide,[46] p-aminophenyl(pAP),[34] 6-(p-cinnamoylphenoxy)hexyl tether,[35] and methyl glycosides.[36] In an attempt to develop physiologically stable oxime bonds for sugar-sugar couplings for entirely carbohydrate-based vaccines, our approach, is to introduce the aminooxy precursor succidimidyl group at an early stage in the synthetic endeavour taking advantage of a flexible, functional group strategy amenable to derivitization.[47] In utilizing chemically compatible reagents, the goal is to develop orthogonal reactions that are non-toxic, high yielding, and are robust toward pre-exposure of an aminooxy moiety for the synthesis of Gb3-α-ONH2. Insofar as we are aware, Gb3-α-ONH2 has never been previously synthesized and the benign nature and orthogonality of the reagents we have employed overcome some of the current challenges regarding succinimidyl lability.
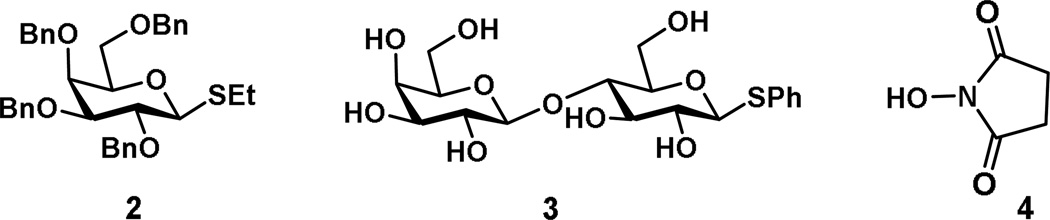
In this context, we describe, a synthesis of a Gb3 trisaccharide derivative noted as [α-d-Gal-(1,4)-β-d-Gal-(1,4)-β-d-Glc-α-aminooxy] (1) (Fig 1). The synthesis of Gb3-α-ONH2 was achieved by sequential convergent glycosylations of a suitably protected monosaccharide (2) as well as disaccharide (3) that were prepared from commercially available reducing sugars using chemically compatible reaction conditions (Fig. 2). The use of N-hydroxysuccinimide (4) (NHS) was essential for installing the -OHN2 functionality. Other similar types of NHS equivalents, such as N-hydroxyphthalamide (PhtOH)’[48–50] N-pentenoyl (NHPent)[51–54] have also been used for the -ONH2 functionality but due to our experience with NHS, others were not utilized.[47,55]
Figure 1.
The Gb3-α-ONH2 (1) as an aminooxy sugar for oxime-forming conjugation.
Figure 2.
Compounds used in the synthesis of target trisaccharide Gb3-α-ONH2.
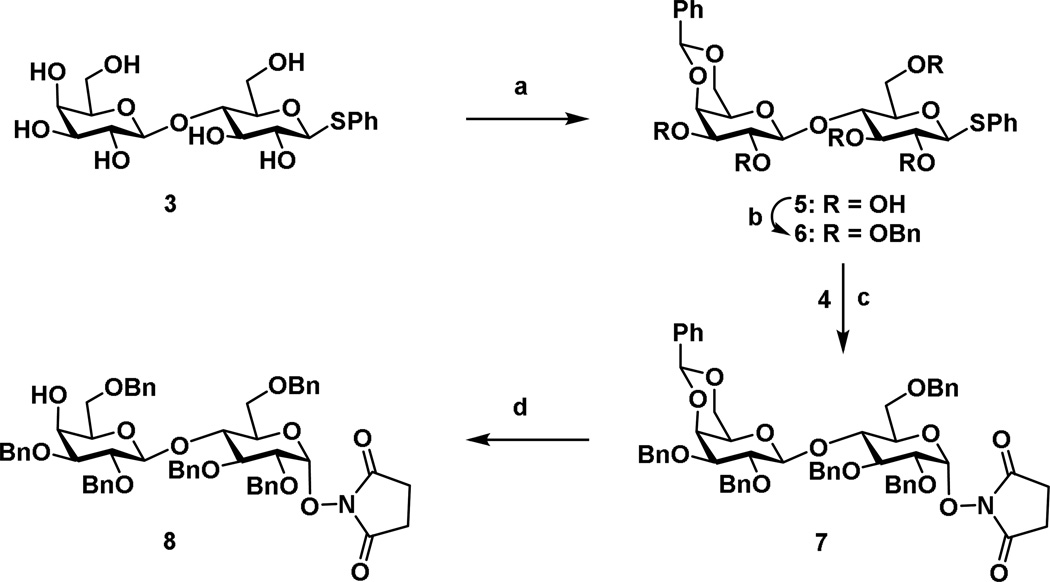
Phenyl-β–d-galactopyranosyl-(1,4)-1-thio-β-d-glucopyranoside (3) was prepared from d-lactose following a literature procedure.[46] Ethyl-2,3,4,6-tetra-O-benzyl-1-thio-β-d-galactopyranoside (2),[56], derived from d-galactose and N-hydroxysuccinamide (4) were commercially available. Compound 3 was treated with benzaldehyde dimethyl acetal to protect the 4- and 6-hydroxy groups in the presence of camphor sulphonic acid to yield compound 5, which was then benzylated using benzyl bromide and sodium hydride furnishing compound 6 in 81% overall isolated yield (for two steps) (Scheme 1). Stereoselective glycosylation of disaccharide donor 6 with N-hydroxy succinimide 4[57] (1.5 eq. due to low solubility in DCM) in the presence of N-iodosuccinimide (NIS) and trimethylsilyl trifluoromethanesulfonate (TMSOTf)[58,59] yielded compound 7 in 90% as a mixture of anomers (10:1, α/β) as determined from 1H NMR. Structural confirmation of compound 7, using spectral analysis, was assigned as follows: δ 5.56 (d, J = 4.0 Hz, 1 H, 1A), 5.51 (s, 1 H, PhCH), 4.35 (d, J = 7.9 Hz, 1 H, 1B) and 2.75 (s, 4 H, -C(=O)CH2-CH2-C(O)). The 13C NMR data was as follows: δ 101.7 (C-1A), 103.2 (C-1B), 101.7 (PhCH), 171.1 (2 C, -C(O)CH2-CH2-C(O)), and 25.8 (2 C, -C(=O)CH2-CH2-C(O)). Successful 1,2-cis-glycosylation of the NHS derivative was supported by the coupling constant (J = 4.0 Hz) of the anomeric H-1 in compound 7. Selective reductive ring opening of the benzylidene acetal of 7 was achieved by treatment with ethereal HCl and sodium cyanoborohydride in THF to produce disaccharide acceptor 8 with a 4-OH exposed as a single regioisomer in an overall isolated 69% yield.
