Highlights
-
•
The Mycobacterium secreted protein PE25/PPE41 drives TNF-α secretion.
-
•
PE25/PPE41 protein induces necrotic cell death, but not apoptosis, in macrophages.
-
•
Necotic cell death induced by PE25/PPE41 is independent of TNF-α/NFκB/AP-1 pathways.
-
•
PE25/PPE41 possibly acts as virulence factor, by an ‘immune quorum sensing’ mechanism.
-
•
Necrotic cell death may help in mycobacterial dissemination and re-activation.
Keywords: Mycobacterium, Tuberculosis, PE/PEE, PE25, PPE41, Rv2431
Abstract
Necrotic cell death during TB infection is an important prerequisite for bacterial dissemination and virulence. The underlying mechanisms and the bacterial factors involved therein are not well understood. The Mycobacterium tuberculosis (M. tuberculosis) co-operonic PE25/PPE41 protein complex, similar to ESAT-6/CFP-10, belonging to the PE/PPE and ESAT-6 families of genes has co-expanded and co-evolved in the genomes of pathogenic mycobacteria. We report a novel role of this highly immunogenic PE25/PPE41 protein complex in inducing necrosis, but not apoptosis, in macrophages. We propose that these protein complexes of M. tuberculosis, secreted by similar/unique transport system (Type VII), have an important role in M. tuberculosis virulence and disease reactivation.
1. Introduction
Cell death either due to apoptosis or necrosis is very common during infection with Mycobacterium tuberculosis (M. tuberculosis), the causative agent of tuberculosis (TB). Apoptosis of antigen presenting cells (APCs) is known to be an innate mechanism of the host to fight infections. M. tuberculosis induces apoptosis in APCs through TNF-α, toll like receptors (TLRs), Fas or by altering the expression of Bax/Bcl-xL via an oxygen dependent pathway [1–4]. Virulent strains of M. tuberculosis have been known to develop mechanisms that resist host apoptotic cell death [5–8], however they are capable of inducing necrotic cell death, which helps in bacterial multiplication and dissemination [9–12]. On the other hand non-virulent strains like Mycobacterium bovis BCG and Mycobacterium smegmatis fail to induce necrosis of the host cells [10,11], suggesting that the factors released by the virulent strains interfere with the host cell death machinery for their release and subsequent infection of the neighboring (fresh) cells [8]. Although necrotic cell death during M. tuberculosis infection is a common observation, very little is known about the mechanisms. A unique property of H37Rv strain in preventing apoptotic envelope formation leading to necrotic cell death of macrophages has been recently reported [13]. Even though it is clear that host-pathogen interactions at the interface of cell death are a key to M. tuberculosis dissemination and virulence, the underlying mechanism(s) and the M. tuberculosis factors involved in the processes are very poorly understood.
RD1 locus, present in the genome of only the virulent mycobacterial strains, is believed to encode virulent factors ESAT-6 and CFP-10 complex secreted via a secretory locus called esx-1 [14]. ESX-1 locus also contains a pair of genes encoding PE/PPE proteins. PE/PPE proteins are coded by a family of genes that include PE, PPE and PGRS (or PE/PPE/PGRS) and these represent 10% of the coding capacity of the mycobacterial genome Although the exact roles of PE/PPE proteins in virulence and regulation of their expression in mycobacteria are not completely understood, it is believed that these have important function in mycobacterial virulence and pathogenesis [15–17]. The findings that PE/PPE genes are duplicated and expanded in the genomes of pathogenic mycobacteria during the course of evolution lends further support to their role in virulence [18]. We earlier reported that the PE/PPE genes in the genome of M. tuberculosis are organized in operons with the members of the same family as well as those belonging to ESAT-6 like family [19]. Proteins belonging to these families are secreted by pathogenic mycobacteria using specialized (Type VII) secretion systems. For example, ESAT-6 protein of RD-1 locus and PE25/PPE41 protein complex are secreted by pathogenic mycobacteria via Esx-1 and Esx-5 loci, respectively [20–22]. This supports the observation that the PE/PPE proteins and the ESAT-6 family of genes are co-evolved and co-expanded in the genome of pathogenic mycobacteria [18,19]. This is also evident from the observations that the pathogenic mycobacteria harbor multiple esx secretory system containing genes encoding both PE/PPE and ESAT-6 family of proteins [15,18,20]. Functionally, the esx-5 locus induces bacterial dissemination by cell lysis during mycobacterial infection, suggesting that the proteins secreted through