Abstract
Aim:
To evaluate the biochemical features and activities of a glyco-engineered form of the anti-human epidermal growth factor receptor monoclonal antibody (EGFR mAb) cetuximab in vitro.
Methods:
The genes encoding the Chinese hamster bisecting glycosylation enzyme (GnTIII) and anti-human EGFR mAb were cloned and coexpressed in CHO DG44 cells. The bisecting-glycosylated recombinant EGFR mAb (bisec-EGFR mAb) produced by these cells was characterized with regard to its glycan profile, antiproliferative activity, Fc receptor binding affinity and cell lysis capability. The content of galactose-α-1,3-galactose (α-Gal) in the bisec-EGFR mAb was measured using HPAEC-PAD.
Results:
The bisec-EGFR mAb had a higher content of bisecting N-acetylglucosamine residues. Compared to the wild type EGFR mAb, the bisec-EGFR mAb exhibited 3-fold higher cell lysis capability in the antibody-dependent cellular cytotoxicity assay, and 1.36-fold higher antiproliferative activity against the human epidermoid carcinoma line A431. Furthermore, the bisec-EGFR mAb had a higher binding affinity for human FcγRIa and FcγRIIIa-158F than the wild type EGFR mAb. Moreover, α-Gal, which was responsible for cetuximab-induced hypersensitivity reactions, was not detected in the bisec-EGFR mAb.
Conclusion:
The glyco-engineered EGFR mAb with more bisecting modifications and lower α-Gal content than the approved therapeutic antibody Erbitux shows improved functionality in vitro, and requires in vivo validations.
Keywords: EGFR mAb; Erbitux; glyco-engineering; antibody-dependent cellular cytotoxicity; antiproliferation; Fc receptor binding; galactose-α-1,3-galactose
Introduction
More than 300 monoclonal antibodies are in clinical trials for the treatment of human malignancies and inflammation1,2. The clinical success of this drug class is demonstrated by the large number of therapeutic antibodies that have been brought to market and the increasing number of therapeutic antibodies in development. However, improving the efficacy of therapeutic antibodies and decreasing their side effects remain challenges1.
Glycosylation is one of the most important post-translational protein modifications and has essential roles in therapeutic antibody function, including effector function, immunogenicity, half-life in serum and other aspects of bioactivity3,4. Some glycoforms of human antibodies exhibit stronger therapeutic effects than other glycoforms, and some glycoforms possess undesired properties. For example, de-fucosylated Herceptin is at least 50-fold more active in Fcγ receptor IIIa-mediated antibody-dependent cellular cytotoxicity (ADCC) assays than the form with alpha-1,6-linked fucose residues5. The anti-CD20 antibody with a higher content of bisecting acetylglucosamine (GlcNAc) residues has 10–20 times higher ADCC than its wild type counterpart6.
The therapeutic anti-human epidermal growth factor receptor monoclonal antibody (EGFR mAb) cetuximab (marketed under the name Erbitux) is expressed in the murine cell line Sp2/0 and approved for the treatment of colorectal cancer. This therapeutic antibody can bind EGFR and lead to EGFR autophosphorylation, as well as subsequent activation of signal transduction pathways that are involved in regulating cellular proliferation, differentiation, and survival7. Furthermore, this therapeutic EGFR mAb exhibits ADCC against cancer cells8.
A high prevalence of hypersensitivity reactions to cetuximab have been reported in some areas of the United States9. In most subjects who had a hypersensitivity reaction, IgE antibodies that recognize the EGFR mAb were present in the circulation before treatment. The IgE antibodies were specific for galactose-α-1,3-galactose (α-Gal), which is present on the Fab portion of the EGFR mAb heavy chain. Those patients who have such IgE antibodies before treatment tend to develop a hypersensitivity reaction after intravenous injection of monoclonal antibodies containing α-Gal.
In this study, we first established a stable Chinese hamster ovary (CHO) cell line that can express the wild-type EGFR mAb and subsequently transfected these cells with cDNA encoding GnTIII (a Golgi-localized enzyme that catalyzes the addition of a bisecting N-acetylglucosamine residue on N-linked oligosaccharide chains). Following coexpression of GnTIII and wild type EGFR mAb in this cell line, we characterized the N-glycan profile of the GnTIII-modified EGFR mAb (bisec-EGFR mAb) using DNA sequencer-assisted-fluorophore-assisted capillary electrophoresis (DSA-FACE)10,11 and quantified the α-Gal content. The effects of the EGFR mAb on cell growth, ADCC and FcγR binding capacity were investigated in vitro to assess the functions of the bisec-EGFR mAb.
Materials and methods
Cell lines
A431 (ATCC CRL-1555) is a cell line expressing EGFR. Daudi (ATCC CCL-213™) is a B lymphoblast cell line expressing the inhibitory Fcγ receptor (FcγRIIb). HEK293 cells expressing human FcγRIa, FcγRIIa, FcγRIIIa-158V, or FcγRIIIa-158F were generated as previously described12.
