Abstract
The aim of this study was to evaluate the effects of rapid cooling prior to freezing on frozen-thawed canine sperm quality. In experiment 1, centrifuged ejaculates from 6 dogs were pooled, split into 4 aliquots and cryopreserved by the Uppsala procedure using different cooling rates (control, cooling speed 18 C/90 min and average cooling rate 0.2 C/min; rapid, cooling speed 18 C/8 min and average cooling rate 2.25 C/min) in combination with 2 glycerol addition protocols (fractionated or unfractionated). In experiment 2, centrifuged ejaculates from 4 dogs were processed individually using the same cooling rates described in experiment 1 in combination with an unfractionated glycerol addition protocol. Each of the experiments was replicated 5 times. Sperm quality was evaluated after 30 and 150 min of post-thawing incubation at 38 C. Total motility (TM), progressive motility (PM) and quality of movement parameters were assessed using a computerized system, and sperm viability (spermatozoa with intact plasma and acrosome membranes) was assessed using flow cytometry (H-42/PI/FITC-PNA). Values for TM, PM, viable spermatozoa and the quality of movement parameters after thawing were not significantly affected by the cooling rate. The interaction between the cooling rate and the added glycerol protocol was not significant. There were significant differences among the males (P<0.01) in the sperm quality parameters evaluated after thawing. The interaction between the males and the cooling rate was not significant. In conclusion, canine spermatozoa can be cryopreserved using the Uppsala method at an average cooling rate of 2.25 C/min prior to freezing together with addition of fractionated or unfractionated glycerol.
Keywords: Canine, Cooling-rate, Cryopreservation, Spermatozoa
In recent years, canine breeding has received increased attention in small animal veterinary practice. Reproductive technologies, such as artificial insemination (AI) and semen conservation, are in high demand not only by breeders but also by owners [1]. Among these technologies, cryopreservation is especially relevant because it allows the storage of spermatozoa for unlimited periods of time. This is of great interest for dog breeding, primarily because it allows the growth of international commerce in canine semen by minimizing the costs of keeping or transporting live animals and by establishing semen banks for males with a high genetic value [2]. Due to the increasing interest in cryopreservation technology for this species in the last decade, many studies have been undertaken to optimize the different aspects of canine semen cryopreservation, including variations in extenders, cryoprotectant types and cooling and thawing rates [3,4,5,6].
It is well known that the cryopreservation procedure affects sperm quality, although sperm susceptibility differs between species and individuals, which has been associated with the composition of the lipid membranes of spermatozoa [7]. During the cryopreservation process, spermatozoa undergo different steps that involve important temperature changes, resulting in the well-known phenomenon of “cold shock” [8]. Specifically, during cryopreservation, spermatozoa are very sensitive to the rapid reduction from room temperature to 5 C [7]. Although dog spermatozoa are considered relatively resistant to cooling at 5 C [9], slow cooling rates prior to freezing are generally used for this species [5, 10,11,12,13]. For this reason, our hypothesis was that a rapid cooling rate prior freezing could be used efficiently for dog semen cryopreservation.
One of the most commonly used cryopreservation protocols for canine spermatozoa is the Uppsala method, which consists of two dilutions before freezing separated by a long cool-down step from room temperature (23 C) to 5 C over 1 to 2 h [5, 12, 14,15,16]. These equilibration periods correspond to estimated mean cooling rates of approximately 0.15–0.3 C/min. Although slow cooling rates are considered optimal, to the best to our knowledge, no studies have been performed to evaluate the effects of a rapid cooling rate and of reducing the cool-down time in the Uppsala protocol. This would allow a considerable reduction in the time required for the semen cryopreservation procedure, which is extremely important from a practical point of view. In fact, successful results have been reported using a rapid cooling rate prior to freezing in other species of mammals [17, 18].
In the Uppsala method, an extender containing 3% glycerol is usually used during the cool-down period, resulting in a fractionated glycerol addition protocol [2, 12, 16]. However, unfractionated glycerol addition at 5 C after slow cooling has also been described [12]. To establish an optimal rapid protocol for dog semen cryopreservation, we were also interested in evaluating the effects of both inclusion and exclusion of glycerol during the cool-down step in combination with a rapid cooling rate.
The aim of the present work was to evaluate the effect of a rapid cooling rate prior to freezing with the Uppsala method in combination with a fractionated or unfractionated glycerol addition protocol. For this purpose, sperm quality was assessed in frozen-thawed semen at different times during incubation after thawing.
Materials and Methods
Animals
The experimental protocols were conducted in accordance with Directive 2000/63/EU EEC for animal experiments and were reviewed and approved by the Ethics Committee for Experimentation with Animals of the University of Murcia, Spain. Six clinically healthy privately owned dogs (Canis lupus familiaris) of different breeds (1 Ibizan hound, 2 Scottish terriers, 1 French bulldog, 1 Jack Russell terrier and 1 Golden retriever) aged 2 to 5 years were used in this study. All dogs were of proven fertility and were trained semen donors, and none of them were preselected according to sperm freezability.
