To the Editor:
Heterozygous gain-of-function mutations in signal transducer and activator of transcription 1 (STAT1) have recently been identified as a cause of chronic mucocutaneous candidiasis (CMC). Uzel et al1 described “STAT1 gain-of-function mutations in patients with FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked syndrome.” They briefly mentioned the presence of poor enamel in 1 patient and structural and functional gastrointestinal defects in another patient. Here we present a patient with CMC associated with dental anomalies, diaphragmatic hernia, and esophageal dysmotility in whom the phenotype led to a broad differential diagnosis ranging from severe combined immunodeficiency inspired by the neonatal onset of infections to humoral immune deficiency, immune dysregulation–polyendocrinopathy–enteropathy–X-linked syndrome, nuclear factor κB essential modulator (NEMO) deficiency, Shwachman-Diamond syndrome, autosomal dominant hyper-IgE syndrome, and, finally, CMC caused by gain-of-function mutation in STAT1. We stress the early onset of respiratory tract infections, as well as the dental and gastrointestinal defects, in this patient.
The patient was born at term small for gestational age (−2 SD) as the third son of unrelated parents. He presented with recurrent lower respiratory tract infections from birth, intractable diarrhea, failure to thrive, seborrheic dermatitis, and CMC. Small-bowel biopsy showed villous atrophy interpreted as celiac-like disease, yet diarrhea was unresponsive to a gluten-free diet. Primary dentition showed enamel defects. Recurrent lower respiratory tract infections with Haemophilus influenzae and Streptococcus pneumoniae led to the development of bronchiectasis. Partial IgG2 deficiency (0.34 g/L; normal range, 0.72-3.4 g/L) was found at 3 years of age. Anti-tetanus antibody levels were protective (1.2 mg/L; protective level, >1 mg/L), suggesting an intact anti-protein antibody response. Intravenous immunoglobulin (IVIG) substitution was initiated. Diaphragmatic hernia, gastroesophageal reflux disease, and disturbed esophageal motility were demonstrated and led to a Nissen fundoplication.
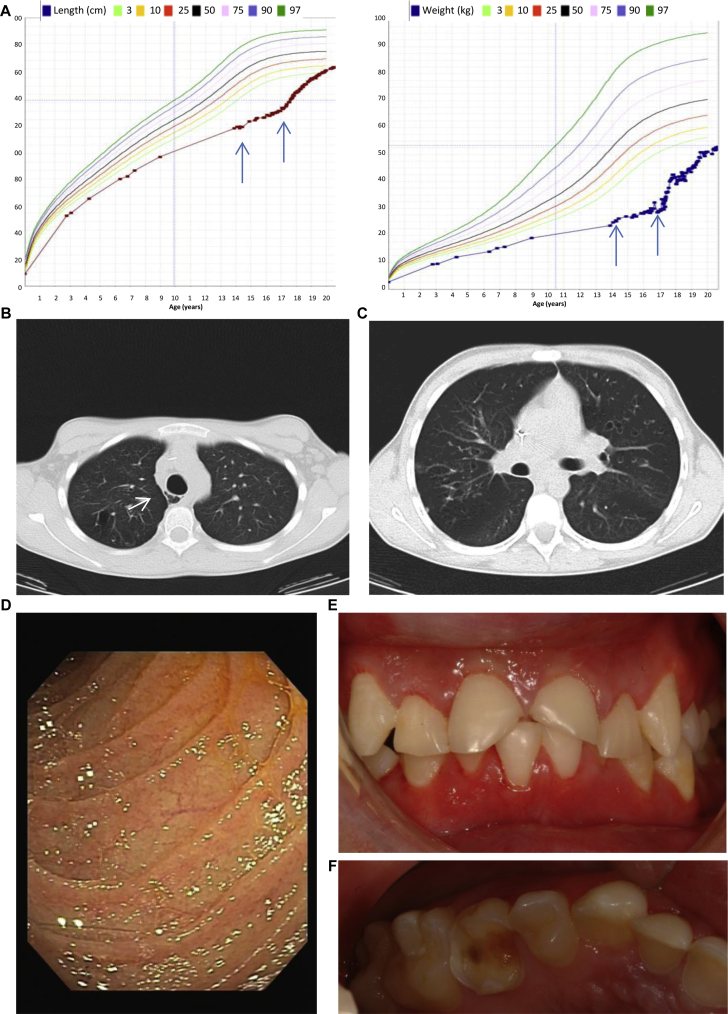
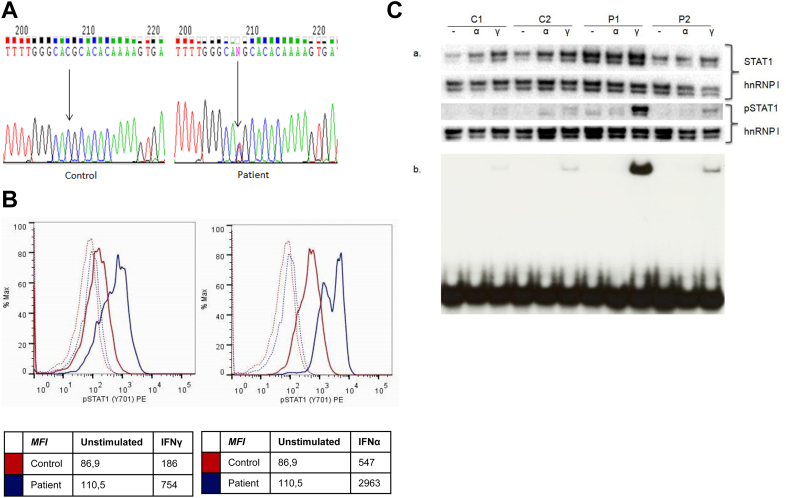