this locus play an important role in virulence [22]. We previously reported that the PPE41 and PE25 proteins interact with each other and form complex in a fashion similar to ESAT-6 and CFP-10 [19]. To form soluble complex, PE25 and PPE41 interact at the translational or translocation levels since PE25 or PPE41, when expressed individually in Escherichia coli cells, remain insoluble as opposed to when co-expressed as an operon [19]. That this is indeed the case is evident from recent observation that co-expression of PE25 and PPE41, as well as the C-terminal of PE25 is indispensable for the secretion of PPE41 protein in pathogenic mycobacteria [20]. We also reported that the PE25/PPE41 complex elicited stronger immune responses in TB patients as well as in a mouse model, compared to individually expressed PE25 or PPE41 proteins [23,24], thereby suggesting that PE25/PPE41 complex is recognized by the host immune system. The esx secretory systems, including esx-1 and esx-5, are also involved in secretion of other PE/PPE and ESAT-6 proteins, which potentially form a part of the virulence component factors that help in reactivation and dissemination of the pathogenic mycobacteria. Based on the evidences that the PE25/PPE41 complex and ESAT-6 family of proteins are exported out of mycobacteria (via special secretory loci, esx-1 and esx-5) and induce necrosis or host cell lysis, but not apoptosis, we hypothesize that the genes belonging to PE/PPE and ESAT-6 family facilitate bacterial dissemination, multiplication and infection of fresh/neighboring cells leading to increased virulence, pathogenicity and disease reactivation.
2. Materials and methods
2.1. Protein expression and purification
The purification of the recombinant protein was carried out as described previously [19]. Briefly, E. coli BL-21 cells were transformed with pETDuet vector carrying both the pe25 and ppe41 genes for the expression of recombinant proteins. Soluble fraction of the co-expressed protein was loaded onto a cobalt affinity column and washed with 1X PBS containing 25 mM Imidazole. The bound proteins were eluted with 200 mM imidazole in 1X PBS and dialyzed against 1X PBS. To obtain the protein complex, the co-purified proteins were loaded onto Superose 6 column and the fractions corresponding to the sharp (∼70 kDa) peak containing both PE25 and PPE41 proteins were collected, as described [19]. The concentration of the recombinant proteins was measured using BCA protein assay kit (Pierce). The purified recombinant protein was treated with Polymyxin B sepharose and the endotoxin level was measured with LAL (Limulus Amebocyte Lysates) method, which was <10 pg/ml.
2.2. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay
RAW264.7 macrophages were seeded and stimulated with different concentrations of the recombinant protein in a final volume of 250 μl of RPMI per well. The cell culture was incubated at 37 °C under 5% CO2 and 70% relative humidity. Cell cytotoxicity was tested by MTT assay by adding 10 μl of 5 mg/ml MTT. Cells were then incubated at 37 °C for 3–4 h and then 100 μl of solubilization buffer (20% SDS, 50% N-Methyl Formamide) was added and absorbance was measured at 570 nm.
2.3. Enzyme linked immunosorbent assay (ELISA)
The levels of cytokines present in the culture supernatants were quantified by commercially available two-site sandwich enzyme-linked immunosorbent assay kit (BD OptEIA™ set Mouse, IL-12, IL-10 and TNF-α) according to the manufacturer’s instructions. Briefly, 96 well plates were coated with coating antibody, incubated at 4 °C overnight, blocked with 2% BSA (blocking buffer) and incubated for 2 h at 37 °C. The culture supernatant was then added followed by detection antibody for 1 h at RT. The plates were then incubated with HRP conjugated secondary antibody for 1 h at 37 °C and the HRP activity was determined by using the substrate, o-phenylenediamine tetra-hydrochloride and H2O2. The plates were washed at every step of the incubation with wash buffer (1XPBS-Tween-20) for 5–7 times. The reaction was terminated using 2 N H2SO4 and the absorbance was measured at 492 nm in an ELISA reader.
2.4. TUNEL assay
Apoptosis was assayed using TUNEL assay kit (Promega), according to the manufacturer’s instructions, in a 4 well chamber slide (BD, Bioscience). In brief, cells were washed with PBS, fixed with 3% paraformaldehyde and incubated for 30 min at RT. Cells were permeabilized with 0.1% Triton X-100 and incubated with reaction mixture containing fluorescein labeled dTTP and rdTTP transferase and allowed for nick end labeling for one hour at 37 °C. Reaction was stopped with 2X SSC. The slides were then rinsed with PBS and analyzed using confocal microscope.
