Abstract
Nuclear transfer is a complex multistep procedure that includes oocyte maturation, cell cycle synchronization of donor cells, enucleation, cell fusion, oocyte activation and embryo culture. Therefore, many factors are believed to contribute to the success of embryo development following nuclear transfer. Numerous attempts to improve cloning efficiency have been conducted since the birth of the first sheep by somatic cell nuclear transfer. However, the efficiency of somatic cell cloning has remained low, and applications have been limited. In this review, we discuss some of the factors that affect the developmental ability of somatic cell nuclear transfer embryos in cattle.
Keywords: Cattle, Cloning, Embryo, Reprogramming, Somatic cell nuclear transfer
The somatic cell nuclear transfer (SCNT) technology is expected to be useful for farm animal breeding and research, the production of transgenic animals for biomedical purposes, and the conservation of endangered species. Cattle are probably the most widely used species for SCNT experiments [1, 2]. Successful production of clones of elite bulls [3, 4], cows with high milk performance [5] and an endangered breed of cattle [6] has been reported. Furthermore, transgenic cattle such as calves lacking the prion protein [7] and cows overexpressing casein proteins in their milk [8] have been produced by SCNT. However, the efficiency of bovine cloning remains low, despite the numerous studies that have been conducted. Nuclear transfer (NT) is a complex multistep procedure including oocyte maturation, cell cycle synchronization of donor cells, enucleation, cell fusion, oocyte activation, and embryo culture. Therefore, many factors are believed to contribute to the success of embryo development following SCNT. In this review, we discuss some of the factors that affect the developmental ability of bovine SCNT embryos based on our studies as well as other previous reports.
Oocyte Source and Quality
Oocytes are usually collected from slaughterhouse-derived ovaries or live cows by ovum pick-up (OPU) and used for bovine SCNT after in vitro maturation. We examined the developmental ability of NT embryos derived from the cumulus cells of a Japanese black cow using slaughterhouse-derived and OPU-derived in vitro matured oocytes. As shown in Table 1, no significant differences in the cleavage and blastocyst formation rates were observed between oocyte sources (OPU and slaughterhouse). Japanese black cows (same breed as donor cells) and Holstein cows were used as the OPU donors, but the breed of oocyte donors did not affect the in vitro developmental ability of SCNT embryos. Sugimura et al. also reported no difference in the blastocyst formation rates of SCNT embryos between oocytes from a slaughterhouse and OPU, but follicle-stimulating hormone (FSH) pretreatment of OPU donor cows improved oxygen consumption and OCT4 and IFN-τ expression of SCNT embryos to levels similar to fertilized embryos [9], suggesting that FSH pretreatment of OPU donor cows has a positive effect on oocyte quality. Furthermore, in vivo-matured oocytes can be collected by OPU from hormone-treated cows [10, 11]. In vivo-matured oocytes are more developmentally competent after in vitro fertilization (IVF) than in vitro-matured oocytes [10,11,12]. We examined the development of in vivo- and in vitro-matured oocytes after SCNT [13]. In vivo-matured oocytes collected by OPU from heifers treated with FSH, prostaglandin-F2α and gonadotropin hormone-releasing hormone, and in vitro-matured oocytes collected from slaughterhouse-derived ovaries were used as recipient cytoplasts. In accordance with the bovine IVF results [10,11,12], the blastocyst formation rate of in vivo-matured oocytes after SCNT was significantly higher than that of in vitro-matured oocytes. The pregnancy rate did not differ between in vivo- and in vitro-matured oocytes. However, a high abortion rate (75% of pregnancies) was observed in SCNT fetuses from in vitro-matured oocytes, whereas no subsequent abortions were observed from in vivo-matured oocytes. These results suggest that inappropriate oocyte maturation of recipient cytoplasts is one of the factors causing embryonic or fetal loss after NT in cattle.
Table 1. Development of nuclear transfer (NT) embryos derived from cumulus cells of a Japanese black cow using ovum pick-up (OPU)-derived and slaughterhouse-derived oocytes.
| Recipient oocytes | No. of NT embryos |
No. of cleaved embryos (%) |
No. of blastocysts (%) |
No. of embryos transferred |
No. of calves (%) |
No. surviving > 60 days |
|
| Source | Breed | ||||||
| Slaughterhouse | Unknown | 89 | 70 (78.7) | 32 (36.0) | 20 | 5 (25) | 3 (15) |
| OPU | Total | 112 | 101 (90.1) | 33 (29.5) | 10 | 5 (50) | 1 (20) |
| Japanese black | 70 | 64 (91.4) | 19 (27.1) | 7 | 2 (28) | 0 (0) | |
| Holstein | 42 | 37 (88.1) | 14 (33.3) | 3 | 3 (100) | 1 (33) | |
In SCNT, donor cells are electrically fused with enucleated recipient oocytes containing a large amount of foreign cytoplasm. Cloned calves with mixed mitochondrial DNA from the donor cell and the recipient oocyte (heteroplasmy) have been reported [14,15,16,17], although the influence of heteroplasmy on the development of SCNT embryos is unclear. Cloned calves can be produced using both oocytes and somatic cells derived from the same cow to avoid cytoplasmic contribution from foreign oocytes (autologous SCNT [18]). Cloned calves produced in this manner do not exhibit heteroplasmy. Yang et al. showed that autologous SCNT embryos resulted in higher developmental rates in vitro and in vivo compared with heterologous SCNT embryos (donor cell not related to recipient cytoplasm) [18]. In contrast, reports by other laboratories [19,20,21] have indicated no such positive effect of autologous SCNT. This discrepancy may be because of the influence of individual oocyte donors. The oocyte donor influences the production of blastocysts in bovine IVF [22] and SCNT [23]. We examined the developmental ability of autologous SCNT embryos using cumulus cells and oocytes collected from six cows by OPU [24]. The developmental rates of autologous SCNT embryos to the blastocyst stage varied widely among individual cows (range, 19–64%) [24]. We produced four cloned calves by autologous SCNT. However, two of the calves were stillborn, and the remaining two died 13 days and 150 days after birth and had anomalies at the postmortem examination. These results suggest that it is difficult to improve the birth rate of healthy cloned calves only using both oocytes and somatic cells derived from the same cow.