At 13 years of age, the patient presented at the immunology clinic with severe growth retardation (Fig 1, A), pubertal delay, bronchiectasis, atonic esophagus with multiple diverticula (Fig 1, B and C), atrophic duodenal mucosa (Fig 1, D) corresponding to villous blunting or atrophy, joint hyperlaxity, osteopenia with recurrent fractures, delayed dental development with retention of primary teeth necessitating dental extractions (Fig 1, E and F), severe erosive tooth wear suggestive of enamel hypoplasia (Fig 1, E and F), seborrheic dermatitis, aphthous stomatitis, and CMC (see Table E1 in this article's Online Repository at www.jacionline.org). The autoimmune regulator (AIRE) and caspase recruitment domain family, member 9 (CARD9), genes were sequenced, but no mutations were found. After withdrawal of IVIG, antibody response to unconjugated pneumococcal vaccine was tested and showed protective titers for 9 of 14 serotypes tested (see Table E2 in this article's Online Repository at www.jacionline.org).2 Increased IgG levels (21 g/L; normal range, 5.76-12.65 g/L) and persistent partial IgG2 deficiency were noted (0.80 g/L; normal range, 1.06-6.10 g/L). An extended autoantibody screening panel was performed, including thyroid-related, adrenal gland–related, and anti–IFN-α and anti–IFN-ω antibodies. Only anti–salivary gland antibodies were demonstrated (for the entire panel, see the Methods section in this article's Online Repository at www.jacionline.org). Immunophenotyping showed a low percentage of switched memory B cells (1.3%; normal range, 5% to 10%; see Table E3 in this article's Online Repository at www.jacionline.org). Because withdrawal of IVIG was associated with an increased incidence of pneumonia, treatment was optimized by restarting IVIG and initiating azithromycin (both for antibacterial prophylaxis and its anti-inflammatory actions) and fluconazole prophylaxis, as well as overnight tube feeding. Growth hormone therapy and puberty induction led to a correction of the growth deficit (Fig 1, A). Finally, at the age of 18 years, hypothyroidism was diagnosed almost simultaneously with the initial reports on STAT1 coiled-coil domain gain-of-function mutations.3, 4 A mutation in the DNA-binding domain of STAT1 was detected (c.1154C>T, p.T385M; Fig 2, A; for information on the analysis, see the Results section in this article's Online Repository at www.jacionline.org). The mutation was not found in the parents or the 2 male siblings of the index patient. As described previously, T385M is a gain-of-function mutation.1, 5, 6, 7 Likewise, we showed increased STAT1 phosphorylation in response to IFN-α and IFN-γ in the patient compared with control values (Fig 2, B). Also, the electrophoretic mobility shift assay (EMSA) showed increased gamma-activated sequence (GAS) binding activity on stimulation with IFN-γ (Fig 2, C).
