Abstract
Aim:
Tanshinone II-A sodium sulfonate (DS-201), a water-soluble derivative of Tanshinone II-A, has been found to induce vascular relaxation and activate BKCa channels. The aim of this study was to explore the mechanisms underlying the action of DS-201 on BKCa channels.
Methods:
Human BKCa channels containing α subunit alone or α plus β1 subunits were expressed in HEK293 cells. BKCa currents were recorded from the cells using patch-clamp technique. The expression and trafficking of BKCa subunits in HEK293 cells or vascular smooth muscle cells (VSMCs) were detected by Western blotting, flow cytometry and confocal microscopy.
Results:
DS-201 (40–160 μmol/L) concentration-dependently increased the total open probability of BKCa channels in HEK293 cells, associated with enhancements of Ca2+ and voltage dependence as well as a delay in deactivation. Coexpression of β1 subunit did not affect the action of DS-201: the values of EC50 for BKCa channels containing α subunit alone and α plus β1 subunit were 66.6±1.5 and 62.0±1.1 μmol/L, respectively. In both HEK293 cells and VSMCs, DS-201 (80 μmol/L) markedly increased the expression of α subunit without affecting β1 subunit. In HEK293 cells, DS-201 enriched the membranous level of α subunit, likely by accelerating the trafficking and suppressing the internalization of α subunit. In both HEK293 cells and VSMCs, DS-201 (≥320 μmol/L) induced significant cytotoxicity.
Conclusion:
DS-201 selectively targets the pore-forming α subunit of human BKCa channels, thus enhancing the channel activities and increasing the subunit expression and trafficking, whereas the β1 subunit does not contribute to the action of DS-201.
Keywords: Danshen, tanshinone, BKCa channel, vascular relaxation, protein trafficking, vascular smooth muscle cell
Introduction
It is well known that arterial tone is regulated by functional balance of the ion channels responsible for cellular depolarization and hyperpolarization1. The large conductance calcium-activated potassium channels (BKCa channels), also called Maxi-K or Slo, are broadly expressed in vascular smooth muscle cells (VSMCs) and play crucial roles in regulating vascular tone2,3. Activation of BKCa channels by elevation of the intracellular calcium concentration due to membrane depolarization increases the K+ conductance of the membrane and drives VSMC membrane hyperpolarization, which in turn closes the L-type voltage-dependent Ca2+ channels (LVDCCs), decreases global [Ca2+]i, and induces vascular relaxation4,5. The BKCa channel consists of a functional Slo α-subunit and an affiliated β subunit6. There are 4 types of affiliated subunits (β1–β4). The β1 subunit is the major affiliated subunit of VSMCs, and it enhances not only BKCa α-subunit expression in the membrane but also the voltage and Ca2+ sensitivity of BKCa channels7. Previous reports have revealed that β1 subunit knock-out rats easily developed hypertension8. Our previous study showed that, in the VSMCs of patients with essential hypertension, the whole-cell current, spontaneous transient outward potassium currents (STOCs) and the Ca2+ sensitivity of BKCa channels were reduced due to the down-regulation of the β1 subunit both at the mRNA and protein levels, whereas α-subunit expression was maintained9. Therefore, rescuing β1 subunit function to restore the activity of BKCa channels could be a therapeutic approach for diseases with β1 subunit malfunction, such as hypertension. Alternatively, enhancing the activity of the α subunit directly might also be a therapeutic strategy for these diseases.