Scheme 1.
Reagents: (a) benzaldehyde dimethyl acetal, DMF, rt, overnight, 89% isolated yield. (b) Benzyl bromide, NaH, TBAB (cat.), DMF, rt, 4 h, 91% overall isolated yield. (c) N-iodosuccinimide (NIS), trimethylsilyl trifluoromethanesulfonate, 4Å MS, CH2Cl2, −20 °C, 1 h, 81% isolated yield. (d) Etheral HCl, sodium cyanborohydride, anhydrous THF, argon, 69% isolated yield.
Stereoselective glycosylation of disaccharide acceptor 8 with thioglycoside derivative 2[56] in the presence of (NIS) and TMSOTf furnished trisaccharide 9 in 57% yield as a mixture of anomers (20:1, α/β). Spectral 1H NMR data analysis confirmed the trisaccharide formation of the 1,2-cis glycosidic linkage: δ 5.51 (d, J = 4.0 Hz, 1 H, 1A), 5.07 (d, J = 3.5 Hz, 1 H, 1C), 4.33 (d, J = 7.74 Hz, 1 H, 1B), 2.75 (s, 4 H, -C(=O)CH2-CH2-C(O)). The 13C NMR data was as follows: δ 171.1 (2 C, -C(O)CH2-CH2-C(O103.2 (C-1B), 101.7 (C-1A), 101.1 (C-1C), 25.9 (2 C-C(=O)CH2-CH2-C(O)). Both 1,2-cis glycosidic linkages were confirmed through the examination of coupling constants (J = 4.0 Hz) of the H-1 of compound 8 and (J = 3.5 Hz) of the H-1 of compound 4.
In the final deprotection phase, we first utilized Pearlman’s catalyst[60] for benzyl group removal to obtain compound 10 followed by treatment with hydrazine hydrate[21] for deprotection of the succinimide group to furnish the final product 1. After completion as noted by TLC, two by-products[57,61] were also characterized, namely acetohydrazide and tetrahydropyriddazine-3,6-dione that are notorious to interfere with product purification. In noting the later to be true when using silica gel chromatography, these two by-products were eventually removed using a Bio-gel P-2 column with water as the eluent giving ultra-pure 1.
In order to test the reactivity of the aminooxy 1, we elected to utilize a simple carbonyl compound in the form of a ketone. As our group has been conjugating to carbonyl aldehydes, such as those noted in our previous published work,[47,55] we were eager to utilize a carbonyl ketone for three reasons: 1) firstly as a means to validate that oxime formation would occur as readily with carbonyl ketones as our experience indicated it would with carbonyl aldehydes, 2) for future development of novel oxime protecting groups and 3) for future studies on the hydrolysis of oxime bonds. Thus 2-propanone was used for the oxime forming reaction which ultimately gave rise to compound 11 in quantitative yield.
In conclusion, a convenient, stereocontrolled synthesis of Gb3-α-ONH2 was accomplished using a succinimidyl group at the anomeric position of lactose which remained intact until the final step of the synthesis. The succinimidyl group was transformed into an aminoxy moiety after global deprotection which readily allows for selective conjugation to carbonyl compounds. The aminooxy Gb3 was also reacted with 2-propanone to from an oxime link with which marks our initial attempts at developing new oxime protecting groups and for determining stability.
Experimental Section
General Experimental Methods
1H, 13C, 2D COSY and HMQC nuclear magnetic resonance spectra were recorded on Bruker 600 (1H NMR-600 MHz; 13C NMR 150) at ambient temperature with CDCl3, D2O as solvent and TMS as internal reference unless otherwise stated. Chemical shifts are reported in δ ppm. Data for 1H NMR are reported as follows: chemical shift, integration, multiplicity (s = singlet, d = doublet, dd = doublet of doublet, t = triplet, q = quartet, m = multiplet) and coupling constants in Hertz. All 13C NMR spectra were recorded with complete proton decoupling. Low resolution mass spectra (LRMS) were acquired on a Waters Acuity Premiere XE TOF LC-MS using electrospray ionization.
All reactions were carried out in oven-dried glassware under an argon atmosphere unless otherwise noted. Reactions were monitored by thin layer chromatography over silica gel coated TLC plates. TLC was visualized by warming ceric sulphate (2% Ce(SO4)2 in 2 N H2SO4)-sprayed plates on a hot plate. Column chromatography was performed on silica gel 60 (0.040–0.063 mm). All chemicals were of commercial grade and were used without further purification. Anhydrous solvents were obtained from Aldrich and EMD. Yields refer to chromatographically and spectroscopically pure material unless otherwise noted.