Fig 1.
Clinical characteristics. A, Growth charts showing severe growth retardation that only picks up after growth hormone therapy and puberty induction with tube feeding overnight. Arrows indicate onset of tube feeding and growth hormone therapy, respectively. B, Computed tomography showing an atonic esophagus (arrow) with air containing paraesophageal diverticula. C, Computed tomography of the chest showing multiple saccular bronchiectases and bronchial wall thickening. D, Atrophy in the duodenum, as seen on endoscopy. E and F, Severe erosive tooth wear, caries, and retained primary teeth.
Fig 2.
The mutant T385M STAT1 allele is a gain-of-phosphorylation and gain-of-function mutation. A, Direct sequence analysis of exon 14 of STAT1 (forward sequence) in a control subject and the patient with a c.1153C>T resulting in p.T385M. B, Intracellular staining of phosphorylated tyrosine 701 STAT1 (STAT1p) in lymphocytes after stimulation with IFN-γ (2000 IU/mL, left panel) or IFN-α (105 IU/mL, right panel) for 15 minutes. STAT1 and STATp are shown in a control subject (red) and in the T385M patient (blue). Unstimulated conditions are represented as dashed lines. Results shown are representative of 2 independent experiments. MFI, Mean fluorescence intensity. C, Evaluation of STAT1, STAT1 phosphorylation, and STAT1p GAS DNA-binding capacity. Fibroblasts derived from wild-type (WT)/WT control subjects (C1 and C2), p.T385M/WT (patient P1), and p.K388E/WT (patient P2) were stimulated with 100 U/mL IFN-α (α) or 100 U/mL IFN-γ (Υ) or left unstimulated (−) for 60 minutes. a, Western blotting was carried out for detection of STAT1 and STAT1p levels in nuclear extracts (5 μg per sample). Heterogeneous nuclear ribonucleoprotein I (hnRNP I) was used as a loading control reference. b, STAT1 GAS DNA-binding capacity was evaluated by using EMSA. One microgram of nuclear extract was preincubated with 20,000 cpm of GAS probe at room temperature before nondenaturing PAGE separating free from STAT-bound probe.
The mechanism that leads to gain of function in the T385M mutation is not entirely clear. Takezaki et al5 suggested an impaired dephosphorylation of STAT1. However, it is also possible that there is impaired dissociation from the DNA or a problem with the reciprocal association of the DNA-binding domain with the coiled-coil domain. After diagnosis, extended immunophenotyping of PBMCs was performed and showed absence of TH17 cells, as described by Liu et al.4
The dental anomalies in the patient were impressive. Both primary and permanent teeth showed rapid loss of tooth substance, with severe caries and erosive tooth wear reminiscent of the dental anomalies encountered in patients with Shwachman-Diamond syndrome or Ora1/Stromal interaction molecule 1 deficiency. Moreover, deciduous teeth had to be extracted because of delayed shedding. The latter feature resembles autosomal dominant hyper-IgE syndrome.
Several hypotheses to explain the dental anomalies were put forward. First, antibiotic and antimycotic therapy and acidic hypercaloric nutrition were blamed. Second, malabsorption of calcium and vitamin D was investigated. Third, in the context of recurrent aphthous stomatitis, a sicca syndrome was suspected. Although salivary flow was low, treatment with oral saliva analogues did not improve the dental condition. There were no biochemical or clinical signs of hypothyroidism until age 18 years in our patient, excluding this as a cause for the delayed shedding of deciduous teeth. Although a role for STAT1 signaling has been demonstrated in amelogenesis and dentinogenesis in rats, further research is needed to investigate the potential causal relationship between STAT1 gain-of-function mutation and abnormal dental development.8, 9
Aside from the persistent villous blunting, diaphragmatic hernia and esophageal dysmotility are remarkable gastrointestinal features. On computed tomographic (CT) scanning, as well as endoscopy, the esophagus appeared wide open and atonic, with multiple diverticula present. Thus defects in the development of the upper gastrointestinal tract seem to be a noteworthy feature of this syndrome, as hypothesized by Uzel et al.1 Whether the gastrointestinal manifestations are all secondary to CMC or a primary manifestation of disturbed STAT1 signaling is yet to be determined.1, 6
In conclusion, we report extensive dental anomalies, as well as diaphragmatic hernia and esophageal dysmotility, in a patient with early onset of lower respiratory tract infections in the context of a gain-of-function mutation in the DNA-binding domain of STAT1. These features add to the complexity of the phenotype observed in patients with a gain-of-function mutation in STAT1.