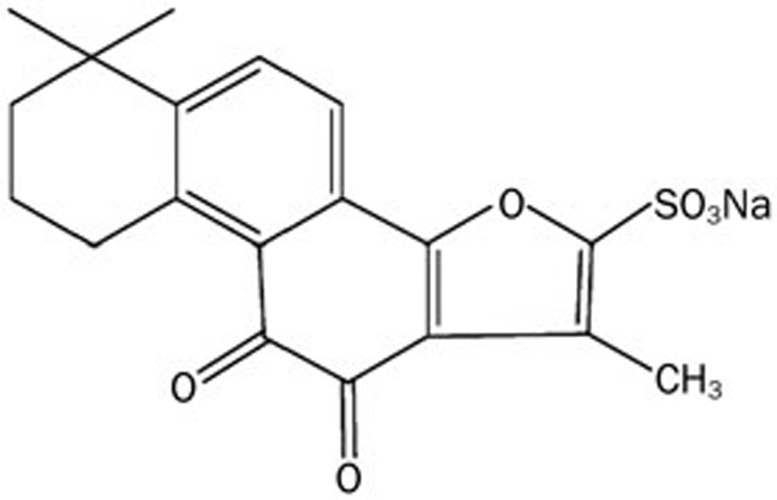
Danshen (Salvia miltiorrhiza), a traditional Chinese medicinal herb, has been widely used in China and many other countries with minimal side effects in therapies for cardiovascular and cerebrovascular diseases10,11,12. Tanshinone II-A is a type of diterpene quinine and a major effective component of Danshen, and tanshinone II-A sodium sulfonate (DS-201) (Figure 1) is a water-soluble derivative of tanshinone II-A after sulfonation. DS-201 retains the pharmacological efficacy of tanshinone II-A and is convenient for injection because of its water-soluble character. We showed in a previous report that DS-201 could induce the relaxation of isolated vascular rings, activate the macro-currents in a whole-cell configuration and increase open probability in inside-out patches in porcine coronary arterial smooth muscle cells13, suggesting that BKCa channel activation is involved in DS-201-mediated vasorelaxation. We further demonstrated that DS-201 activated BKCa channels mainly by shifting the kinetic properties and the Ca2+ dependence of the channel in mouse cerebral arterial smooth muscle cells14, suggesting that DS-201 likely plays a role similar to that of the β1 subunit in modulating the BKCa channel. However, it remains unknown whether the β1 subunit is necessary to the action of DS-201 on the BKCa channel and whether DS-201 can potentiate the expression and trafficking of BKCa channels, just as β1 subunit does.
Figure 1.
The chemical structure of DS-201.
To address the above questions, we manipulated HEK293 cells to express the α subunit alone or with the β1 subunit of the BKCa channels. Using these genetically engineered cell lines and cultured VSMCs and a series of approaches, including patch clamp, Western blotting, flow cytometry (FCM) and confocal microscopy, we investigated the acute effects of DS-201 on BKCa channel kinetics and the relatively chronic effects on the expression and trafficking of BKCa channel subunits, with a particular focus on the target protein of DS-201 in the BKCa channel complex. This study could increase our understanding of the mechanisms of DS-201 in mediating vasorelaxation and could provide further perspectives for promoting the clinical use of Danshen for treating diseases with vascular dysfunction, such as hypertension.
Materials and methods
Materials
DS-201, with the chemical formula of C19H17NaO6S and the structure shown in Figure 1, was purchased from the National Institutes for Food and Drug Control (NIFDC, Beijing, China) (purity ≥98%). The drug was dissolved in de-ionized water to obtain a 4-mmol/L stock solution and was added to the bath solution or culture medium to achieve the desired concentrations. K-aspartate, HEPES, EGTA and iberiotoxin (IbTX) were obtained from Sigma (MO, USA).
Plasmid construction
The plasmid pcDNA3.1-hSlo, containing the human BKCa α subunit (hSlo), was a kind gift from Prof Philip K AHRING (NeuroSearch A/S, Denmark). Using the overlapping PCR protocol, we constructed the expression plasmid pcDNA3.1-Flag-hSlo-EGFP (abbreviated as “Flag-hSlo-GFP” below), using the plasmid pcDNA3.1-hSlo as a template. The enhanced green fluorescence protein (EGFP) was connected to the C-terminal of hSlo, and a Flag tag (DYKDDDDK) was inserted into the extracellular loop between the S1 and S2 segments (SNPIESDYKDDDDKCQNFYKDF). The expression plasmid pEF1-myc-hβ1 (hβ1), containing the human β1 subunit with the His tag, was previously constructed in our laboratory. All of the plasmids were identified by DNA sequencing.