Expression of wild-type EGFR mAb and bisec-EGFR mAb
A GnTIII(−) CHO DG44 cell line was used in expression studies of the wild type EGFR mAb and the bisec-EGFR mAb. GnTIII and the heavy chain and light chain of the EGFR mAb (cDNA synthesized by Invitrogen and subcloned into an expression vector in our lab) were coexpressed in CHO DG44 cells using the OptiCHO™ Antibody Express System (Invitrogen). A cell line that stably expressed the wild type EGFR mAb was screened with 1 μmol/L MTX and validated by whole cell ELISA (A431)6. The synthesized Chinese hamster GnTIII gene (NM_001244074.1) was cloned into the pCDNA 3.1 Hygro(−) vector and the wild type EGFR mAb-expressing cell line was transfected with this vector. The bisec-EGFR mAb (GnTIII gene stably transfected) cell line was selected on 500 μg/mL hygromycin.
Glycosylation analysis of wild type EGFR mAb, bisec-EGFR mAb and the corresponding Fab and Fc fragments
The wild type EGFR mAb and bisec-EGFR mAb from cell supernatants were captured with Protein G-agarose and protein A-agarose, respectively. The corresponding Fc and Fab fragments were isolated using immobilized papain (Thermo Scientific, USA) following the manufacturer's instructions. The digested supernatant was then loaded onto a Protein A column. The Fab fragments were collected as the flow-through fraction. The bound Fc fragments were eluted with 0.01 mol/L glycine, pH 3.0. N-glycan profiling of the wild type EGFR mAb, and bisec-EGFR mAb, and the corresponding Fab and Fc fragments was conducted by DSA-FACE.
ADCC activity assay of wild type EGFR mAb and bisec-EGFR mAb
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized fresh healthy human blood by standard centrifugation procedures using Ficoll/Hypaque (Sigma). The PBMCs used as effector cells were activated in RPMI with 10% FBS and 10 U/mL interleukin-2 (Roche) overnight. The ADCC activity assay was performed according to the manufacturer's instructions (CytoTox 96® Non-Radioactive cytotoxicity Assay, Promega, USA). Briefly, A431 cells were grown to the log phase and resuspended at 4×105 cells/mL after washing in assay medium (DMEM). The target cells (A431) were added at 50 μL/well into a 96-well flat-bottomed cell culture plate. Antibodies were serially diluted in assay medium and then added at 50 μL/well in triplicate well in the plates. The plates were incubated at room temperature for 10 min prior to the addition of 100 μL of serially diluted effector cells (PBMCs)6. The cell mixtures with antibodies were incubated at 37 °C for 4 h in a humidified CO2 incubator. One hundred microliters of supernatant was removed from each well and analyzed by measuring lactate dehydrogenase (LDH) activity released from damaged target cells using a CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, USA). The effector and/or target cells were also included as controls. Specific lysis was calculated relative to a total lysis control generated by incubating the target cells with 100 μL of 2% Triton X-100.
Antiproliferative effects of wild type EGFR mAb and bisec-EGFR mAb
The A431 cell line was employed to test the Fab binding-mediated antiproliferative activity of the antibodies. In brief, A431 cells were incubated with the wild type EGFR mAb and bisec-EGFR mAb diluted in FBS-free medium for 72 h at 37 °C with 5% CO2. After MTS solution (G5340, Promega) was added, the cells were incubated for another 3 h. Colorimetric evaluation was performed at 492 nm using a spectrophotometer. The inhibition of proliferation is reported as the IC50 induced by the wild type EGFR mAb or bisec-EGFR mAb in comparison with that induced by a positive control (Erbitux).
FcR binding affinity of wild type EGFR mAb and bisec-EGFR mAb
HEK293 cells expressing human FcγRIa, FcγRIIa, FcγRIIIa-158V, or FcγRIIIa-158F (1×106 cells) were incubated with the wild type EGFR mAb, bisec-EGFR mAb or Erbitux (10 μg/mL) or 1% BSA in PBS at 4 °C for 1 h and then washed and stained with FITC-labeled anti-human IgG (Sigma, USA). Cells were analyzed using light-scatter parameters on a MACS QUANT flow cytometer (Miltenyi Biotec, Germany). Blank controls were used, setting the cutoff at no more than 0.5% cells binding with FITC labeled anti-human IgG.
α-Gal quantification of Erbitux and bisec-EGFR mAb
The standards for the calibration curve were created through serial dilution of a 100 mmol/L D-galactose stock solution (Sigma). Each sample was mixed with 2 μL of (1-3,4,6)-galactosidase (Prozyme) and diluted to a final concentration of 1 mg/mL in the reaction buffer and incubated overnight at 37 °C. High-performance anion exchange chromatographic separation of the analyte was performed using an ICS-3000 chromatography system (HPAEC, Dionex Corp). The solution was diluted 2× to an antibody concentration of 0.5 mg/mL and detected using a pulsed amperometric detector (PAD) after elution through a CarboPac PA1 column.
Statistical analysis
All statistical analyses were performed with SPSS 16.0 for Windows software (SPSS, Chicago, IL, USA). Quantitative variables were compared using Student's t test and ANOVA. All reported P-values were two-tailed, and P values less than 0.05 were considered to be statistically significant.