Acknowledgments
We thank the patient and his family for their confidence. We also thank the entire paramedical and medical staff of UZ Leuven, as well as Dr De Koster, for their dedicated care. Finally, we thank Mrs R. Bollen and H. De Bruyn for excellent technical assistance.
Footnotes
Supported by a research grant from the Fonds Wetenschappelijk Onderzoek - Vlaanderen and by a research grant from the Research Council of the Catholic University of Leuven. X.B. is a senior clinical investigator of the Fonds Wetenschappelijk Onderzoek - Vlaanderen. I.M. is funded by a K.O.F. grant of the Katholieke Universiteit Leuven and by a grant of the Jeffrey Modell Foundation. L.M., H. S., and L.V.E. are funded by a research grant of the Fonds Wetenschappelijk Onderzoek - Vlaanderen. X.B., I.M., A.L., and G.F. are funded by a G.O.A. grant of the Katholieke Universiteit Leuven. A.L. is funded by a European Research Council grant (IMMUNO).
Disclosure of potential conflict of interest: G. Frans has received research support from the Catholic University of Leuven. L. Moens, H. Schaballie, and E. Vermeulen have received research support from Fonds Wetenschappelijk Onderzoek - Vlaanderen. L. Van Eyck has received research support from Fonds Wetenschappelijk Onderzoek - Vlaanderen and is employed by University Hospital Leuven. J. Dooley and A. Listons have received research support from the European Research Council. B. Grimbacher has received research support from the German Federal Ministry of Education and Research, the European Union, and Helmholtz; is employed by University College London and University Medical Center Freiburg; and has received payment for lectures from CSL, Baxter, and Biotest. M. Renard is employed by University Hospital Leuven. K. De Boeck serves on boards for Vertex, Aptalis, and Pharmaxix and has consultant arrangements with Ablynx, Galapagos, Gilead, and PTC. I. Meyts has received financial support from the Jeffrey Modell Foundation. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Measurement of salivary flow rate
Salivary flow rate was measured through standardized collection of nonstimulated (resting) and stimulated (paraffin-chewing) saliva over a time span of 5 minutes.
Autoantibody analysis
The autoantibody panel analyzed consisted of anti–nuclear antigen, anti–neutrophil cytoplasmic, anti-thyroglobulin, anti–thyroid peroxidase, anti–thyrotropin receptor, anti–parietal cell, anti–intrinsic factor, anti–smooth muscle, anti-mitochondrial, anti-pancreatic, anti-insulin, anti-GAD65kDA, anti–liver kidney microsome, anti–proteinase 3, anti-myeloperoxidase, and anti–IFN-α and anti–IFN-ω antibodies. The latter analysis was performed by Anette S. Boe Wolff and Husebye Eystein, Bergen, Norway, because of the diagnostic value in patients with autoimmune polyendocrine syndrome type 1.E1
Genetic analysis
Genomic DNA was extracted from EDTA blood by using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). The UM13F/R-tagged primers (Life Technologies, Ghent, Belgium) used for genotyping STAT1 hotspots (gene ID 6772, ENSG00000115415) were designed with Oligo7 software (OLIGO Primer Analysis Software Version 7, assembled in 2009 by Wojciech Rychlik, Molecular Biology Insights, Cascade, Colo; http://www.oligo.net/) and were as follows, respectively: AGTACAATAAAGTAAACATTCTGC (F1) and CTAAATCTGATTCTCCCACTT (R1); AGTCACACCCTGAAGAAAACGATG (F2) and CTGCAAAATTTTTCTTCCCAA (R2); AGTCTAAAGTCTTTGGAAGTTGCT (F3) and CTGGCCTGGGTTATCAAGGAA (R3); AGTTCTTCTTTATATATTTACTGG (F4) and GGTGGCTATAATTTTTCCTCT (R4); AGTTGAGAATGAAATGATATTTGC (F5) and GTGTTTATGTGGTTAGCCAGT (R5); AGTCTTCTGGACTGTTTCTCATAG (F6) and ATCATCTGAATTAACGGTAAA (R6); AGTCCTCAACCTTAATGGAAATGC (F7) and CTCAAAAGCACCCTATATAAC (R7); AGTAAACGTTAATAGGGAATTGGC (F8) and CCTAGGGAGGCAAACTTCCAC (R8); and AGTCTCTTATAATTTTGTTAAAGC (F9) and CCTACCAGGTGCCGAAATTCA (R9).
