Abstract
We have previously shown that polymorphonuclear neutrophils (PMNs) are present in bovine oviduct fluid under physiological conditions, and that the oviduct provides a microenvironment that protects sperm from phagocytosis by PMNs. Alpha 1-acid glycoprotein (AGP) is a major acute-phase protein produced mainly in the liver that has immunomodulatory functions. AGP mRNA is expressed in extrahepatic organs, such as the lung, kidney, spleen, lymph node, uterus, and ovary. Therefore, in this study, we investigated, 1) the local production of AGP in the bovine oviduct, 2) the effect of AGP on the phagocytic activity of PMNs for sperm and superoxide production and 3) the impact of AGP desialylation on the PMN phagocytosis of sperm. The AGP gene was expressed in cultured bovine oviduct epithelial cells (BOECs) and AGP protein was detected in oviduct fluid. Preexposure of PMNs to AGP at physiological levels impaired PMN phagocytosis for sperm and superoxide generation. The desialylation of AGP eliminated these suppressive effects of AGP on PMN. Scanning electron microscopy revealed that AGP drastically reduced the formation of DNA-based neutrophil extracellular traps (NETs) for sperm entanglement. Additionally, AGP dose-dependently stimulated BOECs to produce prostaglandin E2 (PGE2) which has been shown to partially contribute to the regulation of sperm phagocytosis in the bovine oviduct. AGP and PGE2 at concentrations detected in the oviducts additively suppressed sperm phagocytosis by PMNs. These results provide evidence that locally produced AGP may be involved in protecting sperm from phagocytosis by PMNs in the bovine oviduct.
Keywords: Alpha 1-acid glycoprotein (AGP), Bovine, Oviduct, Phagocytosis, Sperm
The oviduct is a key component of the female reproductive tract, where essential states such as oocyte maturation, sperm capacitation, fertilization, and initial embryonic development take place [1, 2]. The oviduct is classically described as a sterile milieu, even though pathogens and endotoxins could invade the mucosal surfaces of the oviduct via the uterus, peritoneal cavity, and follicular fluid. Therefore, the oviduct environment should be equipped with an efficient and strictly controlled immune system [3]. We have recently shown that polymorphonuclear neutrophils (PMNs), the first line of defense against microorganisms, are present in the bovine oviduct fluid during preovulatory stages. Moreover, the findings of our recent study strongly suggest that the bovine oviduct provides a prostaglandin E2 (PGE2)-rich microenvironment to protect sperm from phagocytosis by PMNs that they possibly face in vivo, thereby supporting sperm survival in the oviduct [4].
Alpha 1-acid glycoprotein (AGP), a major acute-phase protein produced mainly in the liver, is a single polypeptide chain of 20.4 kDa, with a carbohydrate moiety that accounts for 40% of its total mass [5, 6]. Hepatic production and serum concentrations of AGP are increased in response to systemic injury and inflammation [7]. The precise biological functions of AGP are not completely understood, but numerous activities of potential physiological significance have been described, particularly its effects on immunomodulation and its ability to bind basic drugs [8]. These activities of AGP have been shown to be mostly dependent on carbohydrate composition, and changes in glycosylation can affect the biological properties of AGP [9]. AGP mRNA is expressed in extrahepatic organs, such as the lung, kidney, spleen, lymph node, uterus, and ovary [10].
Therefore, we hypothesized that AGP is secreted locally in the bovine oviduct, and is involved in regulation of the phagocytic activity of neutrophils for sperm.
To test our hypothesis, we investigated, 1) the local production of AGP in the bovine oviduct, 2) the effects of AGP on the phagocytic activity of PMNs for sperm and superoxide production; and 3) the impact of AGP desialylation on PMN phagocytosis for sperm.
Materials and Methods
Oviduct preparation
Paired oviducts along with their ipsilateral ovaries were collected from a local slaughterhouse, and they were closed from both ends to prevent leakage or contamination of the oviduct contents. The stages of the estrous cycle were determined macroscopically by assessing ovarian morphology through the observation of color, size, and weight of the corpus luteum, as described previously [11]. Furthermore, oviducts as well as the attached uteri were macroscopically examined to ensure that they were healthy and free from inflammation. Following these examinations, oviducts were immersed in PBS without Ca2+ and Mg2+ (PBS–/–) (Sigma-Aldrich, St. Louis, MO, USA) and supplemented with 0.3% amphotericin B (Sigma-Aldrich), and 0.3% gentamicin (Sigma-Aldrich).