2.5. Flow cytometry
Cell death assay was performed using Annexin V and propidium iodide (PI) staining kit (BD) according to the manufacturer’s instructions. In brief, 0.2 × 106 cells were stimulated with different concentrations of recombinant protein and incubated for different intervals of time. Cells were then harvested and washed in PBS twice and resuspended in 1X Annexin V binding buffer. Cells were stained with Annexin V and PI dyes, incubated for 15 min at RT and processed for flow cytometric analysis (BD, Vantage SE).
2.6. LDH assay
LDH release assay was carried out with the culture supernatant of RAW 264.7 macrophages stimulated with different concentration of recombinant protein and LPS (5 μg/ml). The reaction was carried out in a buffer containing 80 mM Tris–HCl (pH 7.2), 200 mM NaCl, 1.6 mM pyruvate, 0.2 mM NADH and 50 μl of culture supernatant. Optical density at 340 nm was recorded for 30 min using spectrophotometer. LDH activity was measured by calculating the rate of oxidation of NADH per unit time per mg of protein.
2.7. Statistical analyses
The experiments were carried out in triplicate and repeated 4 or more independent times. Statistical significance was determined using student’s t test.
3. Results
3.1. The PE25/PPE41 complex drives TNF-α production in macrophages
We used the polymyxin B treated recombinant PE25/PPE41 complex protein to stimulate in vitro cultured RAW264.7 macrophages and scored for inflammatory cytokines TNF-α, IL-10 and IL-12. Strong induction of TNF-α, indicative of pro-inflammatory response, could be seen when RAW264.7 macrophages were stimulated with the PE25/PPE41 protein complex. The up regulation of TNF-α by recombinant PE25/PPE41 complex was observed in a dose and time dependent manner (Fig. 1). The production of TNF-α was observed as early as 2 h of incubation (∼400 pg/ml). Recombinant PE or PPE protein when expressed individually could also induce TNF-α expression (data not shown). Control experiments with heat or protease treated protein failed to stimulate TNF-α secretion (Fig. 1), clearly demonstrating that the increase in TNF-α secretion is not due to endotoxin contamination but is due to the PE/PPE complex. The PE/PPE complex failed to drive production of IL-12 and IL-10 after incubation with the macrophages even up to 48 h (data not shown). These results demonstrate that the PE25/PPE41 protein complex is able to mount a strong pro-inflammatory response in the form of TNF-α production.
Fig. 1.

The PE25/PPE41 protein complex induces TNF-α secretion in macrophages. RAW264.7 macrophages were stimulated with 1 μg/ml of PE25/PPE41 protein complex and TNF-α levels were measured at 2, 6 and 12 h, as indicated. In a parallel experiment, macrophages were incubated for 12 h with varying concentrations (0.01, 0.1 and 1 μg/ml) of the PE/PPE protein complex. The PE25/PPE41 complex was treated with protease cocktail followed by inactivation by autoclaving to serve as a control (Heat+Protease K). Supernatants were collected and ESLISA was performed to measure TNF-α level as described in materials and methods. Statistical significance was determined using student’s t test (∗∗p < 0.01; ∗∗∗p < 0.001).
3.2. PE25/PPE41 complex induced cell death is not due to TNF-α, NO or NFκB mediated signaling
In-vitro cultured murine macrophage RAW264.7 cells were treated with recombinant PE25/PPE41 protein complex and cell death was measured using MTT assay. It could be seen that PE25/PPE41 complex induces cell death in a concentration dependent manner, causing approximately 26%, 42% and 60% cell death at a concentration of 0.3, 3 and 10 μg/ml, respectively after 12 h of incubation (Fig. 2).
Fig. 2.

The PE25/PPE41 protein complex induces cell death. MTT assay was carried out (as described in Section 2) to score for cell death in RAW264.7 macrophages stimulated with different concentration of PE25/PPE41 protein complex, as indicated. Cell death was determined by MTT assay (described in Section 2). Data plotted for O.D value as well as percentage cell death. Statistical significance was determined using student’s t test (∗∗∗p < 0.001).