PCR samples were prepared by using AccuPrime SuperMix II (Life Technologies, Belgium) and run on a Veriti Thermal Cycler (Applied Biosystems, Foster City, Calif). Purified PCR products (PCR purification kit, Qiagen) were sequenced by BaseClear (Leiden, The Netherlands) and analyzed with Chromas 2.33 Software.
Analysis of phosphorylated STAT1 by using flow cytometry
Peripheral blood was left unstimulated or stimulated with IFN-γ (2000 IU/mL) or IFN-α (10*5 IU/mL) for 15 minutes. Cells were lysed (Lyse/Fix Buffer; BD Phosflow; BD Biosciences, San Jose, Calif) for 10 minutes at 37°C and washed with 1 mL of Stain Buffer (BD PharMingen, San Jose, Calif). Thereafter, the cells were permeabilized (Perm Buffer III [BD Phosflow]) for 30 minutes on ice. Cells were washed, resuspended in 100 μL of stain buffer, and stained with phycoerythrin-labeled antibody specific for phosphorylated STAT1 (pY701; BD Biosciences). Phosphorylated STAT1 was evaluated in the lymphocyte gate. Analysis was performed on a FACSCanto II instrument (BD Biosciences).
Western blot analysis
Nuclear extracts of patient- or control subject–derived fibroblast (obtained from skin biopsy) cultures (unstimulated or stimulated with IFN-α [100 U/mL] or IFN-γ [100 U/mL] for 1 hour) were prepared, as described previously.E2 Nuclear protein lysates (5 μg per sample) were subjected to SDS-PAGE separation on 4-12% Bis-Tris Plus gels (Life Technologies, Carlsbad, Calif), and proteins were transferred to a polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, United Kingdom) and immunoblotted with primary antibodies (STAT1 p84/p91 [M-22, sc-592; Santa Cruz Biotechnology, Dallas, Tex], phospho-STAT1 [Tyr701, sc-7988, Santa Cruz Biotechnology], and loading control heterogeneous nuclear ribonucleoprotein I [3H7, sc-73391, Santa Cruz Biotechnology]) and detected with horseradish peroxidase–conjugated secondary antibody (sc-2317 and sc-2005, Santa Cruz Biotechnology). All Western blot images were captured and quantified with a ChemiDoc MP imager and Image Lab software (Bio-Rad Laboratories, Hercules, Calif) after adding Pierce ECL Western blotting substrate (Thermo Scientific, Waltham, Mass). Relative phosphorylated STAT1 expression is normalized to the respective value for heterogeneous nuclear ribonucleoprotein I expression, and the results are described as fold increases relative to the baseline level in the unstimulated condition of the sample.