Primary bovine oviduct epithelial cell (BOEC) isolation and cultivation
The isolation and cultivation of BOECs was based on the method described previously [12, 13]. Briefly, oviducts were cut and separated from the surrounding connective tissue. BOECs were mechanically dislodged, purified, and cultured in D-MEM/F12 culture medium supplemented with 2.2% NaHCO3, 0.1% gentamicin, 1% amphotericin, and 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD, USA), in 6-well culture dishes (Nalge Nunc International, Roskilde, Denmark) at 38.5 C in 5% CO2 and 95% air. After monolayer formation, cells were trypsinized (0.05% trypsin EDTA; Amresco, Solon, OH, USA), replated in 12-well culture dishes at a density of 3 × 104 cells/ml, and cultured until formation of a subconfluent monolayer. The growing BOEC monolayer was then cultured in medium supplemented with 0.1% FCS and incubated for 24 h with AGP (bovine AGP; Life Diagnostics, West Chester, PA, USA) (0, 1, 10 or 100 ng/ml). Finally, the culture medium was collected and stored at −80 C until PGE2 determination. Finally, 500 μl TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added to the wells, and the cells were collected, placed in 1.5 ml microcentrifuge tubes and then, stored at −80 C until RNA extraction. The purity of epithelial cell preparations was evaluated with monoclonal antibodies to cytokeratin (anti-cytokeratin-CK1) and immunostaining. Approximately 98% of the cells were positive for anti-cytokeratin (CK1) antibodies.
AGP gene expression
AGP gene expression in PMNs, BOECs and the liver was detected by reverse transcription polymerase chain reaction (RT-PCR), as described previously [14]. The primers used for PCR were as follows: 5’- CCAACCTGATGACAGTGGC-3’, forward and 5’- GCCGACTTATTGTACTCGGG-3’, reverse, for AGP (NM‑001040502, 109 bp) and 5-CCAAGGCCAACCGTGAGAAAAT-3’, forward, and 5-CCACATTCCGTGAGGATCTTCA-3’, reverse, for β-actin (K00622, 256 bp).
AGP concentration determination
Fourteen oviducts at different stages of the estrous cycle (preovulatory, n = 4; postovulatory, n = 5; and mid-luteal stage, n = 5) were very gently flushed with 200 µl PBS–/– and the flushing media were collected into 1.5 ml microcentrifuge tubes. Extreme precautions were taken to avoid any outside contaminations. The flushing media were centrifugated at 1000 g for 10 min at 4 C to remove cellular debris. The AGP concentrations were measured directly in the flushing media using an ELISA kit (Uscn Life Science, Wuhan, China) according to the manufacturer’s protocol. The AGP concentrations were quantified based on a standard curve with optical density (OD) measurements at 450 nm on an ELISA reader (Multiskan MS plate reader, Thermo Labsystems, Vantaa, Finland). The intra- and inter-assays coefficients of variation (CVs) were 10% and 12%, respectively. The range of the standard curves for these assays was 15.5–1000 ng/ml.
PGE2 concentration determination
Previously, we have shown that the bovine oviducts provide a PGE2-rich microenvironment to protect sperm from phagocytosis by PMNs [4]. Therefore, we investigated the effect of AGP on PGE2 production from cultured BOECs. The BOECs were incubated with AGP (0, 1, 10 or 100 ng/ml) for 24 h and then, the culture medium was collected and used for measuring of PGE2 concentrations by using a second antibody enzyme immunoassay as previously described [11]. Thirty-six BOEC supernatants were used for PGE2 concentration determination. The intra and inter-assays CVs were 7.3 and 11.4%, respectively. The ED50 was 260 pg/ml, and the range of the standard curves for these assays was 20–20000 pg/ml.
Preparations of PMNs
Heparinized blood from a multiparous Holstein cow during the luteal stage was collected, and PMNs were isolated as previously described [15]. The PMNs were suspended at a density of 15×106 cells/ml in culture medium supplemented with 0, 1, 10 or 100 ng/ml of AGP (bovine AGP; Life Diagnostics) and incubated at 37 C in 5% CO2 and 95% air for 4 h with gentle shaking. After PMNs incubation, PMNs were washed 2 times with PBS–/– and used for a phagocytosis assay.
Preparation of sperm
In parallel with the PMN preparation, sperm preparation was also carried out. Frozen straws were obtained that contained semen from three highly fertile Holstein bulls of the Genetics Hokkaido Association (Hokkaido, Japan). All semen straws were obtained from a single ejaculate from each bull separately. In vitro capacitation was induced by 4 h incubation of bull sperm in modified Tyrode’s albumin, lactate, and pyruvate medium (Sp-TALP) supplemented with 10 µg/ml heparin, according to the method previously described [16, 17]. Capacitation was verified by the induction of acrosome reactions using 100 µg/ml lysophosphatidylcholine for 15 min. Acrosome reactions were detected by performing a dual staining procedure with Trypan blue supravital stain and Giemsa stain as described by Kovacs and Foote [18]. After the treatment for capacitation, sperm were washed and suspended in Tyrode’s medium containing lactate, pyruvate, and HEPES (TL-HEPES) [19, 20], and they were then used in the phagocytosis assays.