A member of the PE/PPE family – PE_PGRS33 protein – has been reported to induce TNF-α secretion in RAW264.7 macrophages leading to apoptotic cell death [25]. Therefore, experiments were designed to investigate the role of TNF-α, nitric oxide (NO) or nuclear factor kappa B (NFκ-B) mediated signaling in PE25/PPE41 protein complex mediated cell death. MTT assay was carried out after pre-incubation of macrophage cells with anti-TNF-α antibody, amino guanidine (AG, inhibitor of nitric oxide synthesis), or PDTC (inhibitor of nuclear factor kappa B), followed by incubation with varying concentrations (0.3–10 μg/ml) of the PE/PPE complex. Expectedly, while PE/PPE protein complex caused cell death as a direct function of protein concentration, cell death remains unchanged in the absence of TNFα, NO or NFκB (Fig. 3). These results suggest that cell death induced by the PE/PPE protein complex is independent of NFκB, NO or TNF-α mediated signaling.
Fig. 3.
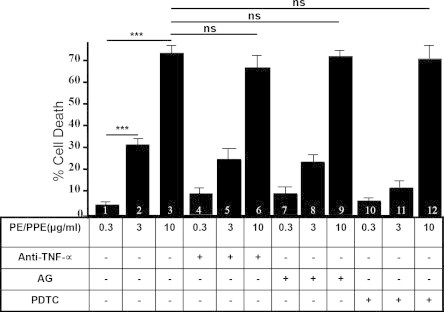
Cell death is independent of TNF-α, NO or (NFκ-B) mediated signaling. MTT assay was carried out with RAW264.7 cells, either incubated with anti-TNF-α antibody (bars 4–6), amino guanidine (AG, 100 μg/ml) (bars 7–9), 10 μg/ml of PDTC (bars 10–12) or none (bars 1–3) prior to stimulation with 0.3 μg/ml (lanes 1, 4, 7 and 10), 3 μg/ml (bars 2, 5, 8 and 11) and 10 μg/ml (lanes 3, 6, 9 and 12) of PE25/PPE41 protein complex. Data plotted for percent cell death by MTT assay (as described in materials and methods). Statistical significance was determined using student’s t test (∗∗∗p < 0.001; ns = not significant).
3.3. The PE25/PPE41 complex induces necrosis, but not apoptosis of mouse macrophages
In order to differentiate necrotic cell death from apoptosis, propidium iodide (PI)/Annexin V staining was carried out using macrophages stimulated with PE25/PPE41 complex (Fig. 4A and B). Propidium iodide easily passes through the ruptured membrane of dead cells and stains nucleic acids, but live cells or cells in early apoptotic phase are impermeable to PI dye. Unlike PI, Annexin V binds to phosphatidylserine with high affinity, which is externalized on the surface of apoptosed or dead cells, thus recognizing cells undergoing apoptosis even at early stage of cell death. Using flow cytometry staining, it could be seen that while PI uptake by the macrophages was a direct function of increasing concentration of the PE/PPE complex (Fig. 4A), the necrotic population of macrophages (PI+/AnnexinV+) increased at 2, 8 and 12 h of incubation, respectively (Fig. 4B). Absence of apoptotic population (PI−/AnnexinV+) suggests that PE/PPE protein complex induces necrosis of macrophages but not apoptosis (Fig. 4B). These results clearly suggest that the PE25/PPE41 protein complex induces necrosis in macrophages in a time dependent manner. Apoptotic cell death was also ruled out by TUNNEL staining. Macrophages incubated with PE25/PPE41 complex at a concentration as high as 10 μg/ml were negative for TUNEL staining (Fig. 4C). Necrotic cell death was further validated by lactate dehydrogenase (LDH) release assay. LDH assay involves measurement of NADH oxidation to NAD by LDH (released in to the medium from ruptured cells) in presence of pyruvic acid. RAW264.7 macrophages were stimulated with PE25/PPE41 at two different concentrations of PE25/PPE41 protein complex and LDH released was measured in the culture supernatant. It could be seen that the PE25/PPE41 protein complex induced release of LDH into the supernatant of RAW264.7 macrophages in a concentration dependent manner (Fig. 5). Very low oxidation of NADH was seen in the supernatant of cells left unstimulated (Fig. 5) or stimulated with concentration as high as 5 μg/ml of LPS (Fig. 5). Taken together these results demonstrate that the PE25/PPE41 protein complex induces necrosis, but not apoptosis, in RAW 264.7 macrophage cells.