EMSA
EMSA was performed, as described previously.E2 Probe (20,000 cpm) was added to 1 μg of nuclear extract in 50 ng/μL dI-dC, 1 mmol/L dithiothreitol, and 0.05% Triton. EMSA results with nonstimulated extracts or extracts stimulated with IFN-α or IFN-γ were compared. The GAS probe (5′-TCGAACATTTCCCGTAAATCATG-3′, Chapgier et alE3) was labeled by a fill-in reaction with the Klenow fragment. EMSA results with nonstimulated extracts (dimethyl sulfoxide vehicle treated) or extracts stimulated with IFN-α (100 U/mL) or IFN-γ (100 U/mL) were compared in 2 control subjects (C1 and C2), the T385M patient (P1), and a patient with CMC with a K388E mutation in the DNA-binding domain (P2). Intensities of GAS-binding activity were determined by using ImageJ software.E4
Stimulation of PBMCs and measurement of cytokines
PBMCs were prepared from heparinized peripheral blood by means of centrifugation through Ficoll (Lymphoprep, Axis-shield, Oslo, Norway). PBMCs were seeded at 2 × 106 cells/mL in a 96-well culture plate and stimulated with heat-inactivated Candida albicans (2.64 × 103 colony-forming units [CFU]/mL), Staphylococcus aureus (108 CFU/mL; InvivoGen, San Diego, Calif), or PHA (0.18 mg/mL). IFN-γ levels were measured in the supernatant after 48 hours of incubation at 37°C (5% CO2) and IL-17A levels were measured after 120 hours of incubation at 37°C (5% CO2) by means of ELISA (commercial kits by BD Biosciences and BioLegend [San Diego, Calif], respectively). For the production of heat-inactivated C albicans, reference strain ATCC 90028 was used. This strain was cultured on Sabouraud agar slants at 37°C. Yeast colonies were resuspended in RPMI agar and checked microscopically for the absence of hyphae. This yeast stock suspension was adjusted to a concentration of 0.4 × 107 CFU/mL. C albicans was subsequently heat killed by exposure to a 60°C water bath for 1.5 hours. The sterility of this suspension was checked by subculturing 50 μL of the yeast suspension on blood agar at 37°C.
Immune response to Pneumovax
Antibodies to pneumococcal polysaccharides were measured by using a mulitplex bead assay.E5
Extended peripheral blood immunophenotying panel
PBMC immunophenotyping was performed, as described previously.E6 In brief, PBMCs were isolated from heparinized blood of the patient and 10 age-matched control subjects (mean age, 22.7 years [SD, 4.62 years]) by using lymphocyte separation medium (MP Biomedicals, Santa Ana, Calif) and frozen in 10% dimethyl sulfoxide (Sigma, St Louis, Mo). Thawed cells were stained with eBioscience (San Diego, Calif) antibodies against CD3 (SK7), CD4 (RPA-T4), and IL-17 (eBio64DEC17). For cytokine staining, T cells were stimulated ex vivo for 5 hours in 50 ng/mL phorbol 12-myristate 13-acetate (Sigma) and 500 ng/mL ionomycin (Sigma) in the presence of GolgiStop (BD Biosciences) before staining. Before intracellular staining, cells were first surface stained as described, fixed, and permeabilized with Cytofix/Cytoperm (BD) for IL-17 stainings. Data were acquired on BD FACSCanto II and analyzed with FlowJo software (Tree Star, Ashland, Ore).
Results
Salivary flow rate
The nonstimulated salivary flow rate in the index patient was less than 0.2 mL/5 minutes; the stimulated flow rate was 3.1 mL/5 minutes.
T385M is a gain-of-function mutation
On stimulation with IFN-α and IFN-γ, higher levels of STAT1 phosphorylation were observed by means of flow cytometry in the patient compared with those seen in a control subject (Fig 2, B). Next, we studied STAT1-binding activity to a GAS oligonucleotide probe by using EMSA. First, we studied the STAT1 phophorylation in skin-derived fibroblasts using Western blotting. We demonstrated an increased expression of total STAT1 in both the T385M patient (P1) and a second patient with CMC caused by a novel mutation in the DNA-binding domain (K388E; P2), although less pronounced than that in P2, compared with that seen in 2 healthy control subjects. Expression of phosphorylated STAT1 was increased in the nuclear extracts of both the T385M and K388E patients, although again less pronounced in the latter, after stimulation for 1 hour with IFN-γ. The phosphorylation of STAT1 in P1 and P2 was 7 and 2.7 times more intense than in C2 (Fig 2, C). On stimulation with IFN-γ, an increase in GAS-binding activity was detected in the T385M patient (P1; Fig 2, C), as well as in the K388E patient (P2). The intensity of STAT1-binding activity to a GAS probe was quantified to be 16.8 and 2.4 times upregulated in P1 and P2, respectively, compared with the intensity in C2, which was set to 1 in ImageJ software.