Cell culture and transfection
HEK293 cells (from the National Platform of Experimental Cell Resources for Sci-Tech, Beijing, China) and Sprague-Dawley (SD) rat thoracic aortic vascular smooth muscle cell lines (VSMCs) (A7r5 cell lines, from the American Type Culture Collection, ATCC, VA, USA) were cultured at 37 °C in a 95% air/5% CO2 humidified incubator with Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) fetal bovine serum (Gibco, Invitrogen, New York, USA). HEK293 cells were used for transfection and VSMCs for investigating BKCa protein expression. Transfection was carried out at 70%–80% cell confluence, and 4 μg of total plasmid DNA (hSlo or Flag-hSlo-GFP, with or without hβ1) was added to 35-mm cell culture dishes for transfection using the lipofection technique (Lipofectamine 2000, Invitrogen, NY, USA).
Patch clamp
Patch clamp experiments were performed in essentially the same manner as described previously13. Channel currents were recorded with configurations of whole-cell, inside-out and outside-out patches using EPC-10 amplifier (HEKA, Lambrecht/Pfalz, Germany). The total open probability (NPo), current amplitude and density, and kinetic characteristics of the BKCa channels were analyzed with the pCLAMP software (version 10.0, Molecular Devices, CA, USA). In the whole-cell configuration, the bath (extracellular) solution consisted of (in mmol/L): NaCl 140, KCl 5, CaCl2 1.8, MgCl2 2, and HEPES 10 (pH 7.4 with NaOH). The pipette (intracellular) solution consisted of (in mmol/L): K-aspartate (K-Asp) 100, KCl 40, MgCl2 5, EGTA 1, and HEPES 10 (pH 7.2 with KOH), and the intracellular Ca2+ concentration [Ca2+]i was adjusted to 0.1 μmol/L. After the series resistance was compensated for by 70% and reached <10 MΩ to minimize voltage errors, the whole-cell macroscopic currents were recorded with step pulses (from −70 mV to +70 mV), followed by N/P leak subtraction. In the inside-out patch experiments, the pipette (extracellular) solution consisted of (in mmol/L): K-Asp 40, KCl 100, HEPES 10, and EGTA 2 (pH 7.2 with KOH); and the bath (intracellular) solution (in mmol/L): K-Asp 100, KCl 40, HEPES 10, and EGTA 1 (pH 7.4 with KOH). In the outside-out patch, the pipette (intracellular) solution consisted of (in mmol/L): K-Asp 100, KCl 40, HEPES 10, and EGTA 1 (pH 7.4 with KOH); and the bath (extracellular) solution (in mmol/L): K-Asp 40, KCl 100, HEPES 10, and EGTA 2 (pH 7.2 with KOH). Because DS-201 activates the BKCa channels mainly from the cytoplasmic side of the membrane, we conducted a special configuration: the inside-out macro-patches. The advantages of this configuration were that the electrode tip had a larger size with low resistance and therefore could cover thousands of BKCa channels when an inside-out patch was formed, if also considering that the channel proteins were overexpressed in HEK293 cells. Thus, nanoampere currents could be obtained as macro-currents. Additionally, it was very convenient to change solution components at the “intracellular” side. Here, we used these inside-out macro-patches to investigate the voltage dependence and kinetics of the BKCa channels. The pipette and bath solutions were the same as those used in the inside-out patch experiments. To create serial intracellular free Ca2+ concentrations ([Ca2+]i) of 0, 0.01, 0.1, 0.5, 1, or 10 μmol/L, the CaCl2 concentration of the bath solution was set to 0, 0.11, 0.55, 0.86, 0.92, or 1 mmol/L, respectively14. The membrane potential (Vm) was expressed as that of the intracellular side. If there was no other special instruction, the intracellular free Ca2+ concentration ([Ca2+]i) was 0.1 μmol/L, and Vm was +40 mV in the inside-out patch. All of the electrophysiological experiments were conducted at room temperature (22±2 °C).