Cell Cycle Combination
The cell cycle of the donor cells is an important factor affecting the development of SCNT embryos, because cell cycle co-ordination of donor cells and recipient oocytes is essential to maintain ploidy and prevent DNA damage [25]. Nonactivated metaphase II (MII) oocytes have been primarily used as recipient cytoplasts for bovine SCNT [26]. Accordingly, G0- or G1-phase cells of the cell cycle have been used in almost all successful reports [27], although M-phase cells can also be reprogrammed in MII oocytes [28]. The efficiency of blastocyst and full-term development was compared between SCNT embryos derived from fibroblast cells at the G0 and G1 phases in several studies [29,30,31,32]. No significant difference was observed in in vitro development between G0- and G1-phase cell SCNT embryos. However, the in vivo developmental ability of SCNT embryos tended to be higher for G1-phase cells than that for G0-phase cells [29,30,31,32]. One study suggested that homogeneous expression among all blastomeres of SCNT embryos derived from G1-phase cells at embryonic gene activation contributes to a higher success rate [33]. The development of SCNT embryos using pre-activated oocytes has been examined in several studies [28, 34,35,36,37]. Oocytes activated 6 h before NT stopped developing at the 8-cell stage after NT, regardless of the cell cycle of the donor cells [28]. However, oocytes within a few hours after activation appear to have a capacity to reprogram the somatic cell nucleus, and this capacity may be largely dependent on the cell cycle stage of the donor cells. Successful production of cloned calves was reported with SCNT embryos using S/G2-phase cells and oocytes activated 2.5 h before NT [35]. In contrast, no cloned calves were obtained with oocytes activated 2 h before NT when we used G0- and G1-phase cells [38].
Cell Type and In Vitro Culture of Donor Cells
Cloned cattle have been produced from various somatic cell types. However, it is still unclear which cell type is the most appropriate for bovine SCNT [27, 39]. Moreover, the differentiation status of somatic cells may have no relationship with cloning efficiency [40]. Bovine SCNT embryos can develop to the blastocyst stage at a rate similar to that of embryos produced by IVF (approximately 30–50%) [41], although the electric conditions for fusion of enucleated oocytes differs among donor cell types [42]. However, high embryonic and fetal losses occur after embryo transfer regardless of donor cell type. Because the efficiency of bovine cloning is low, it may be difficult to show significant differences among donor cell types [43].
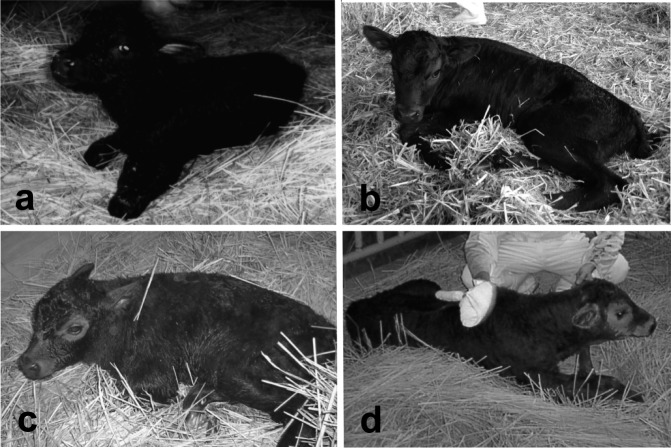
In bovine SCNT, donor cells are usually cultured in vitro before being used for NT [44,45,46]. Not only the nuclei of short-term cultured cells but also the nuclei of long-term cultured cells (cultured for 3 months) [47] or those close to the end of their life span [48] have the ability to generate live healthy calves after NT. We compared the developmental ability of SCNT embryos using bovine cumulus cells under four different culture conditions (non-culture, maturation culture for 20 h, cycling culture and serum-starved culture) to examine the effect of in vitro culture of donor cells on cloning efficiency [49]. The blastocyst formation rate and blastocyst cell number of SCNT embryos derived from cultured cumulus cells (cycling culture and serum-starved culture) were significantly higher than those of SCNT embryos derived from fresh (non-cultured) cells [49]. Cell cycle analysis using flow cytometer showed that the relative percentage of fresh cells in the G0/G1 phase of the cell cycle (89.7 ± 0.4%) was similar to that of serum-starved cells (90.6 ± 0.6%) but lower than that of cycling cells (76.0 ± 1.8%) [49], indicating that the difference in in vitro development between fresh and cultured cells did not result from the cell cycle of the donor cells. The same results have also been obtained for goat [50] and rabbit SCNT [51]. These results suggest that culture of donor cells increases the efficiency of SCNT embryo production in vitro. However, the subsequent viability of blastocyst-stage embryos produced using fresh cells may not be different from that using cultured cells. No difference was observed in the in vivo developmental ability of SCNT embryos between fresh and cultured cells, and live calves were obtained from cumulus cells under all culture conditions (Fig. 1) [49].
Fig. 1.
Cloned calves produced by nuclear transfer using cumulus cells under 4 different conditions: (a) cells removed from cumulus-oocyte complexes (COCs) after aspiration of ovarian follicles; (b) cells removed from COCs after in vitro maturation; (c) cells cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) for 3 days after some subculture; and (d) cells cultured in DMEM with 0.5% FBS for an additional 5 days.