PBMCs of the patient do not induce IL-17
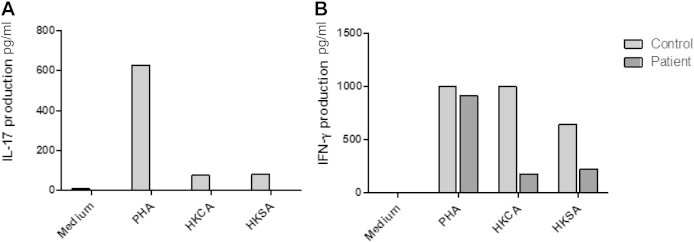
PBMCs of the patient do not induce IL-17 after stimulation with heat-inactivated C albicans, S aureus, or PHA. IL-17 in the supernatant was undetectable after stimulation with the above-mentioned stimuli (Fig E1, A). IFN-γ production after stimulation with PHA, heat-inactivated C albicans, and S aureus in the patient and a control subject are also shown (Fig E1, B).
Extended immunophenotyping at age 20 years
Using extended immunophenotyping, we showed that the patient has no detectable TH17 cells.
Fig E1.
A, IL-17 production in PBMCs of the T385M patient and a healthy control subject after stimulation with PHA, heat-inactivated C albicans, or heat-inactivated S aureus. Results shown are derived from one representative experiment of 4 independent experiments. B, IFN-γ production in PBMCs of the T385M patient and a healthy control subject after stimulation with PHA, heat-inactivated C albicans, or heat-inactivated S aureus. Results shown are derived from one representative experiment of 4 independent experiments. HKCA, Heat-killed C albicans; HKSA, heat-killed S aureus.
Table E1.
Clinical characteristics
| Present age | 20 y |
|---|---|
| Ethnicity | White |
| Initial presentation | At birth |
| CMC | Oral, esophageal, skin |
| Teeth | Retained primary dentition, severe caries and erosive tooth wear |
| Skin | Severe seborrheic dermatitis |
| Pulmonary infections | From birth |
| Haemophilus influenzae, Streptococcus pneumoniae, Pseudomonas aeruginosa | |
| Aspergillus fumigatus | |
| Bronchiectasis at age 3 y, chronic obstructive pulmonary disease | |
| Other infections | Molluscum contagiosum, Pseudomonas folliculitis, herpes zoster (2×) |
| Cardiovascular | − |
| Central nervous system | − |
| Endocrine | At birth: small for gestational age |
| Growth retardation | |
| Delayed puberty | |
| Hypothyroidism (antibodies −) at age 18 y | |
| Gastrointestinal | Oral aphthous ulcers, recurrent ulcerative gastritis, esophagitis |
| Duodenal atrophic mucosa, villous blunting | |
| Diaphragmatic hernia | |
| Bone | Osteopenia, multiple fractures |
| Other | Cystic fibrosis and primary ciliary dyskinesia were excluded. |
Table E2.
Specific anti-pneumococcal antibody concentrations (in milligrams per miter) in the patient 3 weeks after vaccination with unconjugated pneumococcal vaccine
| PS1 | PS3 | PS4 | PS8 | PS9N | PS12F | PS14 | PS19F | PS23F | PS6B | PS7F | PS18C | PS19A | PS9V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 0.28 | 0.37 | 2.33 | 0.20 | 1.31 | 0.38 | 6.37 | 2.71 | 5.44 | 4.11 | 1.35 | 2.52 | 0.91 | 2.81 |
| Cutoff | 0.53 | 0.64 | 0.49 | 1.02 | 0.63 | 0.46 | 0.57 | 0.74 | 0.24 | 0.80 | 1.45 | 0.34 | 0.96 | 0.69 |
The postvaccination serotype-specific fifth percentile (Cutoff) values obtained in 75 healthy subjects are provided as well. PS, Pneumococcal serotype.
Table E3.