Western blotting
Western blotting was conducted as described in a previous report15. Briefly, cells were harvested and lysed with lysis buffer containing 50 mmol/L Tris-Cl (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate and a series of protease inhibitors. Samples containing approximately 50 μg of total protein were separated with SDS-PAGE and were transferred to PVDF membrane, followed by blocking and incubation with anti-Slo (1:500, Alomone, Jerusalem, Israel) or anti-β1 (1:500, Alomone, Jerusalem, Israel) primary antibodies at 4 °C overnight. The blots were then incubated with a horseradish peroxidase-conjugated secondary antibody (1:5000, MBL, Nagoya, Japan) at room temperature for 1 h and were developed using an ECL system (Engreen Biosystem Co, Ltd, Beijing, China). Images were obtained and quantified using Quantity One software (Bio-Rad, CA, USA).
Biotinylation and isolation of cell surface proteins
Membrane biotinylation of HEK293 cells expressing BKCa channels were completed according to the manufacturer's protocol provided in the Cell Surface Protein Isolation Kit (Pierce, IL, USA). Briefly, cells were washed with cold PBS, followed by incubation with 0.25 mg/mL Sulfo-NHS-SS-Biotin in PBS for 30 min on ice. After quenching the biotinylation reaction, the cells were collected, lysed and incubated with beads for the isolation of the labeled proteins. Finally, the labeled proteins, which combined with the beads, were eluted using SDS-PAGE sample buffer and were analyzed by Western blotting.
Co-immunoprecipitation (co-IP)
Co-IP was used to investigate the interaction between hSlo and hβ1 in HEK293 cells coexpressing Flag-hSlo-GFP and hβ1. The experiment was conducted using Protein A/G PLUS Agarose Immunoprecipitation Reagent (Santa Cruz, TX, USA). Briefly, (2–5)×107 cells treated with DS-201 were lysed with mild lysis buffer (50 mmol/L Tris-Cl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 0.5% sodium deoxycholate) containing protease inhibitors. One microgram (mg) of primary antibody (anti-Flag for hSlo or anti-His for hβ1) was added to the sample and was incubated at 4 °C for 2 h. Then, the complex was incubated overnight with protein A/G agarose beads at 4 °C. The mixture was washed three times with cold PBS and was finally resuspended with 2× SDS sample buffer, was heated at 95 °C for 5 min and was centrifuged to acquire the supernatant. The samples were analyzed by Western blotting.
Flow cytometry to measure subcellular localization of channel proteins
Flow cytometry (FCM) was used to investigate the subcellular localization of BKCa channel proteins associated with protein trafficking in HEK293 cells expressing Flag-hSlo-GFP. Because the Flag tag was in the extracellular S1–S2 loop, allophycocyanin (APC)-conjugated anti-Flag antibody could detect the levels of membranous BKCa channels, while GFP represented the global (cytoplasmic and membranous) levels of BKCa channels. To conduct the experiment, cells were collected and washed with cold PBS 3 times. Then, the cells were incubated with APC-conjugated anti-Flag antibody (1:200, Abcam, Cambridge, UK) at 4 °C for 2 h and were washed with cold PBS 3 times. Fluorescence was detected using the Accuri® C6 cytometer (BD, MD, USA). The mean florescence intensity (MFI) was calculated with the following equation:
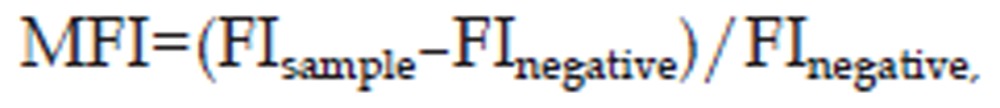 |
where FIsample is the florescence intensity of the sample, FInegative is the florescence intensity of the cells without expressing Flag-hSlo-GFP but incubated with APC conjugated anti-Flag antibody, and MFIFlag and MFIGFP are the expression levels of the membranous BKCa and total BKCa protein levels, respectively. The ratio MFIFlag/MFIGFP, representing the portion of membranous BKCa channel protein among the global pool of BKCa proteins, was used as an indicator of BKCa channel trafficking.