Results
(Bisec-)EGFR mAb expression and N-Glycan analysis
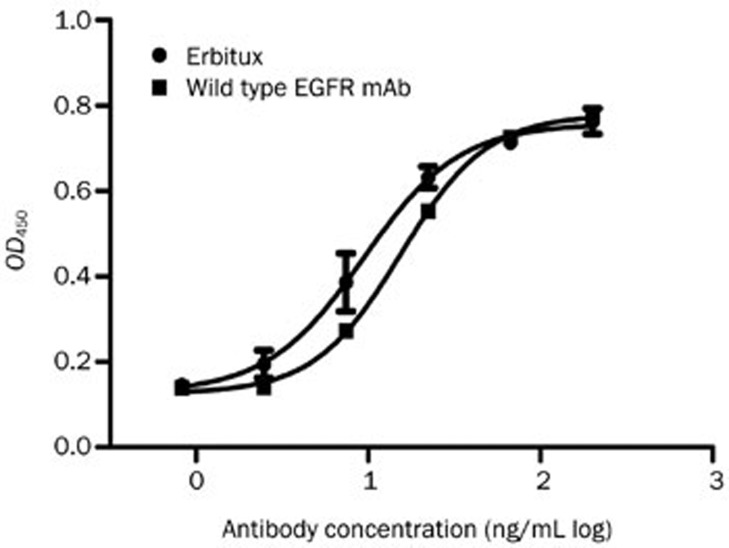
The EGFR mAb was captured from the cell supernatant by using Protein A. Whole cell ELISA showed that the recombinant wild-type EGFR mAb exhibited dose-dependent binding to A431 cells, comparable to Erbitux (Figure 1).
Figure 1.
Whole cell ELISA. The wild type EGFR mAb exhibited dose-dependent binding to A431 cells, comparable to Erbitux.
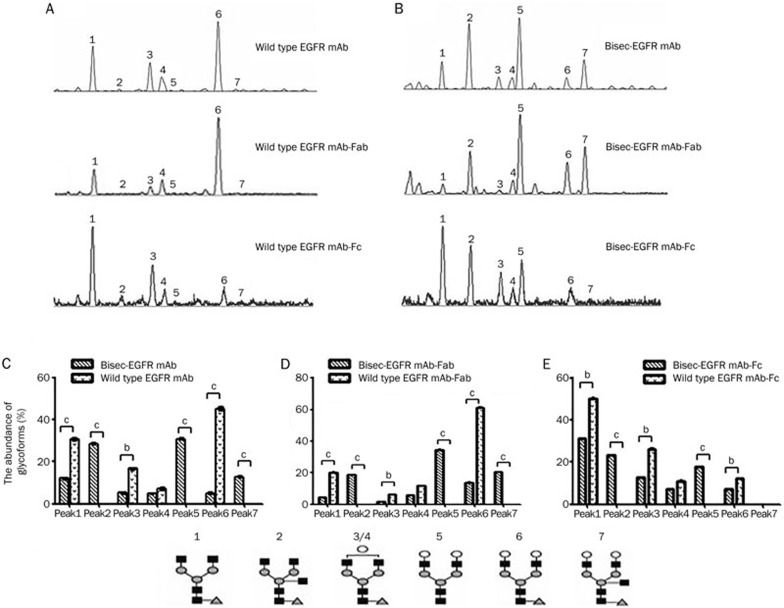
N-glycan analysis revealed 7 dominant N-glycan structures (peaks) in the N-glycan profile, with only peak 2 and peak 7 containing bisecting GlcNAc residues10,11. Thus, the glycan-modified EGFR mAb with elevated abundances of peak 2 and peak 7 (bisec-EGFR mAb) was selected for further study. Typical N-glycan profiles of the wild type EGFR mAb and bisec-EGFR mAb are shown in Figure 2A–2B. For the wild type EGFR mAb, no bisecting GlcNAc glycoform was found in either the corresponding Fab or Fc fragments (Figure 2A). As expected, the bisecting GlcNAc content (peak 2 and peak 7) was elevated dramatically in both the Fab and Fc fragments of bisec-EGFR mAb (Figure 2B) compared to the wide type EGFR mAb. Differences in N-glycan abundances between wild type EGFR mAb and bisec-EGFR mAb are shown in Figure 2C–2E. Notably, only galactosylated bisecting glycan (peak 2, 24%) was detected in Fc fragment of bisec-EGFR mAb (Figure 2E), while both agalactosylated and bigalactosylated bisecting GlcNAc (peak 2, 21%; peak 7, 21%) glycoforms were detected in the intact antibody (Figure 2C) and Fab fragment (Figure 2D).
Figure 2.
Desialylated N-glycan profiling of wild type EGFR mAb and bisec-EGFR mAb. For the wild type EGFR mAb (A), the bisecting GlcNAc glycoforms: an agalacto core-α-1,6-fucosylated bisecting biantennary glycan (NGA2FB, peak 2) and a bigalacto core-α-1,6-fucosylated bisecting biantennary glycan (NA2FB, peak 7) were not found. However, the bisected GlcNAc content was elevated dramatically in both the Fab and Fc fragments of the bisec-EGFR mAb (B). Differences in N-glycan abundance between the wild type EGFR mAb and bisec-EGFR mAb are shown in C–E (bP<0.05, cP<0.01). Notably, only agalactosylated bisecting GlcNAc (peak 2, E) was detected for the Fc of bisec-EGFR mAb, whereas both agalactosylated and bigalactosylated GlcNAc (peak 2 and peak 7) were detected in the consensus intact antibody (C) and Fab fragment (D).