Coating of plates with desialylated AGP (as-AGP)
Plates were coated as described previously [21], with minor modifications. Three 96-well ELISA plates (Nunc, Denmark) were coated with AGP (0, or 10 μg/well) dissolved in 150 μl of PBS for 2 h at 37 C. They were then washed three times with PBS and blocked with 0.1% bovine serum albumin (BSA) in PBS for 2 h at 37 C. Plates were coated with as-AGP by incubating the AGP-coated plates with 200 mU/ml neuraminidase (Streptococcus 6646K, EC 3.2.1.18, Seikagaku, Tokyo, Japan) in 0.1 M PBS (pH 5.2) for 2 h at 37 C. Next, the plates were washed three times with PBS and blocked with 0.1% BSA in PBS for 2 h at 37 C. The plates were washed again three times with PBS before use. PMNs were cultured on the coated plates for 4 h and then, used in the detection of the phagocytic activity and superoxide generation of PMNs in response to sperm.
Phagocytosis assay
The phagocytic activity of PMNs for capacitated sperm was assayed according to the method previously described [22], with minor modifications. Briefly, PMNs incubated for 4 h were suspended in TL-HEPES. A 50 µl aliquot of PMN suspension was mixed with an equal volume of the treated sperm suspension in a 96-well untreated polystyrene microtest plate (Thermo Scientific, Roskilde, Denmark) and incubated at 38 C in 5% CO2 and 95% air for 60 min with gentle swirling on a test-plate shaker. The final concentrations of PMNs and the treated sperm were 15 × 106 and 30 × 106 cells/ml, respectively. After incubation, an equal volume of heparin (40 mg/ml in TL-HEPES) was added to facilitate the dissociation of agglutinated PMNs. Subsamples of 75 µl were fixed by adding 25 µl of 2% (v/v) glutaraldehyde. The fixed samples were mounted into glass slides and examined at × 400 magnification using a phase-contrast microscope connected to a digital camera and a computer system (Suite, Leica Microsystems, Wetzlar, Germany). At least 400 PMNs were counted in different areas of the specimens. The percentage of PMNs with phagocytized sperm was recorded as the phagocytosis rate. Quantification of the number of PMNs with phagocytized sperm was performed independently by two observers.
Superoxide generation determination
PMNs were incubated with AGP (0 or 100 ng/ml) for 4 h, suspended in TL-HEPES either with or without the treated sperm and directly used for measuring superoxide generation. Briefly, 10 µl luminol reagent (Sigma) was pipetted into a 96-well FluoroNunc plate (Nunc, Roskilde, Denmark). Next, 100 µl of PMNs incubated for 4 h (0.5 × 106 cells/ml) and treated sperm (1 × 106 cells/ml) were added. Superoxide generation was detected at 425 nm using an AB-2350 Phelios (ATTO, Tokyo, Japan).
Scanning electron microscopy (SEM)
Neutrophils either directly phagocytize sperm through cell-cell attachment or entrap them within neutrophil extracellular traps (NETs), structures consisting of neutrophil nuclear DNA and associated proteins, that act to ensnare the sperm and hinder their motility [23, 24]. We therefore used SEM to investigate the effect of AGP on NET formation by PMNs for sperm entanglement. Basically, PMNs were incubated in culture medium without any stimulation, or with AGP (100 ng/ml) for 4 h, and then a phagocytosis assay was performed by incubation of PMNs (15 × 106 cells/ml) together with capacitated sperm (30 × 106 cells/ml) for 60 min with gentle swirling on a test-plate shaker. For SEM, each sample, after phagocytosis, was placed onto cover glass coated with 0.1% neoprene in toluene, dried at room temperature, and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4). After fixation, the samples were washed in PB, postfixed in 1% osmium tetroxide in PB and dehydrated in a graded series of ethanol solutions. The specimens were then freeze-dried with t-butyl alcohol using a freeze dryer (ES-2030, Hitachi, Tokyo, Japan). Each dried sample was mounted on a specimen stub with cover glass and sputter-coated with platinum (Pt) (Ion sputter coater E-1045 ion sputter coater, Hitachi High-Technologies, Tokyo, Japan). The specimens were observed using a scanning electron microscope (Hitachi High-Technologies) at an accelerating voltage of 5 kV.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analyses were performed with StatView 5.0 (SAS Institute, Cary, NC, USA). Statistical significance between groups was determined using a one-way ANOVA followed by multiple comparison tests (Fisher’s test for three groups, and Bonferroni’s test for more than three groups). Results were considered to be statistically significant at P < 0.05.