Fig. 4.

The PE25/PPE41 complex induces necrosis, but not apoptosis. Necrosis of macrophages stimulated with the recombinant protein was monitored by propidium iodide (PI) exclusion assay. (A) RAW264.7 macrophages were stimulated with different concentrations of the PE25/PPE41 protein complex, as indicated. The peaks in the M2 region represent the uptake of PI dye by the necrotic cells when stimulated with increased concentration of the recombinant PE25/PPE41 protein complex. The number mentioned in M2 region of each of the graph represents the percentage of cells undergoing necrosis in response to the stimulation with PE25/PPE41 protein complex in a dose dependent manner. Data represent results of 3 independent experiments. (B) RAW264.7 macrophages were stimulated with 10 μg/ml of the PE25/PPE41 protein complex for 0, 2, 8 and 12 h, as indicated. Staining with PI/Annexin-V was carried out, cells acquired and data analyzed on flow cytometer (BD Vantage, SE). Necrotic (PI+/Annexin V+) or apoptotic (PI−Annexin V+) populations were gated and compared between cells stimulated for 0, 2, 8 and 12 h. Statistical significance was determined using student’s t test (∗∗∗p < 0.001). (C) TUNEL assay with macrophages stimulated with 10 μg/ml of recombinant PE25/PPE41 protein complex show absence of TUNEL staining in the presence of the PE25/PPE41 complex. Positive control (cells treated with DNase A) shows staining with fluorescein tagged nucleotides at the nicked ends of nucleotides. DAPI staining shows that the cells were alive.
Fig. 5.

The PE25/PPE41 protein complex induces necrosis. Lactate dehydrogenase (LDH) assay was carried out with the supernatant of cells incubated with 1 or 10 μg/ml of the PE25/PPE41 protein complex or stimulated with LPS or left unstimulated. The PE25/PPE41 protein complex stimulated release of LDH. The oxidation of NADH by LDH was measured in unit/mg/protein. Statistical significance was determined using student’s t test (∗∗∗p < 0.001).
4. Discussion
Macrophages are the cellular host of mycobacterial infection and constitute the first line of defense without which the host is unable to provide complete protection [26]. M. tuberculosis employs various strategies to counteract macrophage protective responses [27]. Induction of necrotic cell death in macrophages is one such strategy that helps in bacterial dissemination and nutrients uptake inside the granulomas, while preventing apoptotic cell death, that acts as an innate defense against M. tuberculosis infection [28]. It is unclear how M. tuberculosis induces host cell necrosis and regulates its growth and multiplication especially during the latent phase of the growth. Here we report that a PE/PPE complex induces macrophage cell death by necrosis. Based on the current findings and previous reports we suggest that one of the important virulent features of the secretory PE/PPE proteins is to induce necrotic cell death of macrophages that helps the bacterium to survive and multiply in the host.
Necrotic cell death commonly observed in mycobacterial lesion and granulomas is driven by bacterial factors but not due to secretion of TNF-α during the infection [29,30]. Production of TNF-α in response to the PE25/PPE41 complex is suggestive of a host response elicited against the protein since it has been reported that TNF-α provides protection against M. tuberculosis infection perhaps through the induction of host cell apoptosis [28,31,32]. While various pathways are known that mediate apoptotic cells death [1–4], not much is known about the mechanisms driving necrotic cell death during mycobacterial infection. Our observations that necrotic cell death, in response to PE25/PPE41 complex, is independent of TNF-α, nitric oxide or NF-κB signaling suggests that PE25/PPE41 utilizes an unknown mechanism(s) to drive macrophage cell death that may ultimately contribute to virulence. Secretory apparatus, esx-5, has been known to be involved in caspase independent, cathepsin-B dependent cell death of macrophages during mycobacterial infection. Since PPE41 protein secretion is mediated by esx-5 apparatus, the role of cathepsin B in induction of cell death in response to PE25/PPE41 protein complex cannot be ruled out [21]. This ability of PE25/PPE41 protein complex to induce necrosis, supports the observation that necrotic cell death during mycobacterial infection is not driven by host response but by bacterial factor(s).