Semen collection
Ejaculates were collected by digital manipulation [19] into calibrated 15 ml Falcon conical tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Only the sperm-rich fraction (SRF) of the ejaculates was processed, while the first and third fractions were discarded. Only samples with a sperm concentration >200 × 106 spermatozoa/ml, a total motility > 80% and a normal morphology > 80% were included in the study.
Chemicals and media
All of the chemicals used in this study were of analytical grade and, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, MO, USA). All media were prepared under sterile conditions in a laminar flow hood (MicroH; Telstar, Terrasa, Spain) using water from a Milli-Q Synthesis System (18 MΩ cm; Milli-Q; Millipore, Billerica, MA, USA).
The extender used during washing and evaluation of the spermatozoa and as a thawing medium was TRIS-citrate-fructose (TCF; composition: 249 mM Trizma base, 80 mM citric acid, 69 mM fructose, pH 6.7; 324 ± 3 mOsm/kg) supplemented with 1 mg/ml penicillin and 1 mg/ml s treptomycin sulfate [12].
The cooling and freezing extenders used in this study were previously described for dog semen cryopreservation and were composed of TCF containing 20% egg yolk and variable glycerol concentrations with or without Equex STM Paste (Nova Chemical Sales, Scituate, MA, USA) [5, 12]. The compositions of the cooling extenders (C-Ext I and II) and the freezing extenders (F-Ext I and II) are shown in Table 1.
Table 1. Composition of extenders used for semen cooling and freezing.
| C-Ext I | C-Ext II | F-Ext I | F-Ext II | |
| Trizma base | 3.025 g | 3.025 g | 3.025 g | 3.025 g |
| Citric acid | 1.7 g | 1.7 g | 1.7 g | 1.7 g |
| Fructose | 1.25 g | 1.25 g | 1.25 g | 1.25 g |
| Streptomycin | 1 mg/ml | 1 mg/ml | 1 mg/ml | 1 mg/ml |
| Penicillin | 1 mg/ml | 1 mg/ml | 1 mg/ml | 1 mg/ml |
| Glycerol | 3% | – | 7% | 10% |
| Equex STM Paste | – | – | 1% | 1% |
| Egg yolk | 20% | 20% | 20% | 20% |
| Milli-Q water | 77 ml | 80 ml | 72 ml | 69 ml |
| pH | 6.76 | 6.72 | 6.70 | 6.76 |
| Osmolarity | 870 mOsm | 335 mOsm | 1570 mOsm | 1978 mOsm |
Cooling extenders (C-Ext I and II)/freezing extenders (F-Ext I and II).
Phosphate-buffered saline solution (PBS: 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4·7H2O, pH 6.8; 289 ± 4 mOsm/kg) was used to dilute the fluorochromes used for flow cytometric evaluation of sperm viability.
Freezing method
The semen was frozen using the Uppsala method, which consisted of 2 dilution steps before freezing [12]. Briefly, after semen collection, the SRFs were washed by centrifugation (700 g/5 min/room temperature; Megafuge 1.0 R, Heraeus, Hanau, Germany). The supernatant was removed, and the resulting sperm samples were pooled or processed individually, depending on the experiment. Samples were diluted at 23 C with a cooling extender (C-Ext I or II) to a concentration of 400 × 106 spermatozoa/ml and were then cooled to 5 C using 2 different cooling rates (see the experimental design). Once at 5 C and just prior to freezing, the samples were diluted slowly by adding an equal volume of freezing extender (F-Ext I or II) that was previously cooled to 5 C using the same cooling rate as the corresponding diluted semen samples, resulting in a final concentration of 200 × 106 spermatozoa/ml. After 10 min of equilibration with the freezing extender, the semen was packed into 0.5 ml plastic straws (Minitüb, Tiefenbach, Germany) placed horizontally on a rack situated above the surface of LN2 at a distance of 4 cm in a closed Styrofoam box for 10 min (freezing unit reference no. 15043/0636, Minitüb). Finally, the straws were plunged directly into the LN2. The semen straws were kept frozen in LN2 for at least 2 weeks before being thawed for evaluation. The straws were thawed in a water bath at 70 C for 8 sec, and the content of each straw was immediately diluted in TCF (1:2, v/v) and incubated at 38 C for 150 min.