Results
AGP mRNA expression and the AGP concentration in oviduct fluid
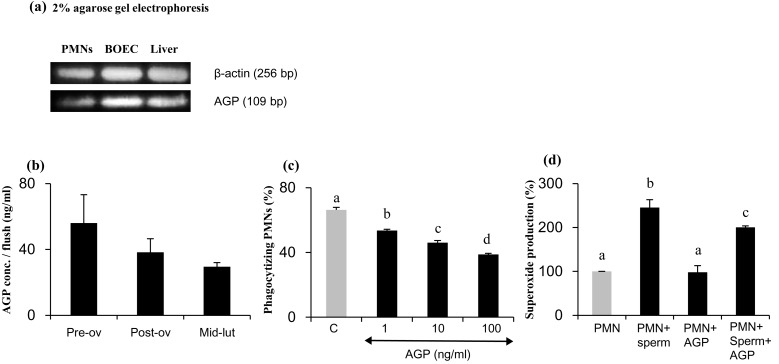
Our results demonstrate local gene expression of AGP by cultured BOECs in vitro that was well comparable to that for the liver and PMNs (Fig. 1a). Thus, the local concentrations of AGP in the oviduct fluid were determined. The AGP concentrations in bovine oviduct flushing media were not significantly changed during different stages of the estrous cycle and were ranged from 30–60 ng/ml (Fig. 1b).
Fig. 1.
(a) AGP gene expression in PMNs, BOECs, and the liver. (b) The AGP concentrations per oviduct flush during the estrous cycle. (preovulatory, n = 4, postovulatory, n = 5, and mid-luteal stage, n = 5) (c) Percentage of PMN phagocytosis for sperm treated to induce capacitation in vitro. PMNs were incubated for 4 h in culture medium supplemented with 0, 1, 10, or 100 ng/ml AGP and then subjected to a 1-h phagocytosis assay. Numerical values are presented as means ± SEM of three experiments. The different letters indicate significant differences between treatments at P < 0.05. (d) The effect of AGP on percentage of superoxide production by PMNs undergoing in vitro phagocytosis of sperm treated to induce capacitation. Numerical values are presented as means ± SEM of four experiments. The different letters indicate significant differences between the marked treatments at P < 0.05.
Dose-dependent effect of AGP on the phagocytic activity and superoxide generation of PMNs for sperm
The AGP system has immunomodulatory functions [9], and may be involved in regulation of the local immune response in the bovine oviduct. It is therefore of interest to determine the effect of AGP on PMN-mediated phagocytosis of sperm. A 4-h preexposure of PMNs to AGP (1, 10 or 100 ng/ml) resulted in a dose-dependent decrease in the PMN phagocytosis activity for capacitated sperm (Fig. 1c). Moreover, the stimulation of PMNs with 100 ng/ml AGP prior to the superoxide assay reduced superoxide production by PMNs incubated with capacitated sperm compared with unstimulated PMNs (Fig. 1d).
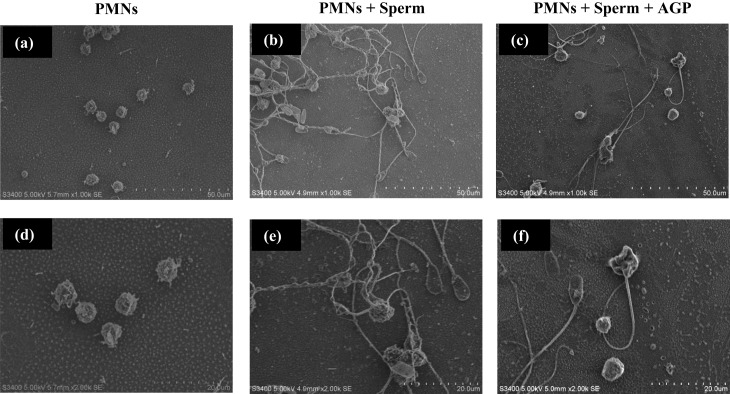
Observation of NET formation by SEM
During the phagocytosis assay, the addition of sperm to PMNs induced NET formation (Fig. 2a, b, d, e). However, the exposure of PMNs to AGP (100 ng/ml) prior to the phagocytosis assay reduced NET formation by PMNs (Fig. 2c, f).
Fig. 2.