The association of PE/PPE and ESAT-6 gene families with virulence is evident from the observations that the duplication and expansion of the PE/PPE and ESAT-6 genes during the course of evolution occurred only in pathogenic mycobacteria [18,19]. These genes are very uniquely organized in the genome with pe and ppe genes in operon with another member of pe/ppe or esat-6 family of genes [19] and evidently both ESAT-6 and PE/PPE family of proteins are secreted by Type VII/ESX secretory apparatus [15]. This also suggests that PE/PPE and ESAT-6 family of genes may have played important role in adaptation and host specificity, as these were earlier shown to be differentially expressed in different species of pathogenic mycobacteria [33–35]. Although exact function of PE/PPE proteins remain unknown, reports suggest that these are surface localized or secreted outside by mycobacteria and may have direct role in virulence and pathogenesis [16,22,36–43]. One of the important virulence mechanisms of pathogenic mycobacteria appears to be their ability to induce necrosis of host immune cells, as not only PE25/PPE41 complex, ESAT-6 protein has also been shown to induce necrosis of immune cells via an unknown mechanism [44]. The fact that the PE/PPE proteins mount both B- and T-cell immune responses [23,24,45] provokes us to also suggest a novel role for these protein families, present exclusively in the genus Mycobacterium, in ‘immune quorum sensing’ (Fig. 6) wherein these proteins are secreted to sense the immune status of the host after which through the possible involvement of cell surface constituents that influence interactions with other cells [38], convey a signal to stay dormant or undergo necrotic cell death. Based on our present and earlier findings, and existing evidences, we believe that the co-evolved and co-expanded PE/PPE and ESAT-6 family of proteins are secreted out, using sophisticated bacterial Type VII secretory systems, interact with host immune cells (Fig. 6), and facilitate mycobacterial dissemination and multiplication, which may ultimately lead to disease reactivation.
Fig. 6.
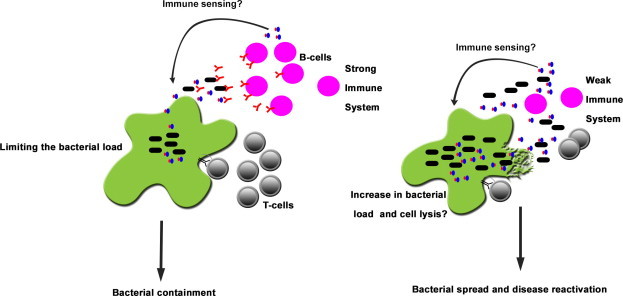
Is the PE25/PPE41 complex involved in quorum sensing of the immune system? A schematic representation of the likely role of the secreted PE25/PPE41 complex on the host immune system. The secretion of the PPE41 or PE25/PPE41 complex is mediated by Type VII secretion system. The PPE41 or the PE25/PPE41 protein complex is secreted outside by the bacterium, which could be recognized by host immune system. But the proteins may help the bacterium by sensing the strength of the host immune system. Weak immune system may induce these proteins to regulate their own expression or signal for multiplication of the bacteria to spread outside the cells by inducing direct lysis of macrophages via necrosis, which in turn helps the bacterium to multiply and spread infection in the surrounding cells resulting into disease reactivation.
Acknowledgments
A Centre of Excellence Grant from the Department of Biotechnology, Ministry of Science & Technology, Government of India to SEH supported this work. KM is a SERB Fellow while SEH is a J.C. Bose National Fellow and Robert Koch Fellow of the Robert Koch Institute, Berlin.