Sperm quality assessment
The motility of the spermatozoa was evaluated objectively using a computer-assisted analysis system (ISAS; Proiser R+D, Paterna, Spain). The samples were analyzed at a concentration of 20 × 106 spermatozoa/ml. For each evaluation, an aliquot of 5 µl was placed in a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel) that was pre-warmed to 38 C. A minimum of 200 spermatozoa per sample were analyzed. Before the track sequence was analyzed, the trajectory of each spermatozoon was identified and recorded in each field, and each was assessed visually to eliminate possible debris and to decrease the risk of including unclear tracks in the analysis. The sperm motility variable recorded was the overall percentage of the total motility (TM) and the progressive motility (spermatozoa moved fast and progressively; PM) in all samples. Spermatozoa with an average path velocity (VAP) ≥ 20 µm/s were considered motile, and spermatozoa with 75% linear movement were designated as spermatozoa with PM, in accordance with the parameters provided by the manufacturer. In the frozen-thawed semen samples, the following quality of movement parameters were measured: curvilinear velocity (VCL; µm/s), straight line velocity (VSL, µm/s), linear coefficient (LIN %) and amplitude of the lateral head displacement (ALH, µm).
Sperm viability was evaluated by simultaneous cytometric assessment of the plasma and acrosomal membrane’s integrity using a triple-fluorescence procedure, as described previously by Ródenas et al.[20] and modified slightly. Briefly, 2 × 106 spermatozoa were transferred to tubes containing 50 µl of TFC-Ext, 4 µl of H-42 (0.05 mg/ml in PBS), 1 µl of propidium iodide (PI, 0.5 mg/ml in PBS, Invitrogen; Molecular Probes, Eugene, OR, USA) and 2 µl of fluorescein-conjugated peanut agglutinin (PNA-FITC, 20 µg/ml in PBS). The samples were incubated at 38 C in the dark for 10 min, and immediately prior to analysis, 400 µl of TCF was added to each sample. The fluorescence spectra of H-42, PI and PNA-FITC were detected using a 450/50 nm band-pass (BP) filter, a 670 nm long-pass (LP) filter and a 530/30 nm BP filter, respectively. The flow cytometry analyses were performed at room temperature under dimmed light using a BD FACSCanto II flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) equipped with three lasers as the excitation sources: blue (488 nm, aircooled, 20 mW solid state), red (633 nm, 17 mW HeNe) and violet (405 nm, 30 mW solidstate). Data were acquired using BD FACSDiva Software (Becton, Dickinson and Company). Non-spermatozoon events were gated out based on their H-42 fluorescence (DNA content), and acquisitions were stopped after 10000 H-42-positive events. Only the results corresponding to viable spermatozoa (intact plasma and acrosomal membranes; PI−/PNA-FITC−) were included in the results.
Experimental design
This study was divided into 2 experiments. Each experiment was replicated 5 times.
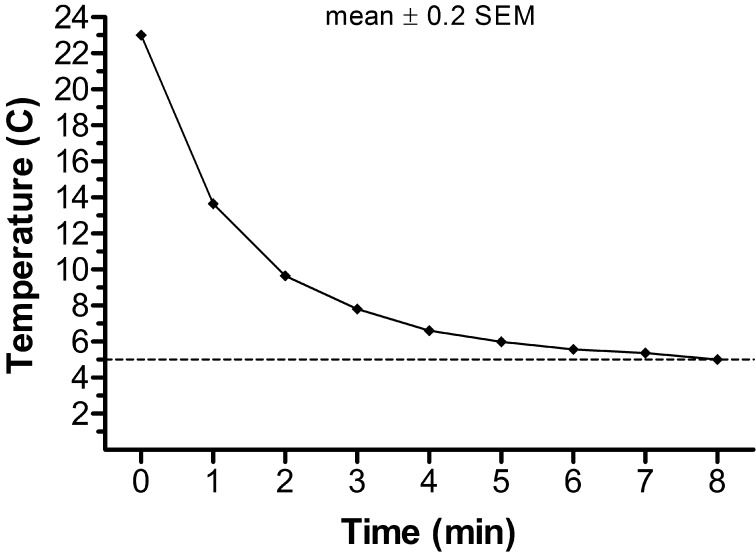
In experiment 1, a total of 30 SRFs from 6 dogs (5 per dog) were collected and centrifuged. The resulting samples from each dog were pooled and divided into 4 aliquots, which were frozen by the Uppsala method, as described above, using 2 different cooling rates (control 0.2 C/min or rapid 2.25 C/min) and 2 glycerol addition protocols (fractionated or unfractionated) in a 2 × 2 factorial design. The rapid cooling rate (2.25 C/min) was predetermined via a preliminary study performed in our laboratory, whereas the control (0.2 C/min) cooling rate was estimated based on previously published studies of dog semen cryopreservation [5, 12]. The 2.25 C/min cooling rate was achieved by plunging the tube of extended semen into a 250 ml glass beaker containing 200 ml of water at 4 C (Fig. 1), while a programmable temperature bath (Programmable Model 9612; PolyScience, Niles, IL, USA) was used to perform the control rate of cooling. The temperature of the samples and the water during the cooling procedure was recorded. For addition of the fractionated glycerol, the semen samples were diluted in C-Ext I at 23 C for the cooling step and in F-Ext I at 5 C prior to freezing. For addition of the unfractionated glycerol, the semen samples were diluted in C-Ext II at 23 C for the cooling step and in F-Ext II at 5 C prior to freezing.