Scanning electron microscopy of sperm phagocytosis by PMNs. The upper panels are images obtained at ×1,000 (a, b, c) and the lower panels are those obtained at ×2,000, respectively. PMNs were incubated without any stimulant (a, d), or with sperm addition to induce neutrophil extracellular traps (NETs) (b, e). NET formation was suppressed in PMNs incubated with AGP (100 ng/ml) prior to a phagocytosis assay (e, f).
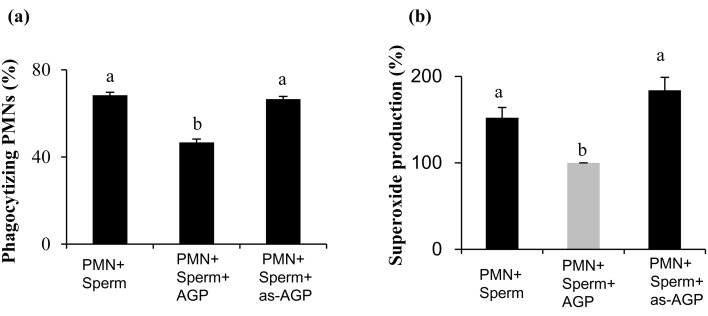
Effect of desialylated-AGP on phagocytic activity and superoxide production by PMNs for sperm
The immunomodulatory functions and binding activities of AGP have been shown to be mostly dependent on carbohydrate composition, and changes in the glycosylation of AGP can affect its biological properties [9]. A four-hour incubation of PMNs on AGP-coated plates prior to the phagocytosis assay resulted in a decrease in the phagocytic activity of PMNs for capacitated sperm (Fig. 3a). However, incubation of PMNs in the as-AGP-coated plates resulted in complete abolishment of the suppressive effect of AGP on the phagocytosis of sperm by PMNs (Fig. 3a). Moreover, the incubation of PMNs in the as-AGP-coated plates resulted in removal of the suppressive effect of AGP on the superoxide production by PMNs in response to sperm (Fig. 3b).
Fig. 3.
Effect of as-AGP on phagocytic activity and superoxide production by PMNs for sperm . PMNs were incubated for 4 h in coated plates supplemented with culture medium only, AGP, or as-AGP and then subjected to a 1-h phagocytosis assay. (a) The percentage PMNs undergoing phagocytosis of sperm treated to induce capacitation, in vitro. The different letters indicate significant differences between the marked treatments at P < 0.001. (b) The percentage of superoxide production by PMNs undergoing in vitro phagocytosis of sperm treated to induce capacitation. The different letters indicate significant differences between the marked treatments at P < 0.05. Numerical values are presented as means ± SEM of four experiments.
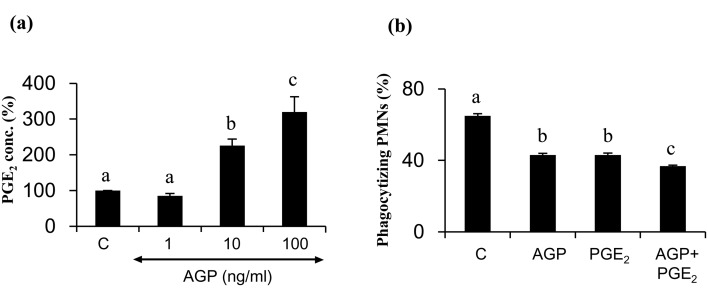
The effect of AGP on PGE2 production from cultured BOECs
Incubation of BOECs with AGP (0, 1, 10, or 100 ng/ml) for 24 hours resulted in stimulation of PGE2 production in BOECs in vitro in a dose-dependent manner (Fig. 4a, P < 0.05).
Fig. 4.
(a) The percentage of PGE2 production in BOEC culture medium supplemented with AGP (0, 1, 10 or 100 ng/ml) for 24 h (n = 9 /group, 100% = 48.1 ± 4.1 ng/ml, means ± SEM). (b) The effect of AGP (50 ng/ml) and PGE2 (10–8 M, 3.52 ng/ml) in combination, on the phagocytic activity of PMNs for sperm treated to induce capacitation. Numerical values are presented as means ± SEM of four experiments. The different letters indicate significant differences between the marked treatments at P < 0.05.
The effect of AGP and PGE2 in combination on the phagocytic activity of PMNs for sperm
A four-hour incubation of PMNs with the local concentration of AGP detected in oviduct flushing media (50 ng/ml), along with that of PGE2 (10-8 M, 3.52 ng/ml, [4]), resulted in an additive effect in suppression of the phagocytic activity of PMNs for treated sperm (Fig. 4b).