Bisec-EGFR mAb had increased ADCC
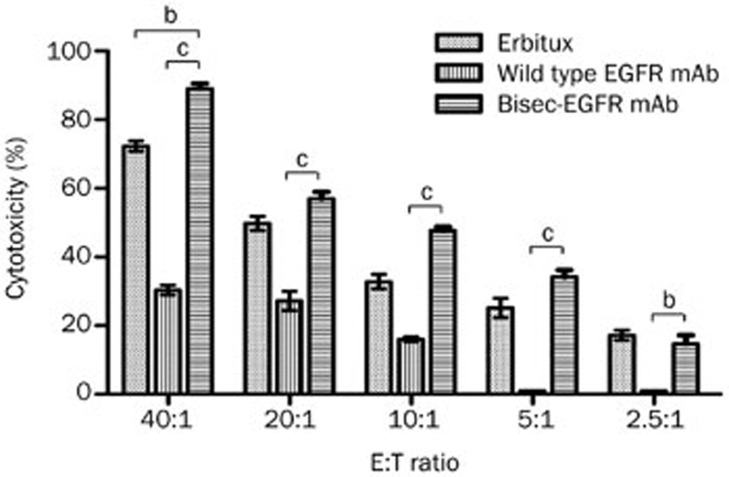
Both the wild type EGFR mAb and bisec-EGFR mAb effectively induced A431 cell lysis at effector:target cell (E:T) ratios of 5:1 and higher (Figure 3). The bisec-EGFR mAb showed a stronger lysis effect even at lower E:T ratios (P<0.05). At an E:T ratio of 40:1, the bisec-EGFR mAb yielded effective specific lysis with an approximately three-fold higher effect than that of the wild type EGFR mAb (89% vs 30%, P<0.001). The results indicated that the EGFR mAb that contained a larger amount of bisecting GlcNAc had a higher ADCC activity.
Figure 3.
Comparisons of wild type EGFR mAb and bisec-EGFR mAb in ADCC. ADCC was evaluated in EGFR-positive A431 cells at different effector:target cell (E:T) ratios. Compared to the wild type EGFR mAb, the bisec-EGFR mAb resulted in comparable effective specific lysis at a lower E:T ratio (P<0.05). At the same E:T ratio (40:1), the bisec-EGFR mAb resulted in effective specific lysis, with approximately three-fold greater lysis than that mediated by the wild type EGFR mAb (88% vs 30%, P<0.001). bP<0.05, cP<0.01.
Bisec-EGFR mAb had higher antiproliferative efficacy than wild type EGFR mAb
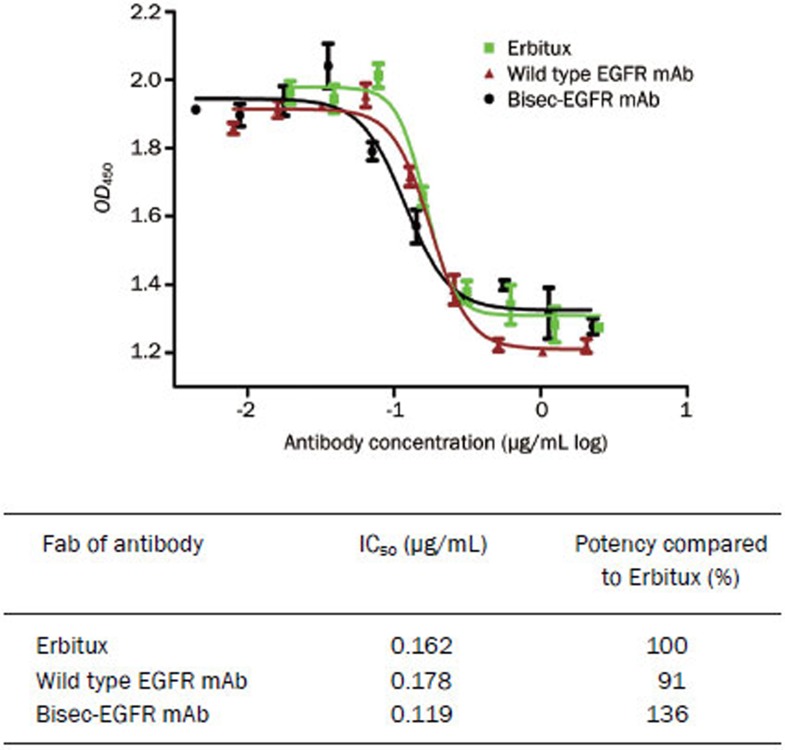
The antiproliferative activity of the EGFR mAb is mediated by the binding of the Fab fragment to EGFR. As indicated in Figure 4, both the wild type EGFR mAb and bisec-EGFR mAb showed significant inhibition of tumor cell growth. The 50% inhibitory concentration (IC50) values of Erbitux, the wild type EGFR mAb and the bisec-EGFR mAb were 0.162 (95% CI: 0.143–0.184), 0.178 (95% CI: 0.159–0.201) and 0.119 (95% CI: 0.095–0.150) mg/mL, respectively. Thus, the bisec-EGFR mAb was approximately 1.36-fold more potent than Erbitux, and the potency of the wild type EGFR mAb was comparable to that of the positive control (Erbitux).