Timing of Fusion and Activation
In SCNT, the lack of sperm-induced fertilization necessitates artificial activation to trigger further development. Direct exposure of chromosomes to nonactivated MII cytoplasm is effective for somatic cell nuclear reprogramming [28, 52], and nonactivated MII oocytes have been used in almost all successful bovine SCNT reports [26, 27]. The timing of activation of MII oocytes can be classified into two protocols as follows: (1) activation performed immediately after fusion (simultaneous fusion and activation method, FA) and (2) activation performed several hours after fusion (delayed activation method, DA). Successful production of cloned offspring using SCNT has been reported for both the FA [45, 47] and DA [44, 46] methods. Donor chromosomes are exposed to factors present in MII cytoplasm for only a short time in the FA method and for a longer time in the DA method. The DA method improves the in vitro development of bovine [6, 53] and mouse [54, 55] NT embryos derived from somatic cells at the G0/G1 stage compared with that of the FA method. We compared the developmental ability of bovine fibroblast cell NT embryos produced using different fusion and chemical activation timings to develop an efficient fusion and activation protocol for producing SCNT embryos [56]. As shown in Table 2, the in vitro development of SCNT embryos was affected by the timing of fusion and chemical activation, and the development of SCNT embryos to the blastocyst stage in the F21A24 group (fusion at 21 h and activation 24 h postmaturation) of the DA method was significantly higher than that in the other groups. However, the development of SCNT embryos activated 6 h after fusion (F21A27 and F24A30 groups) in the DA method was significantly lower than that in the FA method. In reports by Aston et al. [57] and Choi et al. [58], excessive exposure to MII cytoplasm resulted in abnormal chromatin morphology, but SCNT embryos activated less than 2.5 h after fusion resulted in improved nuclear morphology and increased development to the compacted morula/blastocyst stage. These reports and our results suggest that the exposure duration of somatic cell nuclei to oocyte cytoplasm before activation affects the in vitro development of SCNT embryos and that excessive exposure to MII cytoplasm results in a poor developmental rate to the blastocyst stage. However, no influence of the duration of exposure to oocyte cytoplasm on the in vivo developmental ability has been observed. When we examined the in vivo developmental ability of cumulus cell NT embryos and postnatal survivability of cloned calves produced by the DA (F21A24) and FA (F24A24) methods, the pregnancy and calving rates did not differ significantly between the two methods [59]. In addition, high rates of postnatal mortality were observed in both the methods [59]. Sung et al. obtained similar results using two types of donor cells (cumulus and fibroblast cells) [60]. In a report by Aston et al., the time interval between fusion and activation when using the DA method did not affect the in vivo development of SCNT embryos [57].
Table 2. In vitro development of somatic cell nuclear transfer (SCNT) embryos produced using different fusion and activation timings.
| Group | Hours post IVM |
No. SCNT embryos |
No. of cleaved embryos (%) |
No. of blastocysts (%) |
|
| Fusion | Activation | ||||
| F21A21 | 21 | 21 | 89 | 60 (67)cd | 25 (28)c |
| F21A24 | 21 | 24 | 125 | 97 (78)bc | 79 (63)a |
| F21A27 | 21 | 27 | 96 | 52 (54)d | 3 (3)e |
| F24A24 | 24 | 24 | 150 | 123 (82)b | 59 (39)bc |
| F24A27 | 24 | 27 | 134 | 122 (91)a | 63 (47)b |
| F24A30 | 24 | 30 | 93 | 63 (68)cd | 13 (14)d |
| F27A27 | 27 | 27 | 121 | 99 (82)b | 49 (41)bc |
a,b,c,d,e Values without common characters in the same column of each group differ significantly (P < 0.05, chi-square test).
Histone Deacetylase Inhibitor (HDACi) Treatment
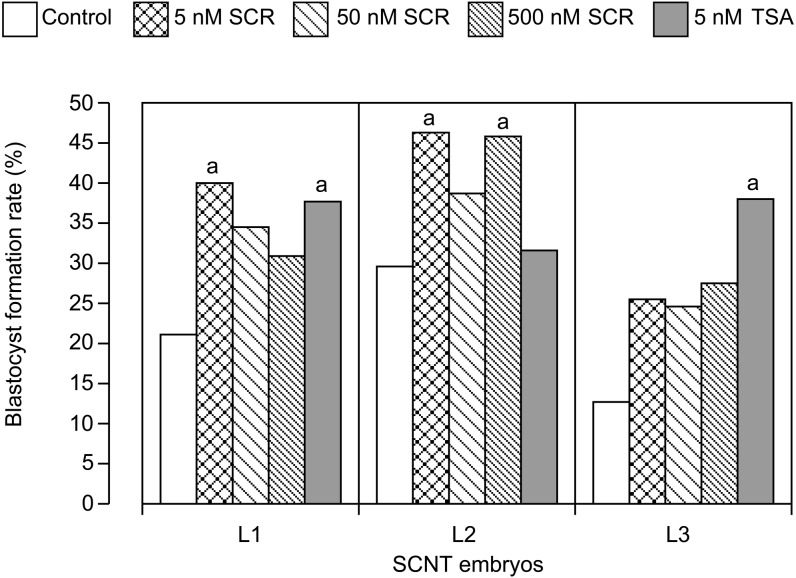
Abnormal epigenetic modifications such as aberrant DNA methylation and histone modification have been observed in SCNT embryos [61,62,63,64]. Therefore, preventing epigenetic errors is expected to lead to improved animal cloning success rates [65]. Several DNA methylation inhibitors and HDACis have been used to improve the developmental ability of bovine SCNT embryos [66]. Treatment of donor cells with 5-aza-2’-deoxycytidine (5-aza-dC), a DNA methylation inhibitor, did not improve the in vitro developmental ability of SCNT embryos [67,68,69], whereas treatment with trichostatin A (TSA) or sodium butyrate, an HDACi, increased the blastocyst formation rate [67,68,69,70]. However, improvement of full-term development following HDACi treatment of donor cells has not been demonstrated. It was reported from two laboratories in 2006 that TSA treatment of mouse SCNT embryos after NT improved the success rate of mouse cloning [65, 71]. In these reports, TSA treatments for 9–20 h led to a significant increase not only in the blastocyst formation rate but also in the full-term developmental rate [65, 71]. In addition, Kohda et al. showed that the gene expression profile of TSA-treated cloned mice came to resemble that of mice produced by intracytoplasmic sperm injection [72]. These reports suggest that inhibiting histone deacetylation in SCNT embryos during a short period of culture after NT promotes reprogramming of the donor nucleus in mice. We examined the effects of treatment with HDACis, TSA and scriptaid (SCR), on the in vitro development of bovine SCNT embryos using three fibroblast cell lines (L1, L2 and L3) [73]. As shown in Fig. 2, TSA treatment improved blastocyst formation rates of SCNT embryos derived from L1 and L3 but had no effect on the rate of embryos derived from L2. Furthermore, SCR treatment increased the blastocyst formation rates of SCNT embryos derived from L1 and L2, but no significant increase was observed in SCNT embryos derived from L3. These results suggest that HDACi treatment of bovine SCNT embryos improves the blastocyst formation rate; however, optimal treatment conditions may differ among donor cell lines. Four laboratories have recently reported the in vivo developmental ability of bovine SCNT embryos treated with TSA [74,75,76,77]. In contrast to the results in mice [65, 71], treatment of bovine SCNT embryos with TSA alone did not significantly improve the full-term developmental rate [74,75,76]. In contrast, a higher calving rate was observed following a combined treatment with a DNA methylation inhibitor [77]. Wang et al. reported that a combined treatment of both donor cells and SCNT embryos with 5-aza-dC and TSA reduced the methylation levels of the NT blastocyst satellite I sequence to levels similar to those in IVF embryos and increased the cloning efficiency from 2.6 to 13.4% [77]. However, it is difficult to correct epigenetic abnormalities completely only by treatment with epigenetic modifiers, as various abnormalities including large offspring syndrome have been observed in cloned calves after combined treatment of both donor cells and cloned embryos with 5-aza-dC and TSA as well as untreated cloned calves [77].