Discussion
Bovine AGP is the main acute phase protein; its concentration in the peripheral blood circulation increases from approximately 0.3 mg/ml to 0.9 mg/ml during disease [25, 26]. AGP is mainly produced by the liver, from which it diffuses into the general circulation [6]. AGP mRNA was detected in extrahepatic organs such as the lung, kidney, spleen, lymph node, uterus, ovary, placenta, and decidua [10, 27]. Additionally, AGP protein has been previously detected in sow oviduct fluid [28]. In this study, we have provided the first evidence for the local gene expression of AGP by bovine oviduct epithelial cells in vitro. The oviduct flushing media contained AGP in the range of 20–60 ng/ml, which is much lower than that seen in bovine plasma. Generally, it has been shown that AGP has immunomodulatory effects [8, 29,30,31]. This prompted us to investigate the effect of AGP on the phagocytosis of sperm by PMNs. Our results show that detectable concentrations of AGP were found in the oviduct flushing media, and AGP dose-dependently suppressed PMN phagocytosis of sperm in vitro. Additionally, SEM analysis demonstrated that AGP drastically reduced NET formation, preventing sperm from being fixed and trapped by PMNs, and thus indirectly resulted in the suppression of PMN phagocytosis for sperm.
Previously, it has been shown that AGP induced a dose-dependent inhibition of superoxide generation in PMNs stimulated by phorbol-12-myristate-13-acetate [32]. Additionally, the ability of PMNs to release superoxide has been used as an indicator for evaluating their phagocytic activity on sperm [33]. Our results show that only in the presence of sperm did AGP (100 ng/ml) significantly suppress superoxide release by PMNs. These findings suggest that the AGP secreted in bovine oviducts contributes to the protection and maintenance of sperm survival through the suppression of phagocytic activity and superoxide release by PMNs. In humans, it has been shown that leukocytes are the predominant source of superoxide production in sperm preparations and that the contribution of spermatozoa was either undetectable or was a small fraction of that contributed by leukocytes [34]. In general, PMNs form NETs for pathogen uptake depending on superoxide release through reactive oxygen species (ROS)-generating pathways [35]. NADPH decomposition results in the release of superoxide, which is converted to hydrogen peroxide (H2O2) either by superoxide dismutase (SOD) or spontaneously [35]. The generated H2O2 is then used in the formation of halogenated ROS, such as hypochlorite (OCL-) by myeloperoxidase (MPO), which induces NETs formation [35]. Therefore, we hypothesize that AGP, via the suppression of superoxide generation, could affect the ROS-generating pathways that lead to suppression of NETs formation, altering the phagocytic behavior of PMNs for sperm.
The mechanism by which AGP suppresses the phagocytosis of sperm by PMNs is still unknown. The binding and immunomodulatory activities of AGP have been shown to be mostly dependent on its carbohydrate composition [9]. Importantly, bovine AGP is one of the most heavily glycosylated proteins [5], and AGP glycosylation is modified during disease [36]. It is conceivable that the terminal sialic acid residues exposed on the surface of AGP block phagocytosis by binding phagocyte sialic acid-binding immunoglobulin-type lectins (Siglec) [37]. Therefore, we hypothesized that the sialic acid contributes to the suppressive effect of AGP on PMN phagocytosis for sperm. In fact, our results show that desialylating AGP completely abolished the AGP-suppressive effect on both PMN phagocytosis of sperm and superoxide release. These results are in agreement with previous studies showing that desialylating AGP abolished an AGP hepato-protective effect [38] and anti-apoptotic activity [39], whereas hyposialylated AGP completely inhibited the phagocytosis of E. coli by feline neutrophils [40].
We previously demonstrated that luteinizing hormone stimulates BOECs to secrete PGE2, which plays a major role in suppressing the phagocytic activity of PMNs for sperm [4]. Interestingly, our results showed that AGP dose-dependently stimulated PGE2 secretion from BOECs. Moreover, AGP and PGE2, within the physiological concentrations detected in oviduct flushing media, additively suppressed the phagocytosis of sperm by PMNs. Thus, these findings suggest that AGP not only directly suppresses sperm phagocytosis but also works cooperatively with PGE2 to suppress the phagocytosis of sperm by PMNs in the bovine oviduct.
Taken together, our findings suggest that AGP has immunomodulatory functions in the bovine oviduct. It is proposed that under physiological conditions, the local AGP system in the bovine oviduct may aid sperm survival through direct suppression of the phagocytic activity of PMNs for sperm, through reduction of superoxide production by phagocytizing PMNs, and by limiting NET formation.