Fig 1.
Temperature changes recorded for the semen samples during the cooling procedure at a mean rate of 2.25 C/min. Values are the means ± SEM of ten measurements. The values of SEMs were not included because they were too small and it would not be possible to present error bars in a visual manner.
Sperm quality in terms of sperm motility and viability were assessed in fresh samples (SRFs after centrifugation at 23 C) and at 30 and 150 min of incubation at 38 C after sperm thawing. A total of 60 frozen-thawed straws (3 straws per each of the 4 treatments and replicate) were analyzed in experiment 1.
In experiment 2, a total of 20 SRFs from 4 dogs (5 per dog) were collected and frozen individually using the same cooling rates described in experiment 1 (control 0.2 C/min and rapid 2.25 C/min) in combination with an unfractionated glycerol protocol. The dogs included in this experiment were selected from those described in experiment 1 based on the best fresh sperm quality parameters. Sperm quality was assessed as described in experiment 1. A total of 30 frozen-thawed straws were analyzed for each male in experiment 2 (3 straw per each of the 2 treatments and replicate).
Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows, Version 19.0 (IBM, Armonk, NY, USA). The data were evaluated using the Kolmogorov-Smirnov test to check the assumption of normality and assessed using an ANOVA with a MIXED procedure. The ANOVA model included the fixed effects of the cooling rates, the glycerol addition protocols and their interactions in experiment 1; the male, the cooling rate and their interaction in experiment 2; and the random effects of replication in each experiment for the different incubation times after thawing. When the ANOVA revealed a significant effect, the values were compared using the Bonferroni test. The values reported are expressed as the least square means (LSM) ± the standard error of the mean (SEM), and statistical significance was considered at P<0.05.
Results
Experiment 1
The mean concentration, volume and total number of spermatozoa in the pool were 670 ± 97 × 106 spermatozoa/ml, 5.0 ± 0.5 ml and 3370 ± 297 × 106 spermatozoa, respectively. The sperm quality parameters in the fresh pooled sperm samples were 87.6 ± 1.8%, 67.6 ± 2.3% and 89.3 ± 1.3% for TM, PM and viable spermatozoa, respectively.
The mean percentages of the TM, PM and viable spermatozoa evaluated at 30 and 150 min after thawing are shown in Tables 2 and 3, respectively. Within the same glycerol addition protocol, use of the rapid or control cooling rate prior to freezing did not significantly affect the percentages of TM, PM and viable spermatozoa at either 30 or 150 min after thawing. The glycerol addition protocol significantly affected the values of the TM, PM and viable spermatozoa (P<0.05) at 30 min and the viable spermatozoa (P<0.05) at 150 min of incubation after thawing in samples frozen using the control cooling rate. The interaction of the cooling rate and the glycerol addition protocol was not significant.
Table 2. Mean percentages of total motility, progressive motility and viability in frozen-thawed semen samples at 30 min after thawing (experiment 1).
| Cooling rate | Glycerol addition protocol | Total motility (%) |
Progressive motility (%) |
Sperm viability (%) |
| Control | Fractionated | 61.5a | 44.9a | 64.5a |
| Unfractionated | 66.4b | 50.5b | 67.7b | |
| Rapid | Fractionated | 62.7ab | 46.0ab | 65.4ab |
| Unfractionated | 64.7ab | 46.0ab | 66.3ab | |
| SEM | 2.4 | 2.0 | 1.6 | |
| Probability | Cooling rate | NS | NS | NS |
| Glycerol addition protocol | 0.003 | 0.025 | 0.000 | |
| Interaction | NS | NS | NS | |
Data are the LSM ± SEM of five replicates per semen pool. a–b Different letters within the same column indicate significant differences (P<0.05) among the glycerol addition protocols.
Table 3. Mean percentages of total motility, progressive motility and viability in frozen-thawed semen samples at 150 min after thawing (experiment 1).
| Cooling rate | Glycerol addition protocol | Total motility (%) |
Progressive motility (%) |
Sperm viability (%) |
| Control | Fractionated | 53.8ab | 39.3ab | 62.1a |
| Unfractionated | 57.8b | 42.6b | 66.5b | |
| Rapid | Fractionated | 52.2a | 37.4a | 61.7a |
| Unfractionated | 56.5ab | 40.4ab | 64.1ab | |
| SEM | 2.2 | 2.2 | 2.7 | |
| Probability | Cooling rate | NS | NS | NS |
| Glycerol addition protocol | 0.001 | 0.013 | 0.000 | |
| Interaction | NS | NS | NS | |
Data are the LSM ± SEM of five replicates per semen pool. a–b Different letters within the same column indicates significant differences (P<0.05) among the glycerol addition protocols.