Fig. 2.
Development to the blastocyst stage of somatic cell nuclear transfer (SCNT) embryos treated with 5, 50 and 500 nM scriptaid (SCR) or 5 nM trichostatin A (TSA). Three fibroblast cell lines (L1, L2 and L3) were used as somatic cell donors. aSignificant difference compared with the control (P < 0.05, chi-square test). Reproduced with permission of the Society for Reproduction and Development from Akagi S, et al.: Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J Reprod Dev 2011; 57: 120–126.
Embryo Aggregation
The cell number in blastocysts has been used as an indicator of embryo quality [78]. The cell number in SCNT embryos is lower than that in in vivo-derived embryos [79, 80]. This poor blastocyst quality appears to contribute to the decreased survival rate of SCNT embryos after embryo transfer. Embryo aggregation is a method that enables an increase in the cell number in embryos [80, 81]. In addition, several studies have indicated that embryo aggregation affects SCNT embryo gene expression [82,83,84]. In mice, aggregation of SCNT embryos at the 4-cell stage led not only to an increase in cell number but also improved Oct 4 expression, and resulted in eight times higher full-term development compared with single embryos [80]. In cattle, a high pregnancy rate was observed in embryos aggregated at 4 days after SCNT (day 4) [85, 86], whereas aggregation of 1-cell stage SCNT embryos did not improve in vivo development [82, 87]. Aggregation of 1-cell stage SCNT embryos resulted in reduced OCT4 expression compared with IVF embryos [82], whereas no significant difference in OCT4 expression was observed between IVF embryos and aggregates of day 2 embryos [88]. Thus, the timing of aggregation may be important for producing high-quality SCNT embryos. We examined the effect of the timing of aggregation on the development of SCNT embryos [89]. One-cell stage embryos after activation, 8-cell stage embryos on day 2 or 16- to 32-cell stage embryos on day 4 were used for embryo aggregation after removing the zona pellucida. Irrespective of the timing of aggregation, aggregates of the three SCNT embryos developed to the blastocyst stage at a high rate (aggregates of 1-cell stage embryos, 17/19, 89%; aggregates of day 2 embryos, 23/23, 100%; aggregates of day 4 embryos, 22/22, 100%). Furthermore, a significant increase in cell number was observed in aggregates of three day 2 and day 4 embryos (223 ± 30 and 163 ± 18, respectively) compared with single SCNT embryos (89 ± 6). A significantly higher pregnancy rate was observed after embryo transfer at 60 days of gestation in aggregates of three day 2 or day 4 embryos than in single SCNT embryos; however, a high incidence of abortion and stillbirth was subsequently observed in aggregates (Table 3). These results suggest that aggregation of SCNT embryos may improve the pregnancy rate after embryo transfer but that it cannot reduce the high incidence of fetal loss and stillbirth, which is often observed in bovine SCNT.
Table 3. In vivo development of bovine fibroblast cell-nuclear transfer (NT) embryos and aggregates after embryo transfer.
| Embryo (time of aggregation) | No. of embryos/aggregates transferred |
No. of pregnancies |
No. of calves (%) | ||
| Day 30 | Day 60 | Day 90 | |||
| Single NT, zona-intact | 10 | 3 (30) | 0 (0) a | 0 (0) | 0 (0) |
| Aggregate (8-cell stage) | 11 | 7 (64) | 6 (55) b | 3 (27) | 2 (18) |
| Aggregate (16- to 32-cell stage) | 7 | 4 (57) | 4 (57) b | 1 (14) | 1 (14) |
a,b Values without common characters in the same column differ significantly (P < 0.05, Fisher’s exact test). Reproduced with permission of the Society for Reproduction and Development from Akagi S, et al.: Developmental ability of somatic cell nuclear transferred embryos aggregated at the 8-cell stage or 16- to 32-cell stage in cattle. J Reprod Dev 2011; 57: 500–506.
Conclusion
NT is a complex multistep procedure, and there are many biological and technical factors affecting the development of bovine SCNT embryos. Numerous studies have led to significant improvements in SCNT protocols [90]; however, the cloning efficiency in terms of healthy cloned calves born still remains low. Failure to reprogram the donor genome is believed to be the main reason for the low cloning efficiency [91]. Further studies to optimize each step of SCNT together with a better understanding of the reprogramming mechanism are necessary in order to improve the efficiency of bovine cloning.