Figure 4.
Antiproliferative efficacies of wild type EGFR mAb and bisec-EGFR mAb. Both the wild type EGFR mAb and bisec-EGFR mAb showed significant inhibition of tumor cell growth. The potency of the wild type EGFR mAb was comparable to that of Erbitux. The bisec-EGFR mAb exhibited a potency that was approximately 1.36-fold that of Erbitux.
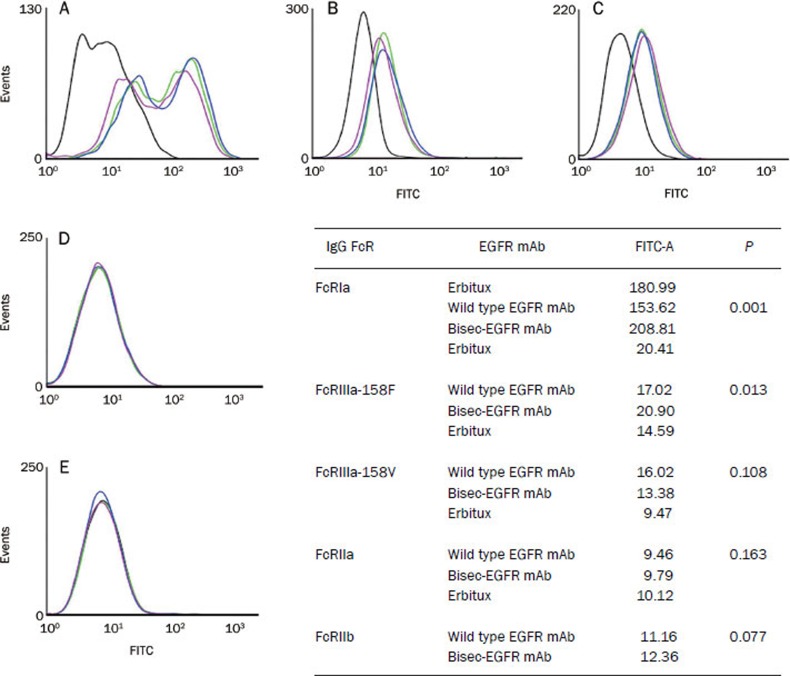
Bisec-EGFR mAb had improved binding affinity for Fcγ receptors
The binding activities of the wild type EGFR mAb, bisec-EGFR mAb, and Erbitux to Fcγ receptors (FcγRs, both inhibitory and activating FcγRs) were tested by flow cytometry. All of the EGFR mAbs bound to human FcγRs with the following rank order of affinity: FcγRIa>FcγRIIIa-158V>FcγRIIIa-158F>FcγRIIa>FcγRIIb (Figure 5). Compared to the wild type EGFR mAb, the bisec-EGFR mAb showed a higher binding affinity for human FcγRIa (P=0.001, Fig ure 5A) and FcγRIIIa-158F (P=0.013, Figure 5B). The binding affinities for 158V (Figure 5C), FcγRIIa (Figure 5D), and FcγRIIb (Figure 5E) of bisec-EGFR mAb and wild-type EGFR mAb were similar.
Figure 5.
Binding affinities of wild type EGFR mAb and bisec-EGFR mAb to FcγRa-positive and FcγRIIb-positive cells. The binding affinity of the wild type EGFR mAb (purple line), bisec-EGFR mAb (blue line), and Erbitux (green line) for FcγRs was tested by flow cytometry. The yellow line represents the blank control. The EGFR mAbs bound to FcγRs with the following rank order: FcγRIa>FcγRIIIa-158V>FcγRIIIa-158F>FcγRIIa>FcγRIIb. Compared to the wild type EGFR mAb, the bisec-EGFR mAb showed significantly enhanced binding affinity for FcγRIa (A, P=0.001) and FcγRIIIa-158F (B, P=0.013). The binding affinities for human FcγRIIIa-158V (C), FcγRIIa (D), and FcγRIIb (E) were comparable between the bisec-EGFR mAb and the wild type EGFR mAb.