Immunologic characteristics
| Age 1 y | Age 3 y | Age 13 y | Age 20 y∗ | |
|---|---|---|---|---|
| Lymphocyte subsets | ||||
| CD19+ B cells | 622/μL (82-476/μL) | |||
| Naive CD19+CD27− cells | 94% of CD19+ (60-80) | |||
| IgM memory CD27+IgM+ cells | 1.3% of CD19+ (1-5) | |||
| Switched memory CD27+IgM− cells | 1% of CD19+ (>5) | |||
| CD3+CD4+T cells | 886/μL (455-1885 μL) | |||
| CD3+CD8+ T cells | 459/μL (219-1124/μL) | |||
| Naive CD4+ cells | 75% of CD3 (30% to 65%) | |||
| TH17 cells | TH17 cells 0% (0.03-0.67) | |||
| Lymphocyte proliferation | ||||
| PHA | Normal | Normal | ||
| Concanavalin A | Normal | Normal | ||
| Tetanus | Normal | Normal | ||
| Candida | Normal | Normal | ||
| Immunoglobulin levels (g/L) | ||||
| IgG | 16.7 (4.8-11.3) | 21.4 (5.8-12.7) | 22.8 (7.51-15.60) | |
| IgG2 | 0.34 (0.72-3.40) | 0.80 (1.06-6.10) | 1.8 (1.5-6.4) | |
| IgA | 0.78 (0.35-1.9) | 1 (0.81-2.32) | 0.28 (0.82-4.53) | |
| IgM | 1.11 (0.34-1.34) | 1.2 (0.30-1.59) | 0.72 (0.43-3.04) | |
Normal values are shown in parentheses.
During IVIG treatment.
References
- 1.Uzel G., Sampaio E.P., Lawrence M.G., Hsu A.P., Hackett M., Dorsey M.J. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131:1611–1623. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgers H., Moens L., Picard C., Jeurissen A., Raes M., Sauer K. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.van de Veerdonck F.L., Plantinga T.S., Hoischen A., Smeekens S.P., Joosten L.A.B., Gilissen C. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–56. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 4.Liu L., Okada S., Kong X., Kreins A., Cypowyj S., Abhynkar A. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takezaki S., Yamada M., Kato M., Park M., Maruyama K., Yamazaki Y. Chronic mucocutaneous candidiasis caused by a gain of function mutation in the STAT1 DNA binding domain. J Immunol. 2012;189:1521–1526. doi: 10.4049/jimmunol.1200926. [DOI] [PubMed] [Google Scholar]
- 6.Soltesz B., Toth B., Shabahova N., Bondarenko A., Okada S., Cypowyj S. New and recurrent gain-of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis from Eastern and Central Europe. J Med Genet. 2013;50:567–578. doi: 10.1136/jmedgenet-2013-101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampaio E.P., Hsu A.P., Pechacek J., Bax H.I., Dias D.L., Paulson M.L. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;131:1624–1634. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsuji W., Tanase S., Yoshida S., Bawden J.W. The immunohistochemical localization of the interferon-gamma and granulocyte colony stimulating factor receptors during early amelogenesis in rat molars. Arch Oral Biol. 1999;44:173–181. doi: 10.1016/s0003-9969(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 9.Tanase S., Bawden J.W. The immunohistochemical localization of signal-transduction pathway components Jak1, Jak2, Jak3, Tyk2 and STAT1 during early enamel and dentine formation in rat molars. Arch Oral Biol. 1996;41:925–940. doi: 10.1016/s0003-9969(96)00048-9. [DOI] [PubMed] [Google Scholar]
References
- Oftedal B.E., Wolff A.S., Bratland E., Kämpe O., Perheentupa J., Myhre A.G. Radioimmunoassays for autoantibodies against interferon omega: its use in the diagnosis of autoimmune polyendocrine syndrome type I. Clin Immunol. 2008;129:163–169. doi: 10.1016/j.clim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Denayer S., Helsen C., Thorrez L., Haelens A., Claessens F. The rules of DNA recognition by the androgen receptor. Mol Endocrinol. 2010;24:898–913. doi: 10.1210/me.2009-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapgier A., Boisson-Dupuis S., Jouanguy E., Vogt G., Feinberg J., Prochnicka-Chalufour A. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2006;2:e131. doi: 10.1371/journal.pgen.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda: US National Institutes of Health. Available at: imagej.nih.gov/ij/.
- Borgers H., Moens L., Picard C., Jeurissen A., Raes M., Sauer K. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Danso-Abeam D., Zhang J., Dooley J., Staats K.A., Van Eyck L., Van Brussel T. Olmsted syndrome: exploration of the immunological phenotype. Orphanet J Rare Dis. 2013;8:79. doi: 10.1186/1750-1172-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]