Acknowledgments
The authors wish to thank the Society for Reproduction and Development (SRD) for conferring the 2013 SRD Outstanding Research Award on our study. The authors are very grateful to Dr T Nagai (Food and Fertilizer Technology Center), Dr N Adachi (Ibaraki Prefectural Livestock Research Center), Dr M Kubo (Zen-noh Institute of Animal Health), Dr E Mizutani (University of Yamanashi), Dr Y Izaike (Iwate University), Mr K Hasegawa (Shimane Prefectural Livestock Technology Center), Mr D Yamaguchi (Ibaraki Prefectural North Livestock Hygiene Service Center), Mr K Fukunari (Iwate Prefecture Central Livestock Hygiene Service Center), Mr T Shiraishi (Aichi Agricultural Research Center), Mr B Tsuneishi (Kochi Prefectural Livestock Experiment Station), Mr T Noguchi (Tokyo University of Agriculture), Mr M Shimizu (NARO Tohoku Agricultural Research Center) and Mrs K Kaneyama (National Livestock Breeding Center) for their support and helpful suggestions. The authors would also like to thank the members of the Animal Reproduction Research Group, Animal Breeding and Reproduction Research Division, NARO Institute of Livestock and Grassland Science, and the members of the Animal Development and Differentiation Research Unit, Division of Animal Sciences, National Institute of Agrobiological Sciences.
References
- 1.Oback B, Wells DN. Cloning cattle: the methods in the madness. Adv Exp Med Biol 2007; 591: 30–57. [DOI] [PubMed] [Google Scholar]
- 2.Ross PJ, Cibelli JB. Bovine somatic cell nuclear transfer. Methods Mol Biol 2010; 636: 155–177. [DOI] [PubMed] [Google Scholar]
- 3.Tian XC, Kubota C, Sakashita K, Izaike Y, Okano R, Tabara N, Curchoe C, Jacob L, Zhang Y, Smith S, Bormann C, Xu J, Sato M, Andrew S, Yang X. Meat and milk compositions of bovine clones. Proc Natl Acad Sci USA 2005; 102: 6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshino Y, Hayashi N, Taniguchi S, Kobayashi N, Sakai K, Otani T, Iritani A, Saeki K. Resurrection of a bull by cloning from organs frozen without cryoprotectant in a -80 degrees c freezer for a decade. PLoS ONE 2009; 4: e4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonai M, Kaneyama K, Miyashita N, Kobayashi S, Goto Y, Bettpu T, Nagai T. Growth, reproduction, and lactation in somatic cell cloned cows with short telomeres. J Dairy Sci 2005; 88: 4097–4110. [DOI] [PubMed] [Google Scholar]
- 6.Wells DN, Misica PM, Tervit HR, Vivanco WH. Adult somatic cell nuclear transfer is used to preserve the last surviving cow of the Enderby Island cattle breed. Reprod Fertil Dev 1998; 10: 369–378. [DOI] [PubMed] [Google Scholar]
- 7.Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, Sathiyaseelan J, Wu H, Matsushita H, Koster J, Kato S, Ishida I, Soto C, Robl JM, Kuroiwa Y. Production of cattle lacking prion protein. Nat Biotechnol 2007; 25: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brophy B, Smolenski G, Wheeler T, Wells D, L’Huillier P, Laible G. Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat Biotechnol 2003; 21: 157–162. [DOI] [PubMed] [Google Scholar]
- 9.Sugimura S, Kobayashi S, Hashiyada Y, Ohtake M, Kaneda M, Yamanouchi T, Matsuda H, Aikawa Y, Watanabe S, Nagai T, Kobayashi E, Konishi K, Imai K. Follicular growth-stimulated cows provide favorable oocytes for producing cloned embryos. Cell Reprogram 2012; 14: 29–37. [DOI] [PubMed] [Google Scholar]
- 10.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61: 234–248. [DOI] [PubMed] [Google Scholar]
- 11.van de Leemput EE, Vos PL, Zeinstra EC, Bevers MM, van der Weijden GC, Dieleman SJ. Improved in vitro embryo development using in vivo matured oocytes from heifers superovulated with a controlled preovulatory LH surge. Theriogenology 1999; 52: 335–349. [DOI] [PubMed] [Google Scholar]
- 12.Leibfried-Rutledge ML, Critser ES, Eyestone WH, Northey DL, First NL. Development potential of bovine oocytes matured in vitro or in vivo. Biol Reprod 1987; 36: 376–383. [DOI] [PubMed] [Google Scholar]
- 13.Akagi S, Kaneyama K, Adachi N, Tsuneishi B, Matsukawa K, Watanabe S, Kubo M, Takahashi S. Bovine nuclear transfer using fresh cumulus cell nuclei and in vivo- or in vitro-matured cytoplasts. Cloning Stem Cells 2008; 10: 173–180. [DOI] [PubMed] [Google Scholar]
- 14.Hiendleder S, Schmutz SM, Erhardt G, Green RD, Plante Y. Transmitochondrial differences and varying levels of heteroplasmy in nuclear transfer cloned cattle. Mol Reprod Dev 1999; 54: 24–31. [DOI] [PubMed] [Google Scholar]
- 15.Steinborn R, Zakhartchenko V, Jelyazkov J, Klein D, Wolf E, Müller M, Brem G. Composition of parental mitochondrial DNA in cloned bovine embryos. FEBS Lett 1998; 426: 352–356. [DOI] [PubMed] [Google Scholar]
- 16.Steinborn R, Schinogl P, Zakhartchenko V, Achmann R, Schernthaner W, Stojkovic M, Wolf E, Müller M, Brem G. Mitochondrial DNA heteroplasmy in cloned cattle produced by fetal and adult cell cloning. Nat Genet 2000; 25: 255–257. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Takahashi S, Onishi A, Goto Y, Miyazawa A, Imai H. Dominant distribution of mitochondrial DNA from recipient oocytes in bovine embryos and offspring after nuclear transfer. J Reprod Fertil 1999; 116: 253–259. [DOI] [PubMed] [Google Scholar]
- 18.Yang XY, Li H, Ma QW, Yan JB, Zhao JG, Li HW, Shen HQ, Liu HF, Huang Y, Huang SZ, Zeng YT, Zeng F. Improved efficiency of bovine cloning by autologous somatic cell nuclear transfer. Reproduction 2006; 132: 733–739. [DOI] [PubMed] [Google Scholar]
