Abstract
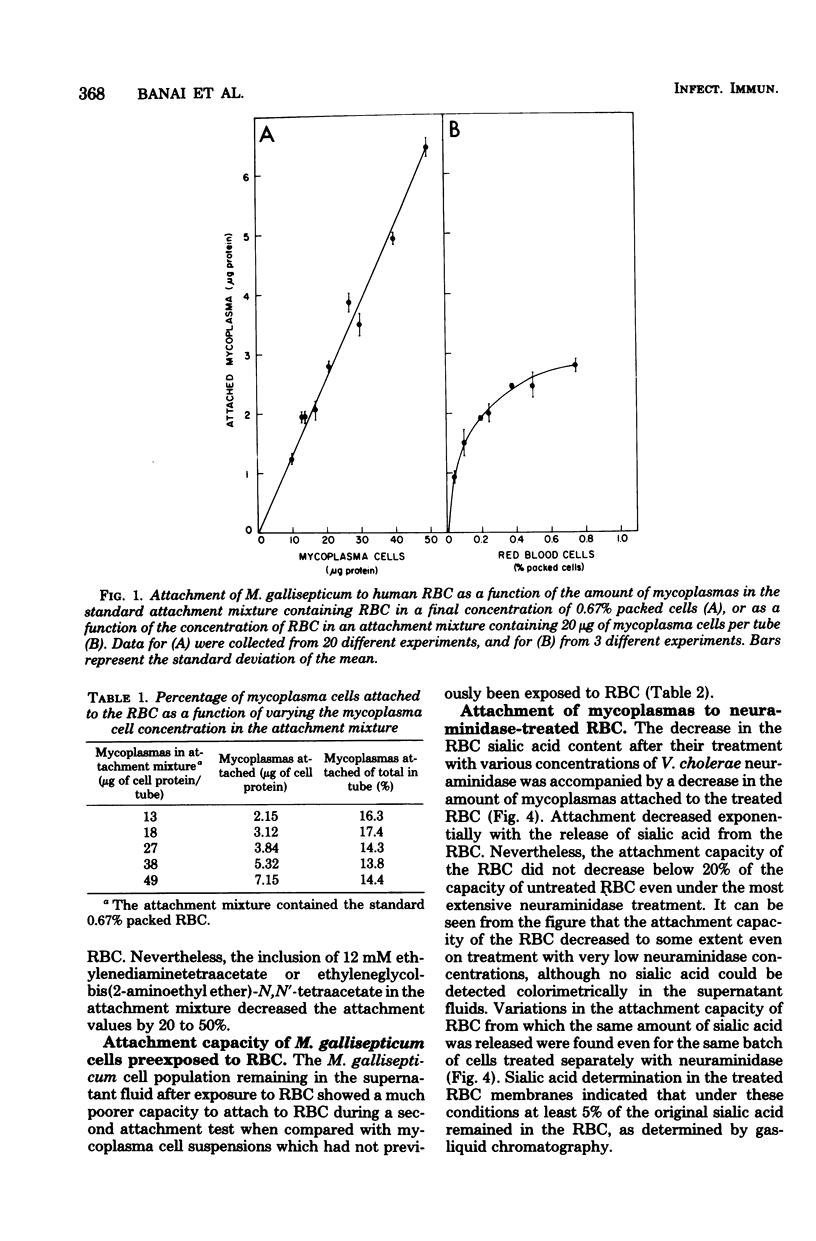
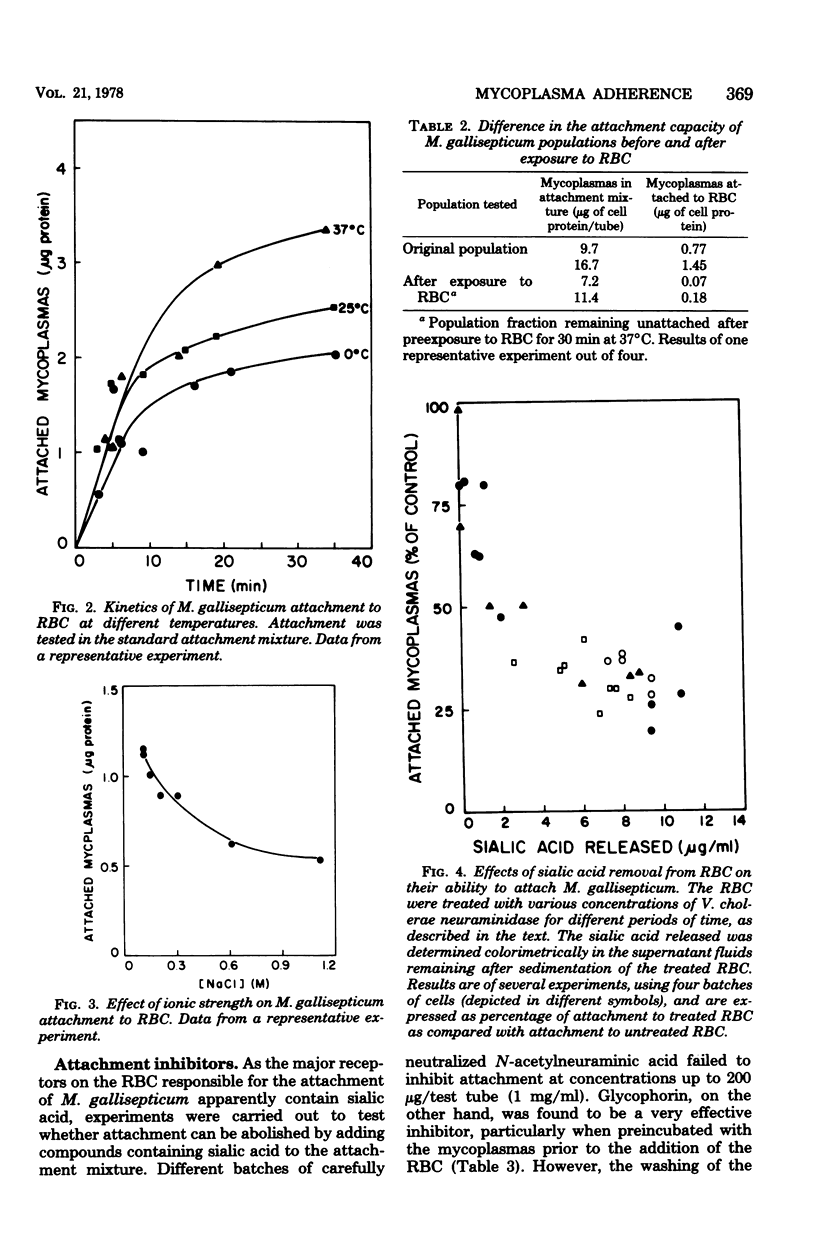
Pathogenic mycoplasmas adhere to and colonize the epithelial lining of the respiratory and genital tracts of infected animals. An experimental system suitable for the quantitative study of mycoplasma adherence has been developed by us. The system consists of human erythrocytes (RBC) and the avian pathogen Mycoplasma gallisepticum, in which membrane lipids were labeled. The amount of mycoplasma cells attached to the RBC, which was determined according to radioactivity measurements, decreased on increasing the pH or ionic strength of the attachment mixture. Attachment followed first-order kinetics and depended on temperature. The mycoplasma cell population remaining in the supernatant fluid after exposure to RBC showed a much poorer ability to attach to RBC during a second attachment test, indicating an unequal distribution of binding sites among cells within a given population. The gradual removal of sialic acid residues from the RBC by neuraminidase was accompanied by a decrease in mycoplasma attachment. Isolated glycophorin, the RBC membrane glycoprotein carrying almost all the sialic acid moieties of the RBC, inhibited M. gallisepticum attachment, whereas asialoglycophorin and sialic acid itself were very poor inhibitors of attachment. Only part of the 125I-labeled glycophorin bound to mycoplasmas could be removed by neuraminidase or by exchange with unlabeled glycophorin. It is suggested that glycophorin, representing the isolated major RBC receptor for M. gallisepticum, binds to the mycoplasmas both specifically, through its sialic acid moieties, and nonspecifically, through its exposed hydrophobic polypeptide moiety.