The quality of movement parameters was not significantly influenced by the cooling rates or by the glycerol addition protocol at any post-thawing evaluation time. The average values of the different experimental groups for VCL, VSL, LIN and ALH ranged from 133.4 to 138.9 µm/sec, from 88.9 to 95.0 µm/sec, from 63.9 to 67.5% and from 4.1 to 4.3 µm for 30 min and from 138.5 to 142.0 to µm/sec, from 82.2 to 86.5 µm/sec, from 60.0 to 62.0% and from 4.7 to 4.9 µm for 150 min, respectively.
Experiment 2
The mean sperm concentration, volume and total number of spermatozoa of the different males ranged from 350 to 1200 × 106 spermatozoa/ml, from 1.0–3.5 ml and 450 to 1320 × 106 spermatozoa, respectively. The individual mean values for sperm quality in the fresh semen ranged from 87 to 92.8%, from 60 to 72% and from 84.5 to 95.1% for TM, PM and viable spermatozoa, respectively.
The individual values for TM, PM and sperm viability in the frozen samples evaluated at 30 and 150 min after thawing are shown in Tables 4 and 5, respectively. There were significant differences among the males (P<0.01) for TM, PM and sperm viability at 30 min after thawing and in sperm viability at 150 min after thawing. There were no significant differences in the percentages of TM, PM and viable spermatozoa among the samples that were frozen using different cooling rates at either 30 or 150 min after thawing. The interaction of the male and the cooling rate was not significant.
Table 4. Individual mean percentages of total motility, progressive motility and viability at 30 min after thawing in frozen-thawed semen samples frozen using different cooling rates and an unfractionated glycerol addition protocol (experiment 2).
| Male | Cooling rate | Total motility (%) |
Progressive motility (%) |
Sperm viability (%) |
| 1 | Control | 71.4ab | 57.7a | 73.2a |
| Rapid | 74.0a | 56.3a | 73.8a | |
| 2 | Control | 64.4abc | 47.8ab | 71.0a |
| Rapid | 60.7bc | 45.5ab | 68.2ab | |
| 3 | Control | 60.1bc | 49.9ab | 60.5b |
| Rapid | 63.7abc | 53.7ab | 61.0b | |
| 4 | Control | 58.7c | 46.5ab | 65.3b |
| Rapid | 60.9bc | 43.9b | 65.5b | |
| SEM | 3.7 | 4.4 | 2.3 | |
| Probability | Male | 0.001 | 0.000 | 0.000 |
| Cooling rate | NS | NS | NS | |
| Interaction | NS | NS | NS | |
Data are the LSM ± SEM of five replicates per male. a–c Different letters within the same column indicates significant differences (P<0.05) among the males.
Table 5. Individual mean percentages of total motility, progressive motility and viability at 150 min after thawing in frozen-thawed semen samples frozen using different cooling rates and a non-fractionated glycerol addition protocol (experiment 2).
| Male | Cooling rate | Total motility (%) |
Progressive motility (%) |
Sperm viability (%) |
| 1 | Control | 45.6 | 34.9 | 69.3a |
| Rapid | 44.8 | 30.9 | 68.9a | |
| 2 | Control | 46.2 | 36.9 | 68.9a |
| Rapid | 40.2 | 28.9 | 66.9ab | |
| 3 | Control | 47.8 | 41.3 | 55.1bc |
| Rapid | 42.5 | 35.5 | 58.1b | |
| 4 | Control | 47.4 | 40.2 | 58.9bc |
| Rapid | 42.8 | 34.5 | 58.7bc | |
| SEM | 4.1 | 4.2 | 2.3 | |
| Probability | Male | NS | NS | 0.000 |
| Cooling rate | NS | NS | NS | |
| Interaction | NS | NS | NS | |
Data are the LSM ± SEM of five replicates per male. a–c Different letters within the same column indicates significant differences (P<0.05) among the males.
The quality of movement parameters was not significantly influenced by the cooling rate at either 30 or 150 min. There were significant differences among the males (P<0.05), and the interaction of the male and the cooling rate was not significant. Values in the different males for VCL, VSL, LIN and ALH ranged from 146.3 to 161.4 µm/sec, from 88.9 to 101.5 µm/sec, from 56.1 to 66.2% and from 4.9 to 5.9 µm for 30 min and from 115 to 155.4 µm/sec, from 67.2 to 86.3 µm/sec, from 54 to 61.1% and from 4.8 to 5.8 µm for 150 min, respectively.