Conflict of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
This study was partly supported by the Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Ellington JE. The bovine oviduct and its role in reproduction: a review of the literature. Cornell Vet 1991; 81: 313–328. [PubMed] [Google Scholar]
- 2.Hunter RH, Huang WT, Holtz W. Regional influences of the fallopian tubes on the rate of boar sperm capacitation in surgically inseminated gilts. J Reprod Fertil 1998; 114: 17–23. [DOI] [PubMed] [Google Scholar]
- 3.Rizos D, Carter F, Besenfelder U, Havlicek V, Lonergan P. Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. J Dairy Sci 2010; 93: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 4.Marey MA, Liu J, Kowsar R, Haneda S, Matsui M, Sasaki M, Takashi S, Hayakawa H, Wijayagunawardane MP, Hussein FM, Miyamoto A. Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: prostaglandin E2 as a major physiological regulator. Reproduction 2014; 147: 211–219. [DOI] [PubMed] [Google Scholar]
- 5.Nakano M, Kakehi K, Tsai MH, Lee YC. Detailed structural features of glycan chains derived from α1-acid glycoproteins of several different animals: the presence of hypersialylated, O-acetylated sialic acids but not disialyl residues. Glycobiology 2004; 14: 431–441. [DOI] [PubMed] [Google Scholar]
- 6.Ceciliani F, Pocacqua V, Provasi E, Comunian C, Bertolini A, Bronzo V, Moroni P, Sartorelli P. Identification of the bovine alpha1-acid glycoprotein in colostrum and milk. Vet Res 2005; 36: 735–746. [DOI] [PubMed] [Google Scholar]
- 7.Martìnez Cordero E, Gonzàlez MM, Aguilar LD, Orozco EH, Hernàndez Pando R. Alpha-1-acid glycoprotein, its local production and immunopathological participation in experimental pulmonary tuberculosis. Tuberculosis (Edinb) 2008; 88: 203–211. [DOI] [PubMed] [Google Scholar]
- 8.Boutten A, Dehoux M, Deschenes M, Rouzeau JD, Bories PN, Durand G. Alpha 1-acid glycoprotein potentiates lipopolysaccharide-induced secretion of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha by human monocytes and alveolar and peritoneal macrophages. Eur J Immunol 1992; 22: 2687–2695. [DOI] [PubMed] [Google Scholar]
- 9.Bories PN, Feger J, Benbernou N, Rouzeau JD, Agneray J, Durand G. Prevalence of tri- and tetraantennary glycans of human alpha 1-acid glycoprotein in release of macrophage inhibitor of interleukin-1 activity. Inflammation 1990; 14: 315–323. [DOI] [PubMed] [Google Scholar]
- 10.Lecchi C, Avallone G, Giurovich M, Roccabianca P, Ceciliani F. Extra hepatic expression of the acute phase protein alpha 1-acid glycoprotein in normal bovine tissues. Vet J 2009; 180: 256–258. [DOI] [PubMed] [Google Scholar]
- 11.Wijayagunawardane MPB, Miyamoto A, Cerbito WA, Acosta TJ, Takagi M, Sato K. Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-1 in the cyclic cow. Theriogenology 1998; 49: 607–618. [DOI] [PubMed] [Google Scholar]
- 12.Wijayagunawardane MP, Miyamoto A, Sato K. Prostaglandin E2, prostaglandin F2 alpha and endothelin-1 production by cow oviductal epithelial cell monolayers: effect of progesterone, estradiol 17 beta, oxytocin and luteinizing hormone. Theriogenology 1999; 52: 791–801. [DOI] [PubMed] [Google Scholar]
- 13.Wijayagunawardane MPB, Kodithuwakku SP, Yamamoto D, Miyamoto A. Vascular endothelial growth factor system in the cow oviduct: a possible involvement in the regulation of oviductal motility and embryo transport. Mol Reprod Dev 2005; 72: 511–520. [DOI] [PubMed] [Google Scholar]
- 14.Shirasuna K, Yamamoto D, Morota K, Shimizu T, Matsui M, Miyamoto A. Prostaglandin F 2 α stimulates endothelial nitric oxide synthase depending on the existence of bovine granulosa cells: analysis by co-culture system of endothelial cells, smooth muscle cells and granulosa cells. Reprod Domest Anim 2008; 43: 592–598. [DOI] [PubMed] [Google Scholar]
- 15.Jiemtaweeboon S, Shirasuna K, Nitta A, Kobayashi A, Schuberth HJ, Shimizu T, Miyamoto A. Evidence that polymorphonuclear neutrophils infiltrate into the developing corpus luteum and promote angiogenesis with interleukin-8 in the cow. Reprod Biol Endocrinol 2011; 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 17.Parrish JJ, Susko-Parrish JL, Handrow RR, Sims MM, First NL. Capacitation of bovine spermatozoa by oviduct fluid. Biol Reprod 1989; 40: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 18.Kovács A, Foote RH. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech Histochem 1992; 67: 119–124. [DOI] [PubMed] [Google Scholar]