Flow cytometry to measure channel protein internalization
Forty-eight hours after transfection, HEK293 cells transiently expressing Flag-hSlo-GFP were harvested and stained with the mouse anti-Flag antibody (1:1000, Sigma, MO, USA) for 2 h at 4 °C. Then, the cells were switched to 37 °C for different time for the BKCa channels to internalize and for the internalization dynamic curves to be established. After this procedure, any remaining surface-labeled channels were stained with DyLight® 650-conjugated donkey anti-mouse secondary antibody for 1 h (1:250, Abcam, Cambridge, UK) at 4 °C, and the fluorescence was detected using the Accuri® C6 cytometer (BD, MD, USA). Because a portion of surface labeled BKCa channels would be internalized into the cytoplasm and could not be labeled by the secondary antibody, a decrease in fluorescence intensity denoted the internalization of the BKCa channels.
Confocal microscopy
HEK293 cells expressing Flag-hSlo-GFP were fixed in 4% paraformaldehyde for 15 min and were blocked with 5% BSA for 1 h. Whether 0.1% Triton-X 100 was used depended on the location at which the protein was detected. In detail, for staining cell surface BKCa channels, membrane permeabilization with Triton-X 100 was not performed, while this procedure was performed for 15 min to stain the global BKCa channel proteins. After blocking, the cells were incubated with the primary mouse anti-Flag antibody (1:2000, Sigma, MO, USA) overnight at 4 °C. DyLight® 550-conjugated donkey anti-mouse secondary antibody (1:200, Abcam, Cambridge, UK) was incubated for 1 h at room temperature. Immunofluorescence-labeled samples were examined using an Olympus confocal laser scanning microscope (Tokyo, Japan). The laser lines (excitation/emission wave) were 358 nm/461 nm, 488 nm/507 nm, and 562 nm/576 nm for DAPI, GFP and DyLight® 550-conjugated antibody, respectively. Negative control experiments were performed by pre-incubation of the primary antibody with the respective antigenic peptide (1:1), and these experiments did not show positive staining under the same experimental conditions.
Cytotoxicity assay with the Cell Counting Kit-8
The Cell Counting Kit-8 (CCK-8) was used to evaluate the potential cytotoxic effects of DS-201 on HEK293 cells and VSMCs. Briefly, cell suspension of 100 μL in volume was dispensed into each well of a 96-well plate (5×103 cells/well) and was pre-incubated for 24 h. The cells were then exposed to various concentrations of DS-201 (0, 10, 20, 40, 80, 160, 320, 640, and 1280 μmol/L) and then were incubated for 12 h at 37 °C in an incubator. The wells were then washed and refilled with fresh culture medium. The cells in each well were incubated with 10 μL of CCK-8 solution for 2 h at 37 °C. Formazan was quantified spectroscopically at 450 nm using a microplate reader (Synergy™ 4, Biotek, VT, USA).
Statistical analysis
The data are expressed as mean±SEM. Student's t test and ANOVA were used for the statistical analysis, according to the experiments. A P value of <0.05 was considered statistically significant.