Phenyl-(2,3-di-O-benzyl-4,6-O-benzylidene-β–D-galactopyranosyl)-(1→4)-2,3,6-tri-O-benzyl-1 thio- β-D-glucopyranoside (6)
To a solution of compound 3 (2 g, 4.60 mmol) in anhydrous DMF (20 mL) were added benzaldehyde dimethyl acetal (0.84 g, 5.52 mmol), camphor sulfonic acid and the reaction mixture was allowed to stir overnight at room temperature. The reaction was quenched with triethylamine and solvents were removed under reduced pressure. The crude material 5 was dissolved in CH2Cl2 (50 mL) and the organic layer was washed 3× with water, dried using Na2SO4, concentrated and dried under vaccum. To a solution of the dried product in anhydrous DMF (30 mL) were added powdered NaH (at 60% w/w dispersion in mineral oil) (1.8 g, 45 mmol), benzyl bromide (3.8 mL, 32 mmol), and tetrabutylammonium bromide (200 mg, 0.62 mmol) and the reaction mixture was allowed to stir briskly at rt for 4 h. The reaction was quenched by adding satd. NH4Cl and concentrated under reduced pressure. The crude mass was dissolved in CH2Cl2 (150 mL) and the organic layer was washed with water, dried using Na2SO4, and finally concentrated. The crude product was purified over SiO2 using hexane-EtOAc (7:1) as eluant to give pure compound 6 (3.28 g, 81%).
1H NMR (600 MHz, CDCl3)
δ 7.61-7.21 (m, 35 H, Ar-H), 5.50 (s, 1 H, PhCH), 5.29 (d J = 10.4 Hz, 1 H, PhCH2), 4.89-4.76 (m, 7 H, PhCH2), 4.69 (d, J = 9.8 Hz, 1 H, 1A), 4.60-4.58 (d, J = 11.9 Hz, 1 H, PhCH2), 4.53 (d, J = 7.9 Hz, 1 H, 1B), 4.40 (1 H, PhCH2), 4.30-4.26 (m, 1 H, 6B), 4.09-4.08 (d, J = 3.7 Hz, 1 H, 4B), 4.06 (t, J1/2, 2/3 = 9.6 Hz, 1 H, 4A), 3.97-3.95 (dd, J1/2,1,3 = 3.9, 11.0 Hz, 1 H, 6A), 3.91-3.89 (m, 1 H, 6B), 3.84-3.79 (m, 2 H, 6A, 2B), 3.70 (t, J = 8. 9 Hz, 1 H, 3A), 3.52 (t, J = 9.9 Hz, 1 H, 2A), 3.48-3.46 (dd, J1/2,1,3 = 3.7, 9.6 Hz, 1 H, 3B), 3.4-3.40 (m, 1 H, 5A), 3.04 (m, 1 H, 5B)
13C NMR (150 MHz, CDCl3)
δ 139.0-126.8 (Ar-C), 103.1 (1B), 101.6 (PhCH), 87.6 (1A), 85.3 (3A), 80.3 (2A), 79.9 (3B), 79.6 (5A), 79.1 (2B), 77.4 (4A), 76.3 (OBn), 75.6 (OBn), 73.9 (4B), 73.2 (OBn), 71.9 (OBn), 69.2 (6B), 68.6 (6A), 66.7 (5B). ESI-MS (LR MS) [C60H60O10SNa]+ found 995.9
Succinamidyl- (2,3-di-O- benzyl-4,6-O-benzylidene- β –D-galactopyranosyl)-(1→4)-2,3,6-tri-O-benzyl-β-D-glucopyranoside (7)
To a solution of compound 6 (3.28 g, 3.71 mmol) and compound 4 (N-hydroxysuccinamide) (1.28 g, 11.12 mmol) in anhydrous CH2Cl2 (50 mL) was added MS-4Å (2.00 g,) and the reaction mixture was allowed to stir at rt for 1 h under argon. The reaction mixture was cooled to −20 °C and N-iodosuccinimide (NIS; 1.00 g, 4.45 mmol) and TMSOTf (80 µL) were then added. After stirring at the same temperature for 3 h, the reaction mixture was quenched with saturated NaHCO3 and sodium thiosulfate, filtered through a pad of celite, and finally washed with CH2Cl2 (100 mL). The organic layer was then washed 3× with aqueous Na2SO4 and water in succession, dried using Na2SO4, and concentrated under reduced pressure to give the crude product, which was purified over SiO2 using hexane-EtOAc (5:1) as the eluant to furnish pure α anomeric product 7 (2.96 g, 81%).
1H NMR (600 MHz, CDCl3)
δ 7.58-7.16 (m, 30 H, Ar-H), 5.56 (d, J = 4.0 Hz, 1 H, 1A), 5.51(s, 1 H, PhCH), 5.23 (d, J = 10.4 Hz, 1 H, PhCH2), 5.02 (d, J = 11.3 Hz, 1 H, PhCH2), 5.03-4.77 (m, 6 H, PhCH2), 4.76-4.75 (m, 1 H, 5A), 4.52 (d, J = 11.9 Hz, 1 H, PhCH2), 4.35 (d, J = 7.9 Hz, 1 H, 1B), 4.30-4.27 (dd, J1/2,1,3 = 1.2, 12.4 Hz, 1 H, 6B), 4.25 (d, J = 11.9 Hz, 1 H, PhCH2), 4.13-4.11 (dd, J1/2,1,3 = 2.3, 11.2 Hz, 1 H, 6A), 4.08-4.05 (m, 3 H, 3A, 4A, 4B), 3.92-3.90 (dd, J1/2,1,3 = 1.7, 12.4 Hz, 1 H, 6B), 3.83-3.80 (dd, J1/2,1,3 = 7.9, 9.6 Hz, 1 H, 2B), 3.76-3.73 (m, 1 H, 2A), 3.52-3.50 (dd, J1/2,1,3 = 1.9, 11.2 Hz, 1 H, 6A), 3.34-3.32 (dd, J1/2,1,3 = 7.9, 9.6 Hz, 1 H, 3B), 2.96 (s, 1 H, 5B), 2.75 (s, 4 H, -C(=O)CH2-CH2-C(O)).