α-Gal quantification of Erbitux and bisec-EGFR mAb
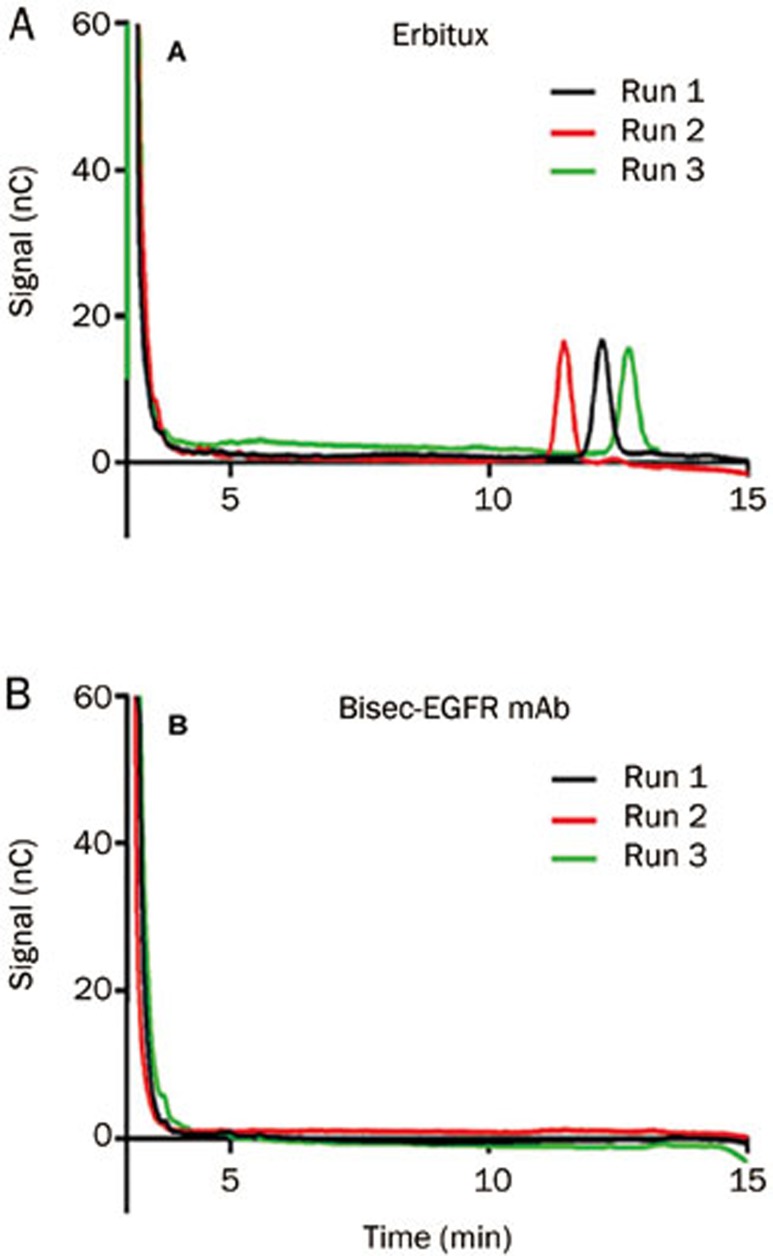
The digested Erbitux and bisec-EGFR mAb were analyzed in triplicate, and the results were measured against a calibration curve (Figure 6). The α-Gal peak was observed at a retention time of approximately 12 min after injection of the digested Erbitux (Figure 7A). The molar ratio of α-Gal per Erbitux molecule was determined to be 2.36±0.09:1. For the bisec-EGFR mAb, no detectable peak was observed at a retention time of approximately 12 min after injection (Figure 7B), and a similar result was found for the wild type EGFR mAb (data not shown).
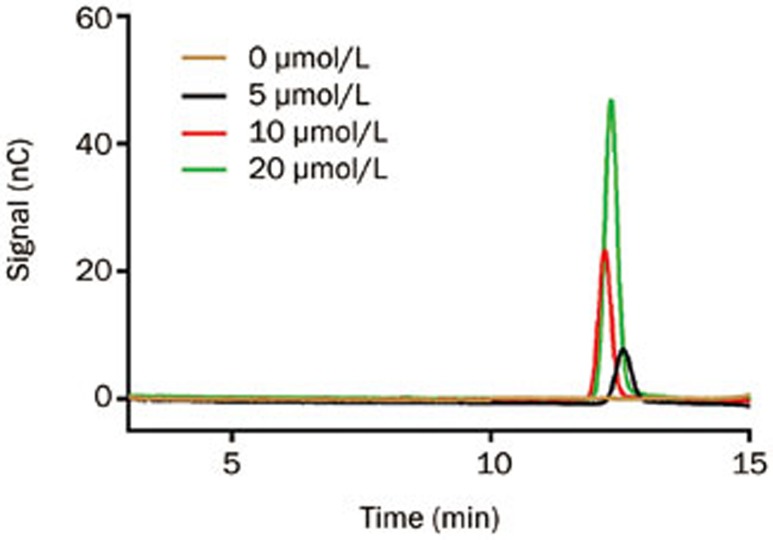
Figure 6.
α-Galactose standard curve building. By high-performance anion exchange chromatography-pulsed amperometric detection (HPAEC-PAD), an α-Galactose standard curve was created by the serial dilution of a 100 mmol/L D-galactose stock solution (Sigma). The α-Gal peak was observed at a retention time of approximately 12 min after the injection of D-galactose solution.
Figure 7.
α-Galactose analysis of Erbitux and bisec-EGFR mAb. Digested Erbitux and bisec-EGFR mAb were analyzed in triplicate, and the results were measured against the standard curve. The α-Gal peak was observed in digested Erbitux (A). For the bisec-EGFR mAb, no detectable α-Gal peak was observed (B).
The retention time of the α-Gal peak variant, at approximately 12 min, is highly sensitive to small changes in the sodium hydroxide concentration and the amount of dissolved carbon dioxide from the air, but the area of the peak is relatively consistent between different runs.
Discussion
The effector functions elicited by therapeutic antibodies strongly depend on the carbohydrate moiety linked to the antibody protein. Therefore, several approaches have been developed to rationally manipulate these glycans and improve the biological functions of antibodies13.