- 19.Edwards JL, Schrick FN, Hockett ME, Saxton AM, Lawrence JL, Payton RR. Development of clones constructed with maternal cytoplasm. Theriogenology 2003; 59: 250 (abstract). [Google Scholar]
- 20.Murakami M, Perez O, Ferguson CE, Behboodi E, Denniston RS, Godke RA. Use of in vivo-recovered oocytes and adult somatic cells from the same donor for nuclear transfer in cattle. Vet Rec 2003; 153: 713–714. [PubMed] [Google Scholar]
- 21.Lee E, Song K. Autologous somatic cell nuclear transfer in pigs using recipient oocytes and donor cells from the same animal. J Vet Sci 2007; 8: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamassia M, Heyman Y, Lavergne Y, Richard C, Gelin V, Renard JP, Chastant-Maillard S. Evidence of oocyte donor cow effect over oocyte production and embryo development in vitro. Reproduction 2003; 126: 629–637. [PubMed] [Google Scholar]
- 23.Yang XY, Zhao JG, Li H, Liu HF, Huang Y, Huang SZ, Zeng F, Zeng YT. Effect of individual heifer oocyte donors on cloned embryo development in vitro. Anim Reprod Sci 2008; 104: 28–37. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa K, Takahashi S, Akagi S, Takeda K, Imai K, Shimizu M, Okazaki T, Abe S, Izaike Y. Bovine somatic cell nuclear transfer using cumulus-oocyte complexes collected from the identical individual by ovum pickup. Reprod Fertil Dev 2007; 19: 138 (abstract). [Google Scholar]
- 25.Campbell KH, Alberio R. Reprogramming the genome: role of the cell cycle. Reprod Suppl 2003; 61: 477–494. [PubMed] [Google Scholar]
- 26.Oback B, Wells DN. Cloning cattle. Cloning Stem Cells 2003; 5: 243–256. [DOI] [PubMed] [Google Scholar]
- 27.Kato Y, Tsunoda Y. Role of the donor nuclei in cloning efficiency: can the ooplasm reprogram any nucleus? Int J Dev Biol 2010; 54: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 28.Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod 2001; 64: 324–330. [DOI] [PubMed] [Google Scholar]
- 29.Goto Y, Hirayama M, Takeda K, Tukamoto N, Sakata O, Kaeriyama H, Geshi M. Effect of synchronization of donor cells in early G1-phase using shake-off method on developmental potential of somatic cell nuclear transfer embryos in cattle. Anim Sci J 2013; 84: 592–599. [DOI] [PubMed] [Google Scholar]
- 30.Ideta A, Urakawa M, Aoyagi Y, Saeki K. Early development in utero of bovine nuclear transfer embryos using early G1 and G0 phase cells. Cloning Stem Cells 2007; 9: 571–580. [DOI] [PubMed] [Google Scholar]
- 31.Ideta A, Hayama K, Urakawa M, Tsuchiya K, Aoyagi Y, Saeki K. Comparison of early development in utero of cloned fetuses derived from bovine fetal fibroblasts at the G1 and G0/G1 phases. Anim Reprod Sci 2010; 119: 191–197. [DOI] [PubMed] [Google Scholar]
- 32.Kasinathan P, Knott JG, Wang Z, Jerry DJ, Robl JM. Production of calves from G1 fibroblasts. Nat Biotechnol 2001; 19: 1176–1178. [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto D, Kasamatsu A, Ideta A, Urakawa M, Matsumoto K, Hosoi Y, Iritani A, Aoyagi Y, Saeki K. Donor cells at the G1 phase enhance homogeneous gene expression among blastomeres in bovine somatic cell nuclear transfer embryos. Cell Reprogram 2012; 14: 20–28. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Kubota C, Shimizu M, Tabara N, Izaike Y, Imai H. Production of cloned calves by somatic cell-nuclear transplantation. In: Roberts RM, Yanagimachi R, Kariya T, Hashizume K (eds), Cloned Animal and Placentation, Tokyo, Japan: Yokendo Publishers; 2000: 30–35.
- 35.Bordignon V, Smith LC. Telophase-stage host ooplasts support complete reprogramming of roscovitine-treated somatic cell nuclei in cattle. Cloning Stem Cells 2006; 8: 305–317. [DOI] [PubMed] [Google Scholar]
- 36.Kurosaka S, Nagao Y, Minami N, Yamada M, Imai H. Dependence of DNA synthesis and in vitro development of bovine nuclear transfer embryos on the stage of the cell cycle of donor cells and recipient cytoplasts. Biol Reprod 2002; 67: 643–647. [DOI] [PubMed] [Google Scholar]
- 37.Shiga K, Fujita T, Hirose K, Sasae Y, Nagai T. Production of calves by transfer of nuclei from cultured somatic cells obtained from Japanese black bulls. Theriogenology 1999; 52: 527–535. [DOI] [PubMed] [Google Scholar]
- 38.Matsukawa K, Akagi S, Fukunari K, Hosokawa Y, Yonezawa C, Watanabe S, Takahashi S. The effects of donor cell cycle and the timing of oocyte activation on development of bovine nuclear transferred embryos in vivo. Reprod Fertil Dev 2011; 23: 124 (abstract). [Google Scholar]
- 39.Galli C, Duchi R, Colleoni S, Lagutina I, Lazzari G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: from the research laboratory to clinical practice. Theriogenology 2014; 81: 138–151. [DOI] [PubMed] [Google Scholar]
- 40.Oback B, Wells DN. Donor cell differentiation, reprogramming, and cloning efficiency: elusive or illusive correlation? Mol Reprod Dev 2007; 74: 646–654. [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet 2007; 39: 295–302. [DOI] [PubMed] [Google Scholar]
- 42.Akagi S, Takahashi S, Ohkoshi K, Takenouchi T, Shimizu M, Geshi M, Adachi N, Fuchimoto D, Izaike Y, Aso H. Nuclear transfer using a bovine mammary epithelial cell line (BMEC). Anim Sci J 2002; 73: 465–469. [Google Scholar]
- 43.Miyoshi K, Rzucidlo SJ, Pratt SL, Stice SL. Improvements in cloning efficiencies may be possible by increasing uniformity in recipient oocytes and donor cells. Biol Reprod 2003; 68: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 44.Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de León FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science 1998; 280: 1256–1258. [DOI] [PubMed] [Google Scholar]