Discussion
To optimize the canine semen cryopreservation protocol by reducing the time necessary for this procedure, we evaluated the effect of a rapid cooling rate protocol on the quality of frozen-thawed spermatozoa. To the best to our knowledge, the present work provides the first evidence that dog spermatozoa are capable of surviving rapid cooling rates (2.25 C/min) before freezing with the Uppsala method. While in the traditional Uppsala protocol, semen reaches 5 C in approximately 90 min, this rapid cooling protocol would support a considerable reduction in the time required for the process of freezing dog spermatozoa, as it allows an interval of approximately 8 min between 23 and 5 C.
It has been reported that the spermatozoa of several species require a long period of cooling before freezing to develop maximal resistance to the effects of freezing [21] and to reduce the well-known “cold shock” process [8]. In dogs, there is a shortage of studies regarding the periods used for cooling to 5 C [22, 23]. To minimize the cold shock process, the majority of the protocols for dog semen cryopreservation include long cooling periods from 23 C to 5 C. The most frequently employed period is from 60 to 120 min, corresponding to approximate mean cooling rates within the range of 0.15–0.3 C/min [5, 13,14,15,16, 24]. Our results clearly demonstrated that the use of rapid cooling at a rate of 2.25 C/min prior to freezing provides the same post-thaw sperm quality, in terms of motility and viability, as the values obtained with the use of a control rate of 0.2 C/min. The results for the sperm quality after thawing, in terms of the motility and viability in all of the experimental groups were relatively high, adequate for artificial insemination and comparable with those reported recently in other studies performed in frozen-thawed canine semen using the Uppsala method [25,26,27]. In addition, we did not observe differences among the groups in the quality of the movement parameters VCL, LIN and ALH, which have been described as indicators of a hyperactivated motility pattern and a pre-capacitation status [28]. Therefore, we suggest that use of the rapid cooling rate did not induce a hyperactivation sperm motility pattern after thawing in comparison with the control cooling rate. Regardless of the experimental group, there was a decrease in sperm quality as a result of incubation after thawing at 38 C, which was especially evident in the percentages of total and progressive motility. Our results are in agreement with those described by other authors [9] and indicate that frozen semen should be used as quickly as possible after thawing.
The positive results obtained using the rapid cooling rate prior to freezing in this study are in agreement with other reports on other species of mammals, such as the boar and red deer [17, 18], showing that dog spermatozoa might be particularly resistant to cold shock during the cooling (to 5 C) process. This resistance of sperm cells to thermal stress may be attributed to the constituents of the plasmatic membrane of canine spermatozoa, which has a low ratio of polyunsaturated to saturated phospholipid fatty acids [7, 22]. Moreover, it should also be considered that the egg yolk included in the extenders for sperm cryopreservation is responsible for membrane stabilization during the cool-down period [17, 29, 30], reducing the damage that results from the cold shock process. The results of the present study suggested that the presence of 20% egg yolk in the sperm’s surrounding media is also adequate when a rapid cooling rate prior to freezing is used.
Our results regarding the glycerol addition protocol suggest that a protocol for addition of fractionated or unfractionated glycerol is adequate when canine semen is frozen by the Uppsala method using a rapid cooling rate of 2.25 C/min. Based in our attempt to optimize the procedure as much as possible, we believe that a protocol for addition of unfractionated glycerol at 5 C may be more practical because it is only necessary to prepare an extender containing glycerol. Although we observed significant differences in sperm quality after thawing among the glycerol addition protocols in the samples frozen using the control cooling rate, from a practical point of view, these differences were not biologically and clinically important, being approximately 4–5%.
In clinical practice, the ejaculates from different males are cryopreserved individually, and differences in freezability have been reported among individual dogs [30,31,32]. With this in mind and in an effort to adapt the rapid cryopreservation procedure for a practical application in dog breeding, a second experiment was performed. Based on the results of the first experiment, the feasibility of use of a rapid cooling rate in different males was evaluated in combination with an unfractionated glycerol addition protocol. In this regard, our results suggest that the rapid protocol is also adequate for individual applications because the different dogs evaluated in this study did not exhibit different responses to the rapid cooling rate of 2.25 C/min prior to freezing. Regardless of the cooling rate used, all of the dogs included in our study showed adequate percentages of sperm motility and viability in the frozen-thawed semen to be used for AI.
As expected and regardless of the cooling rate used, significant differences among individuals in sperm cryosurvival were found in sperm motility and viability at 30 min after thawing. However, these differences in sperm motility were not observed after 150 min of incubation at 38 C. In some dogs, a difference in thermoresistance and a higher decline in sperm motility around the time of incubation after thawing were observed, most likely due to the variations in the metabolic exhaustion of the spermatozoa. One explanation for this fact may be related to the finding that dog ejaculates, as in other species, maintain a constant structure of different motile sperm subpopulations during cold storage [33], after freezing and after thawing [15, 34]. These subpopulations, which are not distributed uniformly among male dogs [35], include spermatozoa that differ in their physiological states, functional integrity and quality of sperm kinematics [34].