GnT-III is a key enzyme in N-glycan biosynthesis and catalyzes the transfer of GlcNAc from UDP-GlcNAc, a glycosyl donor, to a core β-mannose residue in N-linked oligosaccharides via a β1→4 linkage, resulting in the formation of a bisected sugar chain14. The GlcNAc residue added is referred to as a bisecting GlcNAc. The addition of this unique structure inhibits the action of other N-acetylglucosaminyl transferases, such as GnT-IV and GnT-V, both of which are involved in the formation of multi-antennary sugar chains15. Thus, GnT-III is a critical enzyme that has been used to improve the function of therapeutic antibodies.
Increasing evidence shows that N-linked oligosaccharides can affect the solubilities, clearance rates and effector functions of antibody molecules16. GnT-III is an ideal enzyme for manipulating the N-glycosylation of expressed proteins, as it exerts a large degree of control over the glycosylation process by blocking the action of 1,6-FucT, ManII, and GnT-II17,18.
Previous studies have shown that over-expression of GnTIII in CHO DG44 cells leads to ADCC enhancement of an anti-neuroblastoma IgG118, an anti-CD20 IgG119, and an anti-human interleukin 5 receptor IgG120. To further identify the roles of bisecting GlcNAc in EGFR mAb function, we overexpressed GnTIII in an EGFR mAb producing cell line (CHO DG44) that lacks endogenous GnTIII expression21, and we confirmed that an antibody with bisecting GlcNAc (the bisec-EGFR mAb) enhanced ADCC compared to an antibody without bisecting GlcNAc (the wild type EGFR mAb).
However, all of the previous studies have only focused on the glycosylation of intact IgG. Considering the different oligosaccharide profiles of IgG Fab and Fc fragments revealed in some case-control studies22,23, we also analyzed the N-glycan profiles of antibody Fab and Fc fragments. Both the Fab and Fc fragments of the bisec-EGFR mAb showed an increased bisecting GlcNAc content compared to the wild type EGFR mAb, but the Fab and Fc fragments had different N-glycan profiles from each other. This finding demonstrated site specificity for the elongation of the oligosaccharide chains on the glycoprotein22 and may explain why the antibodies with a higher bisecting GlcNAc content had higher ADCC activity. First, the enhanced effect may be due to an increased Fc affinity for Fcγ receptors24. Second, the improved ADCC activity may also result from a better specific binding of Fab to antigen-positive cells, which initiates the process of ADCC and target cell lysis25.
In the Fab-mediated cell antiproliferation assay, we found that the potency of the bisec-EGFR mAb was 50% higher than that of the wild type EGFR mAb. Meanwhile, a comparative study on Fc receptor binding activity revealed that the bisec-EGFR mAb had a higher binding affinity for activated human Fcγ receptors (mainly FcRIa and 158F-FcRIIIa) than did the wild type EGFR mAb. Taken together, these data suggest that the bisecting GlcNAc modified antibody has higher affinities for both activated FcR and EGFR-expressing cells.
Most of the recently marketed therapeutic antibodies are manufactured in CHO cells, in part because of the ability of these cells to produce proteins with desirable properties, including 'human-like' glycosylation profiles. Specific glycan structures may adversely affect an antibody's safety profile. For example, the terminal galactose-α-1,3-galactose (α-Gal) antigen can react with circulating anti α-Gal antibodies, which are present in most individuals, and induce an anaphylactic response26. It is now understood that murine cell lines, such as NS0 or SP2/0, contain the necessary biosynthetic machinery to produce proteins containing α-Gal epitopes27,28,29. However, it is generally accepted that CHO cells lack the biosynthetic machinery to synthesize glycoproteins with α-Gal antigens30. Some reports have revealed that CHO cells do not produce α-1,3-galactosyltransferase31 and have a pattern of glycosylation that differs from that of the Erbitux host cell line Sp2/0. However, data from other groups have identified the presence of the CHO ortholog of N-acetyllactosaminide 3-α-galactosyltransferase-132, which is responsible for the synthesis of the α-Gal epitope. These groups also found that the amounts of terminal α-Gal in CHO clones ranged from 0 to 404 picomol per mg of protein; therefore, selection of a cell line without α-Gal expression is important for CHO-derived therapeutic antibody production.
In this study, we developed and quantified the concentration of α-Gal in the bisec-EGFR mAb using HPAEC-PAD (high-performance anion exchange chromatography-pulsed amperometric detection). Consistent with a previous report33, we found that Erbitux contains an α-Gal epitope (2.36±0.09 μmol per μmol Erbitux), whereas the bisec-EGFR mAb we investigated did not show an obvious α-Gal peak. Although the specific levels of α-Gal required to trigger anaphylactic reactions are not yet clear, the fact that patients have high levels of circulating anti-α-Gal antibodies9 suggests that controlling the levels of the α-Gal epitope during EGFR mAb development may have a positive impact on drug safety. More evidence for avoiding the hypersensitivity reaction to α-Gal epitope could be provided by clinical trials.
In conclusion, we modified the glyco-structure of the EGFR mAb by increasing the bisecting glycan abundance. This bisec-EGFR mAb showed higher ADCC and lower α-Gal levels than the approved therapeutic antibody (Erbitux). Additionally, the glycan-modified antibody showed higher binding to FcγRIa and FcγRIIIa-158F. These results indicate that the glyco-engineering of anti-EGFR antibodies could optimize their function and minimize their side effects (such as hypersensitivity). Validation in vivo will be required to confirm the improved efficacy of the glyco-engineered antibody. This research might provide a new, alternative, strategy for the production of engineered therapeutic monoclonal antibodies.