13C NMR (150 MHz, CDCl3)
δ 171.1 (2 C, -C(O)CH2-CH2-C(O)), 139.3-126.9 (Ar-C), 103.2 (1B), 101.7(PhCH), 101.7 (1A), 79.8 (3A), 78.7 (3B), 78.1 (2A), 77.0 (4A), 76.4 (OBn), 75.9 (OBn), 74.0 (4B), 73.4 (OBn), 73.2 (OBn), 72.6 (5A), 72.0 (OBn), 69.3 (6B), 68.0 (6A), 66.6 (5B), 25.8 (2 C, -C(=O)CH2-CH2-C(O)). ESI-MS (LR MS) [C58H59NO13Na]+ found 1000.2
Succinamidyl - (2,3,6-tri-O- benzyl - α –D-galactopyranosyl)-(1→4)-2,3,6-tri-O-benzyl-β-D-glucopyranoside (8)
To a solution of compound 7 (2.96 g, 3.02 mmol) in THF (10 mL) sodium cyanoborohydride(0.95 g, 15.13 mmol) was added and stirred for 1 h under an atmosphere of argon, then the reaction mixture was cooled to ice temperature and treated with ethereal HCl until gas evolution ceased. The reaction mixture stirred for 15 mins in the same temperature then slowly warmed to room temperature and allowed to strir for 1 h further. After completion, the reaction mixture was filtered through a pad of celite. The filtrate was concentrated and purified on a silica gel column using hexane/EtOAc (2:1) as an eluent to give pure 8 (2.04 g, 69%).
1H NMR (600 MHz, CDCl3)
δ 7.53-7.23 (Ar-H), 5.56 (d, J = 4.0 Hz, 1 H, 1A), 5.06-5.01 (m, 2 H, PhCH2), 4.88-4.76 (m, 6 H, PhCH2), 4.75-4.74 (m, 1 H, 5A), 4.57-4.55 (m, 2 H, PhCH2), 4.47-4.45 (d, J = 12.0 Hz, 1 H, PhCH2), 4.35-4.34 (d, J = 7.8 Hz, 1 H, 1B), 4.34-4.33 (d, J = 11.9 Hz, 1 H, PhCH2), 4.09-3.98 (m, 4 H, 4A, 4B, 6A1, 3A), 3.76-3.74 (dd, J1/2,1,3 = 7.0, 9.8 Hz, 1 H, 6B1), 4.71-3.69 (dd, J1/2,1,3 = 4.0, 9.8 Hz, 1 H, 2A), 3.66-3.63 (dd, J1/2,1,3 = 8.0, 9.1 Hz, 1 H, 2B), 3.60-3.57 (m, 1 H, 6B2), 3.56-3.54 (m, 1 H, 6A2), 3.36-3.33 (m, 2 H, 3B), 2.73 (s, 4 H, -C(=O)CH2-CH2-C(O)).
13C NMR (150 MHz, CDCl3)
δ 171.0 (2 C, -C(O)CH2-CH2-C(O)), 139.2-127.4 (Ar-C), 102.6 (1B), 101.4 (1A), 81.1 (3B), 79.5 (3A), 79.1 (2B), 77.8 (2A), 75.7 (OBn), 75.6 (OBn), 75.5 (4A), 73.6 (OBn), 73.2 (OBn), 73 1 (OBn), 73.0 (5B), 72.4 (5A), 72.1 (OBn), 68.6 (6B), 67.5 (6A), 66.3 (4B), 25.4 (2 C-C(O)CH2-CH2-C(O)). ESI-MS (LR MS) [C58H61NO13Na]+ found 1002.9.
Succinamidyl - (2,3,4,6-tetra-O- benzyl - α –D-galactopyranosyl) -(1→4)- (2,3,6-tri-O-benzyl - β –D-galactopyranosyl)-(1→4)-2,3,6-tri-O-benzyl-β-D-glucopyranoside (9)
To a solution of compound 8 (2.04 g, 2.08 mmol) and compound 2 (1.58 g, 2.70 mmol) in anhydrous CH2Cl2 (40 mL) was added MS-4Å (1.00 g) and the reaction mixture was allowed to stir at rt for 1 h under argon. The vessel was then cooled to −10 °C and N-iodosuccinimide (NIS; 0.73 g, 3.24 mmol) and TMSOTf (40 µL) were added. After stirring at a constant temperature for 4 h, the reaction mixture was quenched with saturated NaHCO3 and sodium thiosulfate, filtered through a pad of celite, and washed 3× with CH2Cl2 (100 mL). The organic layer was washed with aq. Na2SO4 and water in succession, dried using Na2SO4, and then concentrated under reduced pressure to give the crude product. Purification ensued over SiO2 using hexane-EtOAc (2:1) as the eluent to furnish pure 9 (1.69 g, 54%).