- 45.Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science 1998; 282: 2095–2098. [DOI] [PubMed] [Google Scholar]
- 46.Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod 1999; 60: 996–1005. [DOI] [PubMed] [Google Scholar]
- 47.Kubota C, Yamakuchi H, Todoroki J, Mizoshita K, Tabara N, Barber M, Yang X. Six cloned calves produced from adult fibroblast cells after long-term culture. Proc Natl Acad Sci USA 2000; 97: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 2000; 288: 665–669. [DOI] [PubMed] [Google Scholar]
- 49.Akagi S, Takahashi S, Adachi N, Hasegawa K, Sugawara T, Tozuka Y, Yamamoto E, Shimizu M, Izaike Y. In vitro and in vivo developmental potential of nuclear transfer embryos using bovine cumulus cells prepared in four different conditions. Cloning Stem Cells 2003; 5: 101–108. [DOI] [PubMed] [Google Scholar]
- 50.Akshey YS, Malakar D, De AK, Jena MK, Garg S, Dutta R, Pawar SK, Mukesh M. Hand-made cloned goat (Capra hircus) embryos—a comparison of different donor cells and culture systems. Cell Reprogram 2010; 12: 581–588. [DOI] [PubMed] [Google Scholar]
- 51.Cervera RP, García-Ximénez F. Oocyte age and nuclear donor cell type affect the technical efficiency of somatic cloning in rabbits. Zygote 2003; 11: 151–158. [DOI] [PubMed] [Google Scholar]
- 52.Du F, Sung LY, Tian XC, Yang X. Differential cytoplast requirement for embryonic and somatic cell nuclear transfer in cattle. Mol Reprod Dev 2002; 63: 183–191. [DOI] [PubMed] [Google Scholar]
- 53.Shin SJ, Lee BC, Park JI, Lim JM, Hwang WS. A separate procedure of fusion and activation in an ear fibroblast nuclear transfer program improves preimplantation development of bovine reconstituted oocytes. Theriogenology 2001; 55: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 54.Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394: 369–374. [DOI] [PubMed] [Google Scholar]
- 55.Wakayama T, Tateno H, Mombaerts P, Yanagimachi R. Nuclear transfer into mouse zygotes. Nat Genet 2000; 24: 108–109. [DOI] [PubMed] [Google Scholar]
- 56.Akagi S, Takahashi S, Yokota M, Noguchi T, Taniyama A, Fuchimoto D, Izaike Y. The timing of fusion and chemical activation in nuclear transfer affects developmental potential of bovine embryos. Theriogenology 2001; 55: 252 (abstract). [Google Scholar]
- 57.Aston KI, Li GP, Hicks BA, Sessions BR, Pate BJ, Hammon D, Bunch TD, White KL. Effect of the time interval between fusion and activation on nuclear state and development in vitro and in vivo of bovine somatic cell nuclear transfer embryos. Reproduction 2006; 131: 45–51. [DOI] [PubMed] [Google Scholar]
- 58.Choi JY, Kim CI, Park CK, Yang BK, Cheong HT. Effect of activation time on the nuclear remodeling and in vitro development of nuclear transfer embryos derived from bovine somatic cells. Mol Reprod Dev 2004; 69: 289–295. [DOI] [PubMed] [Google Scholar]
- 59.Akagi S, Adachi N, Matsukawa K, Kubo M, Takahashi S. Developmental potential of bovine nuclear transfer embryos and postnatal survival rate of cloned calves produced by two different timings of fusion and activation. Mol Reprod Dev 2003; 66: 264–272. [DOI] [PubMed] [Google Scholar]
- 60.Sung LY, Shen PC, Jeong BS, Xu J, Chang CC, Cheng WT, Wu JS, Lee SN, Broek D, Faber D, Tian XC, Yang X, Du F. Premature chromosome condensation is not essential for nuclear reprogramming in bovine somatic cell nuclear transfer. Biol Reprod 2007; 76: 232–240. [DOI] [PubMed] [Google Scholar]
- 61.Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet 2001; 28: 173–177. [DOI] [PubMed] [Google Scholar]
- 62.Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci USA 2001; 98: 13734–13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos F, Zakhartchenko V, Stojkovic M, Peters A, Jenuwein T, Wolf E, Reik W, Dean W. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr Biol 2003; 13: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 64.Senda S, Wakayama T, Arai Y, Yamazaki Y, Ohgane J, Tanaka S, Hattori N, Yanagimachi R, Shiota K. DNA methylation errors in cloned mice disappear with advancement of aging. Cloning Stem Cells 2007; 9: 293–302. [DOI] [PubMed] [Google Scholar]
- 65.Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun 2006; 340: 183–189. [DOI] [PubMed] [Google Scholar]
- 66.Akagi S, Geshi M, Nagai T. Recent progress in bovine somatic cell nuclear transfer. Anim Sci J 2013; 84: 191–199. [DOI] [PubMed] [Google Scholar]
- 67.Enright BP, Jeong BS, Yang X, Tian XC. Epigenetic characteristics of bovine donor cells for nuclear transfer: levels of histone acetylation. Biol Reprod 2003; 69: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 68.Ding X, Wang Y, Zhang D, Wang Y, Guo Z, Zhang Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2′-deoxycytidine and trichostatin A. Theriogenology 2008; 70: 622–630. [DOI] [PubMed] [Google Scholar]
- 69.Jafarpour F, Hosseini SM, Hajian M, Forouzanfar M, Ostadhosseini S, Abedi P, Gholami S, Ghaedi K, Gourabi H, Shahverdi AH, Vosough AD, Nasr-Esfahani MH. Somatic cell-induced hyperacetylation, but not hypomethylation, positively and reversibly affects the efficiency of in vitro cloned blastocyst production in cattle. Cell Reprogram 2011; 13: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi W, Hoeflich A, Flaswinkel H, Stojkovic M, Wolf E, Zakhartchenko V. Induction of a senescent-like phenotype does not confer the ability of bovine immortal cells to support the development of nuclear transfer embryos. Biol Reprod 2003; 69: 301–309. [DOI] [PubMed] [Google Scholar]