References
- 1.Molloy A., Laochumroonvorapong P., Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keane J., Balcewicz-Sablinska M.K., Remold H.G., Chupp G.L., Meek B.B., Fenton M.J., Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Placido R. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J. Pathol. 1997;181:31–38. doi: 10.1002/(SICI)1096-9896(199701)181:1<31::AID-PATH722>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Perskvist N., Long M., Stendahl O., Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J. Immunol. 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]
- 5.Balcewicz-Sablinska M.K., Keane J., Kornfeld H., Remold H.G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 6.Keane J., Remold H.G., Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 7.Sly L.M., Hingley-Wilson S.M., Reiner N.E., McMaster W.R. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 2003;170:430–437. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Jiang R., Takayama H., Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol. Immunol. 2005;49:845–852. doi: 10.1111/j.1348-0421.2005.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 9.Rook G.A., al Attiyah R. Cytokines and the Koch phenomenon. Tubercle. 1991;72:13–20. doi: 10.1016/0041-3879(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 10.Rook G.A., al Attiyah R., Filley E. New insights into the immunopathology of tuberculosis. Pathobiology. 1991;59:148–152. doi: 10.1159/000163633. [DOI] [PubMed] [Google Scholar]
- 11.Saunders B.M., Britton W.J. Life and death in the granuloma: immunopathology of tuberculosis. Immunol. Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 12.Dobos K.M., Spotts E.A., Quinn F.D., King C.H. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect. Immun. 2000;68:6300–6310. doi: 10.1128/iai.68.11.6300-6310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan H., Lee J., Ren F., Chen M., Kornfeld H., Remold H.G. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat. Immunol. 2008;9:1189–1197. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsson F., Joshi S.A., Rangell L., Brown E.J. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog. 2009;5:e1000285. doi: 10.1371/journal.ppat.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeone R., Bottai D., Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr. Opin. Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Akhter Y., Ehebauer M.T., Mukhopadhyay S., Hasnain S.E. The PE/PPE multigene family codes for virulence factors and is a possible source of mycobacterial antigenic variation: perhaps more? Biochimie. 2012;94:110–116. doi: 10.1016/j.biochi.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Mohareer K., Tundup S., Hasnain S.E. Transcriptional regulation of Mycobacterium tuberculosis PE/PPE genes: a molecular switch to virulence? J. Mol. Microbiol. Biotechnol. 2011;21:97–109. doi: 10.1159/000329489. [DOI] [PubMed] [Google Scholar]
- 18.Gey van Pittius N.C., Sampson S.L., Lee H., Kim Y., van Helden P.D., Warren R.M. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 2006;6:95. doi: 10.1186/1471-2148-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tundup S., Akhter Y., Thiagarajan D., Hasnain S.E. Clusters of PE and PPE genes of Mycobacterium tuberculosis are organized in operons: evidence that PE Rv2431c is co-transcribed with PPE Rv2430c and their gene products interact with each other. FEBS Lett. 2006;580:1285–1293. doi: 10.1016/j.febslet.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Daleke M.H., Ummels R., Bawono P., Heringa J., Vandenbroucke-Grauls C.M., Luirink J., Bitter W. General secretion signal for the mycobacterial type VII secretion pathway. Proc. Natl. Acad. Sci. U.S.A. 2012;109:11342–11347. doi: 10.1073/pnas.1119453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdallah A.M. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J. Immunol. 2011;187:4744–4753. doi: 10.4049/jimmunol.1101457. [DOI] [PubMed] [Google Scholar]
- 22.Abdallah A.M. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol. Microbiol. 2006;62:667–679. doi: 10.1111/j.1365-2958.2006.05409.x. [DOI] [PubMed] [Google Scholar]
- 23.Choudhary R.K., Mukhopadhyay S., Chakhaiyar P., Sharma N., Murthy K.J., Katoch V.M., Hasnain S.E. PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect. Immun. 2003;71:6338–6343. doi: 10.1128/IAI.71.11.6338-6343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tundup S., Pathak N., Ramanadham M., Mukhopadhyay S., Murthy K.J., Ehtesham N.Z., Hasnain S.E. The co-operonic PE25/PPE41 protein complex of Mycobacterium tuberculosis elicits increased humoral and cell mediated immune response. PLoS One. 2008;3:e3586. doi: 10.1371/journal.pone.0003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu S. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J. Biol. Chem. 2007;282:1039–1050. doi: 10.1074/jbc.M604379200. [DOI] [PubMed] [Google Scholar]