1H NMR (600 MHz, CDCl3)
δ 7.49-7.14 (m, 50 H, Ar-H), 5.51 (d, J = 4.0 Hz, 1 H, 1A), 5.13 (J= 10.9 Hz, 1 H, PhCH2), 5.07 (d, J = 3.5 Hz, 1 H, 1C), 4.94 (J = 11.4 Hz, 1 H, PhCH2), 4.89 (J = 11.1 Hz, 1 H, PhCH2), 4.85-4.70 (m, 7 H, PhCH2), 4.70-4.69 (d, J = 9.9 Hz, 1 H, 5A), 4.56-4.45 (m, 5 H, PhCH2), 4.40-4.37 (m, 1 H, 5C), 4.33 (d, J = 7.74 Hz, 1 H, 1B), 4.34-4.31 (m, 2 H, PhCH2), 4.27-4.25 (d, J = 11.9 Hz, 1 H, PhCH2), 4.21-4.19 (dd, J1/2,1,3 = 8.4, 9.6 Hz, 1 H, 6B1), 4.15-4.10 (m, 3 H, 6B1, 2 PhCH2), 4.07 (brs, 1 H, 2C), 4.05-4.00 (m, 4 H, 4A, 4B, 4C, 3A), 3.99-3.96 (t, J = 9.3 Hz, 1 H, 3A), 3.67-3.64 (m, 2 H, 2A, 2B), 3.57-3.55 (dd, J1/2,1,3 = 5.3, 9.6 Hz, 1 H, 6B2), 3.53-3.50 (t, J = 9.0 Hz, 1 H, 6C1), 3.49-3.47 (m, 1 H, 6A), 3.30-3.28 (m, 1 H, 5B), 3.23-3.21 (dd, J1/2,1,3 = 4.7, 8.6 Hz, 1 H, 3B), 3.20-3.18 (q, 1 H, 6C), 2.75 (s, 4 H, -C(=O)CH2-CH2-C(O)).
13C NMR (150 MHz, CDCl3)
δ 171.1 (2 C, -C(O)CH2-CH2-C(O)), 139.5-127.5 (Ar-C), 103.2 (1B), 101.7 (1A), 101.1 (1C), 81.9 (3B), 79.7 (3A), 79.7 (3C), 79.3 (2B), 77.9 (2A), 76.9 (2C), 76.5 (4C), 76.0 (OBn), 75.9 (OBn), 75.5 (4A), 75.2 (OBn), 75.1 (4B), 74.0 (OBn), 73.7 (5B), 73.5 (OBn), 73.4 (OBn), 73.3 (OBn), 72.7(OBn), 72.6 (5A), 72.4, (OBn), 69.7 (5C), 68.2 (6B), 68.1 (6C), 67.8 (6A), 25.9 (2 C-C(=O)CH2-CH2-C(O)). ESI-MS (LR MS) [C92H95NO18Na]+ found 1525.4.
Succinamidyl - (α –D-galactopyranosyl) -(1→4)- (β –D-galactopyranosyl)-(1→4) -β-D-glucopyranoside (10)
To a solution of compound 9 (1.69 g, 1.12 mmol) in CH3OH (30 mL) was added 20% Pd(OH)2-C (200 mg) and mixture was allowed to stir at rt under a positive pressure of hydrogen for 10 h. The reaction mixture was filtered through a pad of celite and then washed with CH3OH-H2O (60 mL; 1:3 v/v). The combined filtrate was evaporated under reduced pressure to furnish compound 10, which was purified through a Sephadex LH-20 column using H2O as an eluent to give pure compound 10 (514 mg, 76%).
1H NMR (600 MHz, CDCl3)
δ 5.63 (d, J = 4.0 Hz, 1 H, 1A), 5.15 (J = 3.6 Hz, 1 H, 1C), 4.72 (J = 7.7 Hz, 1 H, 1B), 4.62 (d, J = 10.2 Hz, 1 H, 5A), 4.53 (m, 1 H, 5B), 423-4.21 (m, 2 H, A4, B4), 4.15-4.09 (m, 4 H, C3, C5, A6, C6), 4.05-3.95 (m, 4 H, A2, A3, C2, B6, C6), 3.94-3.89 (m, 4 H, B3, C4, A6, B6), 3.80-3.78 (m, 1 H, B2), 3.00 (s, 4 H, -C(=O)CH2-CH2-C(O)).
13C NMR (150 MHz, CDCl3)
δ 175.1 (2 C, -C(=O)CH2-CH2-C(O)),103.4 (1B), 103.3 (1A), 100.5 (1C), 77.3 (B4), 75.4 (C4), 78.3 (C5), 72.7 (A5), 72.4 (B3), 71.2 (B2), 71.1 (2 C, A4, B5), 70.6 (A3), 69.4 (A2), 69.2 (C2), 68.8 (C3), 25.6 (2 C-C(=O)CH2-CH2-C(O)). ESI-MS (LR MS) [C22H35NO18Na]+ found 623.9.
Aminooxy- (α –D-galactopyranosyl) -(1→4)- (β –D-galactopyranosyl)-(1→4) -β-D-glucopyranoside (1)
To a solution of compound 10 (514 mg, 0.85 mmol) in H2O (20 mL) was added hydrazine hydrate and the mixture was allowed to stir at rt for 4 h. After completion of the reaction (TLC solvent (Rf 0.3) [4:3:1, n-butanol:H2O:AcOH]), H2O was evaporated under reduced pressure to furnish compound 1, which was purified through a P-2 biogel column using H2O as the eluent to give pure compound 1 (302 mg, 68%).