Author contribution
Chang-hong YI, Can-ping RUAN, and Chun-fang GAO designed the research; Chang-hong YI, Xin-yun XU, Yun-peng ZHAO, and Meng FANG performed research; Jun JI, Hao WANG, and Xing GU analyzed data; Chang-hong YI and Chun-fang GAO wrote the paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No 81301877 and 81271925); State Key Project on Infectious Diseases of China (No 2012ZX10002-016); Science and Technology Commission of Shanghai Municipality (No 11JC1416400).
References
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Targeted cancer therapies based on antibodies directed against epidermal growth factor receptor: status and perspectives. Acta Pharmacol Sin. 2007;28:1476–93. doi: 10.1111/j.1745-7254.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–6. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin. 2010;31:1198–207. doi: 10.1038/aps.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-Linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FcgRIII. Biotechnol Bioeng. 2001;74:288–94. [PubMed] [Google Scholar]
- Matsuo T, Nishizuka SS, Ishida K, Iwaya T, Ikeda M, Wakabayashi G. Analysis of the anti-tumor effect of cetuximab using protein kinetics and mouse xenograft models. BMC Res Notes. 2011;4:140–7. doi: 10.1186/1756-0500-4-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–61. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1,3-Galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA-sequencer based total serum protein glycomics. Nat Med. 2004;10:429–34. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, et al. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426–35. doi: 10.1002/hep.21855. [DOI] [PubMed] [Google Scholar]
- Arora T, Padaki R, Liu L, Hamburger AE, Ellison AR, Stevens SR, et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine. 2009;45:124–31. doi: 10.1016/j.cyto.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Brünker P, Suter T, Moser S, Püntener U, Umaña P. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1,4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng. 2006;93:851–61. doi: 10.1002/bit.20777. [DOI] [PubMed] [Google Scholar]
- Narasimhan S. Control of glycoprotein synthesis. UDP-GlcNAc: glycopeptide beta 4-N-acetylglucosaminyl transferase III, an enzyme in hen oviduct which adds GlcNAc in beta 1,4 linkage to the beta-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J Biol Chem. 1982;257:10235–42. [PubMed] [Google Scholar]
- Taniguchi N, Miyoshi E, Ko JH, Ikeda Y, Thara Y. Implication of N-acetylglucosaminyltransferases III and V in cancer: gene regulation and signaling mechanism. Biochim Biophys Acta. 1999;1455:287–300. doi: 10.1016/s0925-4439(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986;64:163–81. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–80. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- Umaña P, Jean-Mairet J, Bailey JE. Tetracycline-regulated over-expression of glycosyltransferases in Chinese hamster ovary cells. Biotechnol Bioeng. 1999;65:542–9. [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-Acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Cambell C, Stanley P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc: Glycopeptidebeta-4-N-acetylglucosaminyl transferase III activity. J Biol Chem. 1984;10:13370–8. [PubMed] [Google Scholar]
- Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, et al. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 2006;1760:669–77. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Youings A, Chang SC, Dwek RA, Scragg IG. Site-specific glycosylation of human immunoglobulin G is altered in four rheumatoid arthritis patients. Biochem J. 1996;314:621–30. doi: 10.1042/bj3140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–45. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Abès R, Teillaud JL. Impact of glycosylation on effect or functions of therapeutic IgG. Pharmaceuticals. 2010;3:146–57. doi: 10.3390/ph3010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher BA, Galili U. The Galα1, 3Galβ1,4GlcNAc-R (α-Gal) epitope: A carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RD, Rajan VP, Ruff MM, Kukowska-Latallo J, Cummings RD, Lowe JB. Isolation of a cDNA encoding a murine UDP galactose: beta-D-galactosyl-1,4-N-acetyl-D-glucosaminide alpha-1,3-galactosyltransferase: expression cloning by gene transfer. Proc Natl Acad Sci U S A. 1989;86:8227–31. doi: 10.1073/pnas.86.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeley DM, Merrill BM, Taylor LC. Characterization of Monoclonal Antibody Glycosylation: Comparison of Expression Systems and Identification of Terminal α-Linked Galactose. Anal Biochem. 1997;247:102–10. doi: 10.1006/abio.1997.2036. [DOI] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1,3-Galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N, Parekh RB, James DC. Getting the glycosylation right: implications for the biotechnology industry. Nat Biotechnol. 1996;14:975–81. doi: 10.1038/nbt0896-975. [DOI] [PubMed] [Google Scholar]
- Jefferis R.Glycosylation of human IgG antibodies: relevance to therapeutic applications BioPharm 20021419–26.Recent research developments in immunology. Vol 4 (2002); Part II, pp. 769-780 [Google Scholar]
- Bosques CJ, Collins BE, Meador JW, 3rd, Sarvaiya H, Murphy JL, Dellorusso G, et al. Chinese hamster ovary cells can produce galactose-α-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28:1153–6. doi: 10.1038/nbt1110-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, et al. Anti-galactose-α-1,3-galactose IgE from allergic patients does not bind α-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29:574–6. doi: 10.1038/nbt.1912. [DOI] [PubMed] [Google Scholar]