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R. Analysis of glycoproteins. Biochem Soc Symp. 1974;(40):3–16. [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt J. A., Gabridge M. G. Effect of squamous metaplasia on infection of hamster trachea organ cultures with Mycoplasma pneumoniae. Infect Immun. 1977 Feb;15(2):647–655. doi: 10.1128/iai.15.2.647-655.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Barden-Stahl Y. D., Polisky R. B., Engelhardt J. A. Differences in the attachment of Mycoplasma pneumoniae cells and membranes to tracheal epithelium. Infect Immun. 1977 Jun;16(3):766–772. doi: 10.1128/iai.16.3.766-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N., Collins J. Serological comparison and haemagglutinating activity of Mycoplasma dispar. J Hyg (Lond) 1974 Dec;73(3):457–466. doi: 10.1017/s0022172400042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Furthmayr H., Marchesi V. T. Isolation of membrane glycoproteins by affinity chromatography in the presence of detergents. Biochim Biophys Acta. 1976 Mar 19;426(3):464–476. doi: 10.1016/0005-2736(76)90391-6. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Cholesterol-phosphatidylcholine dispersions as donors of cholesterol to Mycoplasma membranes. Biochim Biophys Acta. 1977 Nov 15;471(1):32–38. doi: 10.1016/0005-2736(77)90390-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Adherence of mycoplasmas to glass and plastic. J Bacteriol. 1967 Nov;94(5):1781–1782. doi: 10.1128/jb.94.5.1781-1782.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Spermadsorption and spermagglutination by mycoplasmas. Nature. 1967 Jul 29;215(5100):484–487. doi: 10.1038/215484a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]