In conclusion, the results of this study suggest that dog spermatozoa could be cryopreserved using a rapid cooling rate of 2.25 C/min prior to freezing in combination with the Uppsala method, obtaining values of sperm quality comparable with those obtained by using a control cooling rate. Thus, use of this rapid cooling rate allows a considerable reduction in the time spent on the procedure. Moreover, the glycerol addition procedure does not affect the feasibility of a rapid protocol.
Acknowledgments
This study was supported by the University of Murcia (R-549/2009) and the Seneca Foundation of Murcia (GERM04543/07).
References
- 1.Thomassen R, Farstad W. Artificial insemination in canids: a useful tool in breeding and conservation. Theriogenology 2009; 71: 190–199. [DOI] [PubMed] [Google Scholar]
- 2.Peña FJ, Núñez-Martínez I, Morán JM. Semen technologies in dog breeding: an update. Reprod Domest Anim 2006; 41(Suppl 2): 21–29. [DOI] [PubMed] [Google Scholar]
- 3.Okano T, Murase T, Asano M, Tsubota T. Effects of final dilution rate, sperm concentration and times for cooling and glycerol equilibration on post-thaw characteristics of canine spermatozoa. J Vet Med Sci 2004; 66: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 4.Nöthling JO, Shuttleworth R. The effect of straw size, freezing rate and thawing rate upon post-thaw quality of dog semen. Theriogenology 2005; 63: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 5.Schäfer-Somi S, Kluger S, Knapp E, Klein D, Aurich C. Effects of semen extender and semen processing on motility and viability of frozen-thawed dog spermatozoa. Theriogenology 2006; 66: 173–182. [DOI] [PubMed] [Google Scholar]
- 6.Bencharif D, Amirat-Briand L, Garand A, Anton M, Schmitt E, Desherces S, Delhomme G, Langlois ML, Barrière P, Destrumelle S, Vera-Munoz O, Tainturier D. Freezing canine sperm: comparison of semen extenders containing Equex and LDL (Low Density Lipoproteins). Anim Reprod Sci 2010; 119: 305–313. [DOI] [PubMed] [Google Scholar]
- 7.White IG. Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reprod Fertil Dev 1993; 5: 639–658. [DOI] [PubMed] [Google Scholar]
- 8.Watson PF. The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci 2000; 60-61: 481–492. [DOI] [PubMed] [Google Scholar]
- 9.Batista M, Santana M, Alamo D, González F, Niño T, Cabrera F, Gracia A. Effects of incubation temperature and semen pooling on the viability of fresh, chilled and freeze-thawed canine semen samples. Reprod Domest Anim 2012; 47: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 10.Seager SW, Fletcher WS. Progress on the use of frozen semen in the dog. Vet Rec 1973; 92: 6–10. [DOI] [PubMed] [Google Scholar]
- 11.Eilts BE. Theoretical aspects of canine semen cryopreservation. Theriogenology 2005; 64: 692–697. [DOI] [PubMed] [Google Scholar]
- 12.Hermansson U, Linde Forsberg C. Freezing of stored, chilled dog spermatozoa. Theriogenology 2006; 65: 584–593. [DOI] [PubMed] [Google Scholar]
- 13.Futino DO, Mendes MCB, Matos WNL, Mondadori RG, Lucci CM. Glycerol, methyl-formamide and dimethyl-formamide in canine semen cryopreservation. Reprod Domest Anim 2010; 45: 214–220. [DOI] [PubMed] [Google Scholar]
- 14.Peña A, Linde-Forsberg C. Effects of Equex, one- or two-step dilution, and two freezing and thawing rates on post-thaw survival of dog spermatozoa. Theriogenology 2000; 54: 859–875. [DOI] [PubMed] [Google Scholar]
- 15.Peña AI, Barrio M, Becerra JJ, Quintela LA, Herradón PG. Motile sperm subpopulations in frozen-thawed dog semen: changes after incubation in capacitating conditions and relationship with sperm survival after osmotic stress. Anim Reprod Sci 2012; 133: 214–223. [DOI] [PubMed] [Google Scholar]
- 16.Neagu VR, García BM, Sandoval CS, Rodríguez AM, Ferrusola CO, Fernández LG, Tapia JA, Peña FJ. Freezing dog semen in presence of the antioxidant butylated hydroxytoluene improves postthaw sperm membrane integrity. Theriogenology 2010; 73: 645–650. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Santos MR, Esteso MC, Montoro V, Soler AJ, Garde JJ. Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: effects of egg yolk, glycerol and cooling rate. Theriogenology 2006; 66: 1931–1942. [DOI] [PubMed] [Google Scholar]