1H NMR (600 MHz, CDCl3)
δ 4.98 (d, J = 4.0 Hz, 1 H, 1A), 4.92 (J = 3.9 Hz, 1 H, 1C), 4.49 (J = 7.8 Hz, 1 H, 1B), 4.34-4.32 (m, 2 H, 4A, 4B), 3.92-3.87 (m, 4 H, C5, A6, C6, A5), 3.82-3.79 (m, 3 H, C2, A6, B6), 3.77-3.74 (m, 1 H, C4),3.73-3.71 (m, 1 H, A3, B3), 3.67-3.64 (m, 3 H, B6, C6, C5), 3.62-3.60 (m, 1 H, A2), 3.57-3.54 (m, 1 H, B2).
13C NMR (150 MHz, CDCl3)
δ 103.1 (1B), 101.2 (1A), 100.2 (1C), 78.3 (C5), 77.3 (B4), 75.4 (C4), 72.1 (A3), 71.5 (B3), 70.8 (B2), 70.7 (A2), 70.5 (C2), 70.4 (A4), 68.9 (B5), 68.5 (C3). ESI-MS (LR MS) [C18H33NO16Na]+ found 542.0.
Scheme 2.
Reagents: (a) N-iodosuccinimide, trimethylsilyl trifluoromethanesulfonic, MS 4Å, CH2Cl2, −20 °C, 1 h, 54% isolated yield. (b) H2, 20% Pd(OH)2-C, rt, 8 h, 76% isolated yield. (c) hydrazine monohydrate, MeOH:H2O (1:1), rt, 3 h, 68% isolated yield.
Scheme 3.
Reagents: (a) 2-propanone, H2O, rt.
Acknowledgements
This work was supported by the National Institute of Health (NCI - R01 CA156661-01). S.G. and P.R.A. would like to acknowledge Dr. Yong Wah Kim (University of Toledo) for NMR assistance.
References
- 1.Ghosh S, Misra AK. Synthesis of a tetrasaccharide corresponding to the teichoic acid from the cell wall of Streptomyces sp. VKM Ac-2275. Tetrahedron: Asymmetr. 2009;20:2688–2693. [Google Scholar]
- 2.Pandey S, Ghosh S, Misra AK. Synthesis of a trisaccharide and a tetrasaccharide from the cell-wall lipopolysaccharides of Azospirillum brasilense S17. Synthesis. 2009:2584–2590. [Google Scholar]
- 3.Ghosh S, Misra AK. Concise synthesis of a hexasaccharide present in the cell wall lipopolysaccharide of Azospirillum lipoferum Sp59b. Tetrahedron: Asymmetr. 2010;21:725–730. [Google Scholar]
- 4.Ghosh S, Misra AK. Synthesis of the hexasaccharide repeating unit corresponding to the cell wall lipopolysaccharide of Azospirillum irakense KBC1. Tetrahedron: Asymmetr. 2010;21:2755–2761. [Google Scholar]
- 5.Ghosh S, Misra AK. Concise synthesis of a hexasaccharide related to the adhesin receptor of Streptococcus oralis ATCC 55229. J. Carbohyd. Chem. 2009;28:447–462. [Google Scholar]
- 6.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertozzi CR, Kiessling LL. Chemical Glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 8.Hannun Y, Bell R. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 9.Kolter T, Sandhoff K. Sphingolipids - Their metabolic pathways and the pathobiochemistry of neurodegenerative diseases. Angew. Chem., Int. Ed. 1999;38:1532–1568. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1532::AID-ANIE1532>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Cremesti A, Fischl A. Current methods for the identification and quantitation of ceramides: An overview. Lipids. 2000;35:937–945. doi: 10.1007/s11745-000-0603-1. [DOI] [PubMed] [Google Scholar]
- 11.Harouse J, Bhat S, Spitalnik S, Laughlin M, Stefano K, Silberberg D, Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- 12.Lourenço A, Lobo AM, Rodríguez B, Jimeno M-L. Ceramides from the fungus Phellinus pini. Phytochemistry. 1996;43:617–619. [Google Scholar]
- 13.Hakomori S. Glycolipids of animal cell membranes. Int. Rev. Sci., Org. Chem. Ser. Two. 1976;223 [Google Scholar]
- 14.Sharon N, Lis H. Glycoproteins: research booming on long-ignored, ubiquitous compounds. Chem. Eng. News. 1981;59:21–44. doi: 10.1007/BF00238511. [DOI] [PubMed] [Google Scholar]
- 15.Li Y-T, Li S-C. Biosynthesis and catabolism of glycosphingolipids. Adv. Carbohydr. Chem. Biochem. 1982;40:235–286. doi: 10.1016/s0065-2318(08)60110-9. [DOI] [PubMed] [Google Scholar]
- 16.Sharon N. Glycoproteins. Trends Biochem. Sci. 1984;9:198–202. [Google Scholar]
- 17.Alper J. Searching for medicine's sweet spot. Science. 2001;291:2338–2343. doi: 10.1126/science.291.5512.2338. [DOI] [PubMed] [Google Scholar]
- 18.Dunbar C, Kohn D, Karlsson S, Barton N, Brady R, Cottler-Fox M, Crooks G, Emmons R, Esplin J, Leitman S, Lenarsky C, Nolta J, Parkman R, Pensiero M, Schifmann R, Tolstoshev P, Weinberg K. Retroviral mediated transfer of the cDNA for human glucocerebrosidase into hematopoietic stem cells of patients with gaucher disease. A phase I study. National Institutes of Health, Bethesda, Maryland. Hum. Gene Ther. 1996;7:231–253. doi: 10.1089/hum.1996.7.2-231. [DOI] [PubMed] [Google Scholar]
- 19.Brady RO, Tallman JF, Johnson WG, Gal AE, Leahy WR, Quirk JM, Dekaban AS. Replacement therapy for inherited enzyme deficiency. New Engl. J. Med. 1973;289:9–14. doi: 10.1056/NEJM197307052890103. [DOI] [PubMed] [Google Scholar]