- 19.Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod 1983; 28: 235–247. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie HD, Liu J, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on motility of bovine spermatozoa. Biol Reprod 2002; 67: 1811–1816. [DOI] [PubMed] [Google Scholar]
- 21.Azuma Y, Sakanashi M, Matsumoto K. The effect of alpha2,6-linked sialic acid on anti-IgM antibody-induced apoptosis in Ramos cells. Glycoconj J 2001; 18: 419–424. [DOI] [PubMed] [Google Scholar]
- 22.Matthijs A, Harkema W, Engel B, Woelders H. In vitro phagocytosis of boar spermatozoa by neutrophils from peripheral blood of sows. J Reprod Fertil 2000; 120: 265–273. [PubMed] [Google Scholar]
- 23.Alghamdi AS, Foster DN. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod 2005; 73: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 24.Alghamdi AS, Lovaas BJ, Bird SL, Lamb GC, Rendahl AK, Taube PC, Foster DN. Species-specific interaction of seminal plasma on sperm-neutrophil binding. Anim Reprod Sci 2009; 114: 331–344. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Yatsu T, Itoh H, Motoi Y. Isolation, characterization, and quantitative measurement of serum alpha 1-acid glycoprotein in cattle. Nippon Juigaku Zasshi 1989; 51: 987–994. [DOI] [PubMed] [Google Scholar]
- 26.Eckersall PD, Young FJ, McComb C, Hogarth CJ, Safi S, Weber A, McDonald T, Nolan AM, Fitzpatrick JL. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet Rec 2001; 148: 35–41. [DOI] [PubMed] [Google Scholar]
- 27.Thomas T, Fletcher S, Yeoh GC, Schreiber G. The expression of alpha(1)-acid glycoprotein mRNA during rat development. High levels of expression in the decidua. J Biol Chem 1989; 264: 5784–5790. [PubMed] [Google Scholar]
- 28.Georgiou AS, Snijders AP, Sostaric E, Aflatoonian R, Vazquez JL, Vazquez JM, Roca J, Martinez EA, Wright PC, Fazeli A. Modulation of the oviductal environment by gametes. J Proteome Res 2007; 6: 4656–4666. [DOI] [PubMed] [Google Scholar]
- 29.Costello M, Fiedel BA, Gewurz H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature 1979; 281: 677–678. [DOI] [PubMed] [Google Scholar]
- 30.Bennett M, Schmid K. Immunosuppression by human plasma alpha 1-acid glycoptrotein: importance of the carbohydrate moiety. Proc Natl Acad Sci USA 1980; 77: 6109–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med 1993; 178: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lainé E, Couderc R, Roch-Arveiller M, Vasson MP, Giroud JP, Raichvarg D. Modulation of human polymorphonuclear neutrophil functions by alpha 1-acid glycoprotein. Inflammation 1990; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- 33.Hansen PJ, Hoggard MP, Rathwell AC. Effects of stallion seminal plasma on hydrogen peroxide release by leukocytes exposed to spermatozoa and bacteria. J Reprod Immunol 1987; 10: 157–166. [DOI] [PubMed] [Google Scholar]
- 34.Whittington K, Ford WCL. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int J Androl 1999; 22: 229–235. [DOI] [PubMed] [Google Scholar]
- 35.Kirchner T, Möller S, Klinger M, Solbach W, Laskay T, Behnen M.The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat Inflamm 2012; 2012: 849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceciliani F, Pocacqua V. The acute phase protein alpha1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci 2007; 8: 91–108. [DOI] [PubMed] [Google Scholar]
- 37.Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 2012; 37: 325–332. [DOI] [PubMed] [Google Scholar]
- 38.Kagaya N, Kamiyoshi A, Tagawa Y, Akamatsu S, Isoda K, Kawase M, Yagi K. Suppression of cell death in primary rat hepatocytes by alpha1-acid glycoprotein. J Biosci Bioeng 2005; 99: 81–83. [DOI] [PubMed] [Google Scholar]
- 39.Ceciliani F, Pocacqua V, Miranda-Ribera A, Bronzo V, Lecchi C, Sartorelli P. alpha(1)-Acid glycoprotein modulates apoptosis in bovine monocytes. Vet Immunol Immunopathol 2007; 116: 145–152. [DOI] [PubMed] [Google Scholar]
- 40.Rossi G, Capitani L, Ceciliani F, Restelli L, Paltrinieri S. Hyposialylated α1-acid glycoprotein inhibits phagocytosis of feline neutrophils. Res Vet Sci 2013; 95: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]