- 71.Rybouchkin A, Kato Y, Tsunoda Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol Reprod 2006; 74: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 72.Kohda T, Kishigami S, Kaneko-Ishino T, Wakayama T, Ishino F. Gene expression profile normalization in cloned mice by trichostatin A treatment. Cell Reprogram 2012; 14: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akagi S, Matsukawa K, Mizutani E, Fukunari K, Kaneda M, Watanabe S, Takahashi S. Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J Reprod Dev 2011; 57: 120–126. [DOI] [PubMed] [Google Scholar]
- 74.Sangalli JR, De Bem TH, Perecin F, Chiaratti MR, Oliveira LJ, de Araújo RR, Valim Pimentel JR, Smith LC, Meirelles FV. Treatment of nuclear-donor cells or cloned zygotes with chromatin-modifying agents increases histone acetylation but does not improve full-term development of cloned cattle. Cell Reprogram 2012; 14: 235–247. [DOI] [PubMed] [Google Scholar]
- 75.Sawai K, Fujii T, Hirayama H, Hashizume T, Minamihashi A. Epigenetic status and full-term development of bovine cloned embryos treated with trichostatin A. J Reprod Dev 2012; 58: 302–309. [DOI] [PubMed] [Google Scholar]
- 76.Srirattana K, Imsoonthornruksa S, Laowtammathron C, Sangmalee A, Tunwattana W, Thongprapai T, Chaimongkol C, Ketudat-Cairns M, Parnpai R. Full-term development of gaur-bovine interspecies somatic cell nuclear transfer embryos: effect of trichostatin A treatment. Cell Reprogram 2012; 14: 248–257. [DOI] [PubMed] [Google Scholar]
- 77.Wang YS, Xiong XR, An ZX, Wang LJ, Liu J, Quan FS, Hua S, Zhang Y. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2/-deoxycytidine and trichostatin A. Theriogenology 2011; 75: 819–825. [DOI] [PubMed] [Google Scholar]
- 78.van Soom A, Ysebaert MT, de Kruif A. Relationship between timing of development, morula morphology, and cell allocation to inner cell mass and trophectoderm in in vitro-produced bovine embryos. Mol Reprod Dev 1997; 47: 47–56. [DOI] [PubMed] [Google Scholar]
- 79.Koo DB, Kang YK, Choi YH, Park JS, Kim HN, Oh KB, Son DS, Park H, Lee KK, Han YM. Aberrant allocations of inner cell mass and trophectoderm cells in bovine nuclear transfer blastocysts. Biol Reprod 2002; 67: 487–492. [DOI] [PubMed] [Google Scholar]
- 80.Boiani M, Eckardt S, Leu NA, Schöler HR, McLaughlin KJ. Pluripotency deficit in clones overcome by clone-clone aggregation: epigenetic complementation? EMBO J 2003; 22: 5304–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee SG, Park CH, Choi DH, Kim HS, Ka HH, Lee CK. In vitro development and cell allocation of porcine blastocysts derived by aggregation of in vitro fertilized embryos. Mol Reprod Dev 2007; 74: 1436–1445. [DOI] [PubMed] [Google Scholar]
- 82.Misica-Turner PM, Oback FC, Eichenlaub M, Wells DN, Oback B. Aggregating embryonic but not somatic nuclear transfer embryos increases cloning efficiency in cattle. Biol Reprod 2007; 76: 268–278. [DOI] [PubMed] [Google Scholar]
- 83.Kurosaka S, Eckardt S, Ealy AD, McLaughlin KJ. Regulation of blastocyst stage gene expression and outgrowth interferon tau activity of somatic cell clone aggregates. Cloning Stem Cells 2007; 9: 630–641. [DOI] [PubMed] [Google Scholar]
- 84.Zhou W, Xiang T, Walker S, Abruzzese RV, Hwang E, Farrar V, Findeisen B, Sadeghieh S, Arenivas F, Chen SH, Polejaeva I. Aggregation of bovine cloned embryos at the four-cell stage stimulated gene expression and in vitro embryo development. Mol Reprod Dev 2008; 75: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 85.Vajta G, Lewis IM, Trounson AO, Purup S, Maddox-Hyttel P, Schmidt M, Pedersen HG, Greve T, Callesen H. Handmade somatic cell cloning in cattle: analysis of factors contributing to high efficiency in vitro. Biol Reprod 2003; 68: 571–578. [DOI] [PubMed] [Google Scholar]
- 86.Pedersen HG, Schmidt M, Sangild PT, Strøbech L, Vajta G, Callesen H, Greve T. Clinical experience with embryos produced by handmade cloning: work in progress. Mol Cell Endocrinol 2005; 234: 137–143. [DOI] [PubMed] [Google Scholar]
- 87.Oback B, Wiersema AT, Gaynor P, Laible G, Tucker FC, Oliver JE, Miller AL, Troskie HE, Wilson KL, Forsyth JT, Berg MC, Cockrem K, McMillan V, Tervit HR, Wells DN. Cloned cattle derived from a novel zona-free embryo reconstruction system. Cloning Stem Cells 2003; 5: 3–12. [DOI] [PubMed] [Google Scholar]
- 88.Zhou W, Xiang T, Walker S, Farrar V, Hwang E, Findeisen B, Sadeghieh S, Arenivas F, Abruzzese RV, Polejaeva I. Global gene expression analysis of bovine blastocysts produced by multiple methods. Mol Reprod Dev 2008; 75: 744–758. [DOI] [PubMed] [Google Scholar]
- 89.Akagi S, Yamaguchi D, Matsukawa K, Mizutani E, Hosoe M, Adachi N, Kubo M, Takahashi S. Developmental ability of somatic cell nuclear transferred embryos aggregated at the 8-cell stage or 16- to 32-cell stage in cattle. J Reprod Dev 2011; 57: 500–506. [DOI] [PubMed] [Google Scholar]
- 90.Niemann H, Lucas-Hahn A. Somatic cell nuclear transfer cloning: practical applications and current legislation. Reprod Domest Anim 2012; 47(Suppl 5): 2–10. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Osorio N, Urrego R, Cibelli JB, Eilertsen K, Memili E. Reprogramming mammalian somatic cells. Theriogenology 2012; 78: 1869–1886. [DOI] [PubMed] [Google Scholar]