- 20.Handa S, Ariga T, Miyatake T, Yamakawa T. Presence of alpha-anomeric glycosidic configuration in the glycolipids accumulated in kidney with Fabry's disease. J. Biochem. 1971;69:626–627. [PubMed] [Google Scholar]
- 21.Kallenius G, Molby R, Svenson SB, Winberg J, Lundblad A, Svensson S, Cedergren B. The Pk antigen as receptor for the haemagglutinin of pyelonephritic Escherichia coli. FEMS. Lett. 1980;7:297–302. [Google Scholar]
- 22.Jeyakumar M, Butters TD, Dwek RA, Platt FM. Glycosphingolipid lysosomal storage diseases: Therapy and pathogenesis. Neuropath. Appl. Neuro. 2002;28:343–357. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 23.Fan E, Merritt EA, Verlinde CLMJ, Hol WG. AB(5) toxins: structures and inhibitor design. J Curr. Opin. Struct. Biol. 2000;10:680–686. doi: 10.1016/s0959-440x(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 24.Merritt EA, Hol WG. AB5 toxins. J. Curr. Opin. Struct. Biol. 1995;5:165–171. doi: 10.1016/0959-440x(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 25.Race RR, Sanger R. Blood Groups in Man. 6th Ed. Oxford: Blackwell; 1975. [Google Scholar]
- 26.Keusch GT, Jacewicz M, Acheson DWK, Donohue- Rolfe A, Kane AV, McCluer RH. Globotriaosylceramide, Gb3, is an alternative functional receptor for Shiga-like toxin 2e. Infect. Immun. 1995;63:1138–1141. doi: 10.1128/iai.63.3.1138-1141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyholm P-G, Magnusson G, Zheng Z, Norel R, Binnington-Boyd B, Lingwood CA. Two distinct binding sites for globotriaosyl ceramide on verotoxins: identification by molecular modelling and confirmation using deoxy analogues and a new glycolipid receptor for all verotoxins. Chem. Biol. 1996;4:263–275. doi: 10.1016/s1074-5521(96)90106-4. [DOI] [PubMed] [Google Scholar]
- 28.Ling H, Boodhoo A, Hazes B, Cummings MD, Armstrong GD, Brunton JL, Read RJ. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry-US. 1998;37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- 29.Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature. 1992;355:748–750. doi: 10.1038/355748a0. [DOI] [PubMed] [Google Scholar]
- 30.Hovde CJ, Calderwood SB, Mekalanos JJ, Collier RJ. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc. Natl. Acad. Sci. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd B, Richardson S, Gariepy J. Serological responses to the B subunit of Shiga-like toxin 1 and its peptide fragments indicate that the B subunit is a vaccine candidate to counter action of the toxin. Infect. Immun. 1991:750–757. doi: 10.1128/iai.59.3.750-757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu T, Grassel C, Levine MM, Barry EM. Live attenuated Shigella dysenteriae type 1 vaccine strains overexpressing shiga toxin B subunit. Infect. Immun. 2011:4912–4922. doi: 10.1128/IAI.05814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcato P, Griener TP, George L, Mulvey GL, Armstrong GD. Recombinant Shiga toxin B-subunit-keyhole limpet hemocyanin conjugate vaccine protects mice from Shigatoxemia. Infect. Immun. 2005:6523–6529. doi: 10.1128/IAI.73.10.6523-6529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dohi H, Nishida Y, Takeda T, Kobayashi K. Convenient use of non-malodorous thioglycosyl donors for the assembly of multivalent globo- and isoglobosyl trisaccharides. Carbohyd. Res. 2002;337:983–989. doi: 10.1016/s0008-6215(02)00093-9. [DOI] [PubMed] [Google Scholar]
- 35.Aly MRE, Rochaix P, Amessou M, Ludger J, Florent JC. Synthesis of globo-and isoglobotriosides bearing a cinnamoylphenyl tag as novel electrophilic thiol-specific carbohydrate reagents. Carbohyd. Res. 2006;341:2026–2036. doi: 10.1016/j.carres.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Li Q, Wang H, Zhang LH, Ye XS. A new one-pot synthesis of Gb3 and isoGb3 trisaccharide analogues. Tetrahedron. 2006;62:11657–11662. [Google Scholar]
- 37.Zhou G, Liu X, Su D, Li L, Xiao M, Wang PG. Large scale enzymatic synthesis of oligosaccharides and a novel purification process. Bioorg. Med. Chem. Lett. 2011;21:311–314. doi: 10.1016/j.bmcl.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto S, Sakamoto H, Honda T, Abe H, Nakamura S, Ikegami S. “Armed-disarmed” glycosidation strategy based on glycosyl donors and acceptors carrying phosphoroamidate as a leaving group: A convergent synthesis of globotriaosylceramide. Tetrahedron Lett. 1997;38:8969–8972. [Google Scholar]
- 39.Garegg PG, Hultberg H. Synthesis of di- and tri-saccharides corresponding to receptor structures recognised by pyelonephritogenic E. coli fimbriae (pili) Carbohyd. Res. 1982;110:261–266. doi: 10.1016/0008-6215(82)84007-x. [DOI] [PubMed] [Google Scholar]