- 26.Clay H., Davis J.M., Beery D., Huttenlocher A., Lyons S.E., Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeuillet C., Martinon F., Perez C., Munoz M., Thome M., Meylan P.R. Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J. Immunol. 2006;177:6245–6255. doi: 10.4049/jimmunol.177.9.6245. [DOI] [PubMed] [Google Scholar]
- 28.Behar S.M., Martin C.J., Booty M.G., Nishimura T., Zhao X., Gan H.X., Divangahi M., Remold H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4:279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiling N., Schneider D., Ehlers S. Mycobacterium tuberculosis-induced cell death of primary human monocytes and macrophages is not significantly modulated by tumor necrosis factor-targeted biologicals. J. Investig. Dermatol. Symp. Proc. 2007;12:26–33. doi: 10.1038/sj.jidsymp.5650033. [DOI] [PubMed] [Google Scholar]
- 30.Florido M., Appelberg R. Granuloma necrosis during Mycobacterium avium infection does not require tumor necrosis factor. Infect. Immun. 2004;72:6139–6141. doi: 10.1128/IAI.72.10.6139-6141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fratazzi C., Arbeit R.D., Carini C., Balcewicz-Sablinska M.K., Keane J., Kornfeld H., Remold H.G. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 1999;66:763–764. doi: 10.1002/jlb.66.5.763. [DOI] [PubMed] [Google Scholar]
- 32.Spira A., Carroll J.D., Liu G., Aziz Z., Shah V., Kornfeld H., Keane J. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis: a pivotal role for tumor necrosis factor. Am. J. Respir. Cell Mol. Biol. 2003;29:545–551. doi: 10.1165/rcmb.2002-0310OC. [DOI] [PubMed] [Google Scholar]
- 33.Rehren G., Walters S., Fontan P., Smith I., Zarraga A.M. Differential gene expression between Mycobacterium bovis and Mycobacterium tuberculosis. Tuberculosis (Edinb) 2007;87:347–359. doi: 10.1016/j.tube.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golby P. Comparative transcriptomics reveals key gene expression differences between the human and bovine pathogens of the Mycobacterium tuberculosis complex. Microbiology. 2007;153:3323–3336. doi: 10.1099/mic.0.2007/009894-0. [DOI] [PubMed] [Google Scholar]
- 35.Kohli S., Singh Y., Sharma K., Mittal A., Ehtesham N.Z., Hasnain S.E. Comparative genomic and proteomic analyses of PE/PPE multigene family of Mycobacterium tuberculosis H(3)(7)Rv and H(3)(7)Ra reveal novel and interesting differences with implications in virulence. Nucleic Acids Res. 2012;40:7113–7122. doi: 10.1093/nar/gks465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pajon R., Yero D., Lage A., Llanes A., Borroto C.J. Computational identification of beta-barrel outer-membrane proteins in Mycobacterium tuberculosis predicted proteomes as putative vaccine candidates. Tuberculosis (Edinb) 2006;86:290–302. doi: 10.1016/j.tube.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Delogu G., Pusceddu C., Bua A., Fadda G., Brennan M.J., Zanetti S. Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol. Microbiol. 2004;52:725–733. doi: 10.1111/j.1365-2958.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 38.Brennan M.J., Delogu G., Chen Y., Bardarov S., Kriakov J., Alavi M., Jacobs W.R., Jr. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 2001;69:7326–7333. doi: 10.1128/IAI.69.12.7326-7333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampson S.L., Lukey P., Warren R.M., van Helden P.D., Richardson M., Everett M.J. Expression, characterization and subcellular localization of the Mycobacterium tuberculosis PPE gene Rv1917c. Tuberculosis (Edinb) 2001;81:305–317. doi: 10.1054/tube.2001.0304. [DOI] [PubMed] [Google Scholar]
- 40.Cascioferro A., Delogu G., Colone M., Sali M., Stringaro A., Arancia G., Fadda G., Palù G., Manganelli R. PE is a functional domain responsible for protein translocation and localization on mycobacterial cell wall. Mol. Microbiol. 2007;66:1536. doi: 10.1111/j.1365-2958.2007.06023.x. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Miltner E., Wu M., Petrofsky M., Bermudez L.E. A Mycobacterium avium PPE gene is associated with the ability of the bacterium to grow in macrophages and virulence in mice. Cell. Microbiol. 2005;7:539–548. doi: 10.1111/j.1462-5822.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishnan L., Federspiel N.A., Falkow S. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- 43.Dong D., Wang D., Li M., Wang H., Yu J., Wang C., Liu J., Gao Q. PPE38 modulates innate immune response and is required for Mycobacterium marinum virulence. Infect. Immun. 2012;80:43–54. doi: 10.1128/IAI.05249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welin A., Eklund D., Stendahl O., Lerm M. Human macrophages infected with a high burden of ESAT-6-expressing M. tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PLoS One. 2011;6:e20302. doi: 10.1371/journal.pone.0020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakhaiyar P., Nagalakshmi Y., Aruna B., Murthy K.J., Katoch V.M., Hasnain S.E. Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open-reading frame, Rv2608, show a differential humoral response and a low T cell response in various categories of patients with tuberculosis. J. Infect. Dis. 2004;190:1237–1244. doi: 10.1086/423938. [DOI] [PubMed] [Google Scholar]