- 18.Juarez JD, Parrilla I, Vazquez JM, Martínez EA, Roca J. Boar semen can tolerate rapid cooling rates prior to freezing. Reprod Fertil Dev 2011; 23: 681–690. [DOI] [PubMed] [Google Scholar]
- 19.Kutzler MA. Semen collection in the dog. Theriogenology 2005; 64: 747–754. [DOI] [PubMed] [Google Scholar]
- 20.Rodenas C, Lucas X, Tarantini T, Del Olmo D, Roca J, Vazquez JM, Martínez EA, Parrilla I. The effects of hoechst 33342 staining and the male sample donor on the sorting efficiency of canine spermatozoa. Reprod Domest Anim 2014; 49: 115–121. [DOI] [PubMed] [Google Scholar]
- 21.England GC. Cryopreservation of dog semen: a review. J Reprod Fertil Suppl 1993; 47: 243–255. [PubMed] [Google Scholar]
- 22.Bouchard GF, Morris JK, Sikes JD, Youngquist RS. Effect of storage temperature, cooling rates and two different semen extenders on canine spermatozoal motility. Theriogenology 1990; 34: 147–157. [DOI] [PubMed] [Google Scholar]
- 23.du Bois S, Len JA, Parlevliet JM, Eilts BE. Effects of cooling time on membrane integrity and motility of frozen-thawed canine spermatozoa using two different commercial egg yolk-based extenders at two different cooldown equilibration times. Reprod Domest Anim 2012; 47(Suppl 6): 278–280. [DOI] [PubMed] [Google Scholar]
- 24.Nöthling JO, Dolieslager SMJ, Fillekes R, Colenbrander B. Thawing dog spermatozoa in just-boiled water: submersion time and effect on sperm quality compared to thawing in water at 70 degrees C. Theriogenology 2007; 68: 530–537. [DOI] [PubMed] [Google Scholar]
- 25.Rota A, Milani C, Cabianca G, Martini M. Comparison between glycerol and ethylene glycol for dog semen cryopreservation. Theriogenology 2006; 65: 1848–1858. [DOI] [PubMed] [Google Scholar]
- 26.Martins-Bessa A, Rocha A, Mayenco-Aguirre A. Comparing ethylene glycol with glycerol for cryopreservation of canine semen in egg-yolk TRIS extenders. Theriogenology 2006; 66: 2047–2055. [DOI] [PubMed] [Google Scholar]
- 27.Santana M, Batista M, Alamo D, González F, Niño T, Cabrera F, Gracia A. Influence of cool storage before freezing on the quality of frozen-thawed semen samples in dogs. Reprod Domest Anim 2013; 48: 165–170. [DOI] [PubMed] [Google Scholar]
- 28.Rijsselaere T, Van Soom A, Maes D, Nizanski W. Computer-assisted sperm analysis in dogs and cats: an update after 20 years. Reprod Domest Anim 2012; 47(Suppl 6): 204–207. [DOI] [PubMed] [Google Scholar]
- 29.Medeiros CMO, Forell F, Oliveira ATD, Rodrigues JL. Current status of sperm cryopreservation: why isn’t it better? Theriogenology 2002; 57: 327–344. [DOI] [PubMed] [Google Scholar]
- 30.Farstad W. Cryopreservation of canine semen - new challenges. Reprod Domest Anim 2009; 44(Suppl 2): 336–341. [DOI] [PubMed] [Google Scholar]
- 31.Yu I, Songsasen N, Godke RA, Leibo SP. Differences among dogs in response of their spermatozoa to cryopreservation using various cooling and warming rates. Cryobiology 2002; 44: 62–78. [DOI] [PubMed] [Google Scholar]
- 32.Batista M, Alamo D, González F, Cruz MG, Gracia A. Influence of the freezing technique (nitrogen liquid vs ultrafreezer of -152 degrees C) and male-to-male variation over the semen quality in Canarian Mastiff breed dogs. Reprod Domest Anim 2006; 41: 423–428. [DOI] [PubMed] [Google Scholar]
- 33.Dorado J, Gálvez MJ, Murabito MR, Muñoz-Serrano A, Hidalgo M. Identification of sperm subpopulations in canine ejaculates: effects of cold storage and egg yolk concentration. Anim Reprod Sci 2011; 127: 106–113. [DOI] [PubMed] [Google Scholar]
- 34.Dorado J, Alcaráz L, Duarte N, Portero JM, Acha D, Hidalgo M. Changes in the structures of motile sperm subpopulations in dog spermatozoa after both cryopreservation and centrifugation on PureSperm(®) gradient. Anim Reprod Sci 2011; 125: 211–218. [DOI] [PubMed] [Google Scholar]
- 35.Núñez-Martínez I, Moran JM, Peña FJ. A three-step statistical procedure to identify sperm kinematic subpopulations in canine ejaculates: changes after cryopreservation. Reprod Domest Anim 2006; 41: 408–415. [DOI] [PubMed] [Google Scholar]