- 40.Caulfield T, Kataoka H, Kumazawa T, Nicolaou KC. A practical and enantioselective synthesis of glycosphingolipids and related compounds. Total synthesis of globotriaosylceramide (Gb3) J. Am. Chem. Soc. 1988;110:7910–7912. [Google Scholar]
- 41.Pozsgay V, Trinh L, Shiloach J, Robbins JB, Rolfe AD, Calderwood SB. Purification of subunit B of Shiga toxin using a synthetic trisaccharide-based affinity matrix. Bioconjugate Chem. 1996;7:45–55. doi: 10.1021/bc9500711. [DOI] [PubMed] [Google Scholar]
- 42.Koike K, Sugimoto M, Sato S, Ito Y, Nakahara Y, Ogawa T. Total synthesis of globotriaosyl-E and Z-ceramides and isoglobotriaosyl-E-ceramide. Carbohydr. Res. 1987;163:189–208. doi: 10.1016/0008-6215(87)80181-7. [DOI] [PubMed] [Google Scholar]
- 43.Qiu D, Schmidt RR. Glycosyl imidates, 52. Synthesis of globotriaosylceramide (Gb3) and isoglobotriaosylceramide (isoGb3) Liebigs Ann. Chem. 1992:217–224. [Google Scholar]
- 44.Nishida Y, Dohi H, Uzawa H, Kobayashi K. Synthesis of artifical glycoconjugate polymers carrying biologically active trisaccharides with α-d-galactopyranosyl (1→3) and (1→4)-linkage. Tetrahedron Lett. 1998;39:8681–8684. [Google Scholar]
- 45.Gege C, Kinzy W, Schmidt RR. Total synthesis of the natural antigen involved in the hyperacute rejection response to xenotransplants. Carbohydr. Res. 2000;328:459–466. doi: 10.1016/s0008-6215(00)00145-2. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaou KC, Caulfield T, Kataoka H, Kumazawa T. A practical and enantioselective synthesis of glycosphingolipids and related compounds. Total synthesis of globotriaosylceramide (Gb3) J. Am. Chem. Soc. 1988;110:7910–7912. [Google Scholar]
- 47.Bourgault JP, Trabbic KR, Shi M, Andreana PR. Synthesis of the tumor associative α-aminooxy disaccharide of the TF antigen and its conjugation to a polysaccharide immune stimulant. Org. Biomol. Chem. 2014;12:1699–1702. doi: 10.1039/c4ob00128a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicolaou KC, Groneberg RD. Novel strategy for the construction of the oligosaccharide fragment of calichemicin .gamma.1.alpha.I. Synthesis of the ABC skeleton. J. Am. Chem. Soc. 1990;112:4085–4086. [Google Scholar]
- 49.Grochowski E, Jurczak J. A new class of monosaccharide derivatives: O-phthalimidohexoses. Carbohydrate Res. 1976;50:C15–C16. [Google Scholar]
- 50.Renaudet O, Dumy P. Expedient synthesis of aminooxylated-carbohydrates for chemoselective access of glycoconjugates. Tetrahedron Lett. 2001;42:7575–7578. [Google Scholar]
- 51.Hudak JE, Yu HH, Bertozzi CR. Protein glycoengineering enabled by the versatile synthesis of aminooxy glycans and the genetically encoded aldehyde tag. J. Am. Chem. Soc. 2011;133:16127–16135. doi: 10.1021/ja206023e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez JC, Fraser-Reid B. n-Pentenyl esters versus n-pentenyl glycosides. Synthesis and reactivity in glycosidation reactions. J. Chem. Soc., Chem. Comm. 1991;3:159–161. [Google Scholar]
- 53.Debenham JS, Madsen R, Roberts C, Fraser-Reid B. Two new orthogonal amine-protecting groups that can be cleaved under mild or neutral conditions. J. Am. Chem. Soc. 1995;117:3302–3303. [Google Scholar]
- 54.Madsen R, Roberts C, Fraser-Reid B. The pent-4-enoyl group: A novel amine-protecting group that is readily cleaved under mild conditions. J. Org. Chem. 1995;60:7920–7926. [Google Scholar]
- 55.Silva RAD, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: Design of synthetic vaccines based on Tn–PS A1 conjugates. J. Am. Chem. Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 56.Kihlberg JO, Leigh DA, Bundle DR. The in situ activation of thioglycosides with bromine: an improved glycosylation method. J. Org. Chem. 1990;55:2860–2863. [Google Scholar]
- 57.Cao S, Tropper FD, Roy R. Stereoselective phase transfer catalyzed syntheses of glycosyloxysuccinimides and their transformations into glycoprobes. Tetrahedron. 1995;51:6679–6686. [Google Scholar]
- 58.Veeneman GH, van Leeuwen SH, van Boom JH. Iodonium ion promoted reactions at the anomeric centre II. An efficient thioglycoside mediated approach toward the formation of 1,2-trans linked glycosides and glycosidic esters. Tetrahedron Lett. 1990;31:1331–1334. [Google Scholar]
- 59.Konradsson P, Udodong UE, Fraser-Reid B. Iodonium promoted reactions of disarmed thioglycosides. Tetrahedron Lett. 1990;31:4313–4316. [Google Scholar]
- 60.Pearlman WM. Noble metal hydroxides on carbon nonpyrophoric dry catalysts. Tetrahedron Lett. 1967;8:1663–1664. [Google Scholar]
- 61.Renaudet O, Dumy P. Chemoselectively template-assembled glycopeptide presenting cancer related T-antigen. Tetrahedron Lett. 2004;45:65–68. [Google Scholar]