The relationship between drug concentration and normalized NPo was fitted to the Hill equation: y=xb/(cb+xb). Here, x is the concentration of DS-201 or calcium, c is the half maximal effective concentration (EC50) of DS-201 or Ca2+, the latter (EC50 of Ca2+) reflecting the apparent Ca2+ sensitivity, and b is the slope factor (Hill coefficient, nH). The conductance (G) of the BKCa channel macro-currents in the inside-out macro-patches was calculated by the slope of the I–V curve, which was fitted to the following polynomial equation: y=a0+a1×x1+a2×x2+...+a9×x9. G/Gmax represents the normalized conductance (G) of BKCa channels at a Vm to the maximal conductance (Gmax). Then, the G/Gmax–Vm curve was fitted by the Boltzmann function: G=1–1/{1+exp[(V–V1/2)/K]}. Here, V1/2 is the half maximal activation voltage, and K is the slope of the curve.
Results
The electrophysiological properties of BKCa channels expressed in HEK293 cells
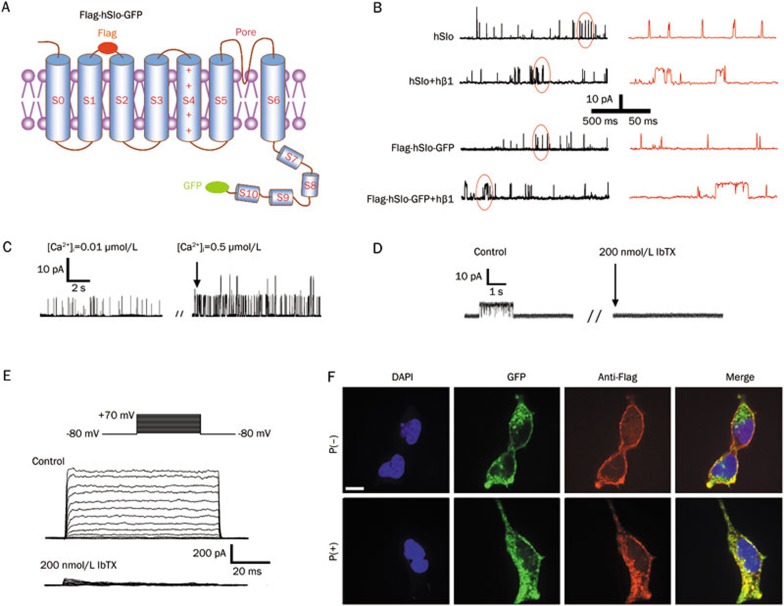
We first verified the electrophysiological characteristics of the BKCa channels heterologously expressed in HEK293 cells. Figure 2A shows a sketch map of the BKCa channel α subunit (hSlo) tagged with Flag and GFP. The Flag tag was inserted into the extracellular S1–S2 loop of the α subunit and thus could detect the surface expression of hSlo by FCM and confocal microscopy, whereas the GFP tag was connected to the C-terminal of the α subunit and was used to detect the total cellular expression of hSlo. Figures 2B–2E show the main electrophysiological properties of the BKCa channels expressed in HEK293 cells, which were consistent with those obtained from the native cells13,14. The BKCa currents could not be recorded in HEK293 cells without the transfection of the BKCa channels (data not shown). Figure 2B shows typical recordings of the single BKCa channel currents under the inside-out patch configuration with the symmetrical 140 mmol/L K+ (Vm=+40 mV and [Ca2+]i=0.1 μmol/L). Coexpression of the hβ1 subunit increased the open time duration. Flag and GFP tags did not affect the open time duration. The Table 1 provides the statistical results, showing that the Flag and GFP tags did not affect the kinetics and conductance of the single BKCa channel, while coexpression of the hβ1 subunit increased the mean open time (To) and the mean closed time (Tc) of BKCa channels, compared with that of the α subunit expression alone. Figure 2C shows the Ca2+-dependent property of the BKCa channel opening. The BKCa currents were blocked by IbTX (a selective BKCa channel blocker) (200 nmol/L) when applied to the bath solution of the outside-out patch (Vm=+40 mV and [Ca2+]i=0.1 μmol/L) (Figure 2D) and whole-cell experiments (Figure 2E).
Figure 2.
The basic properties of BKCa channels expressed in HEK293 cells. (A) A sketch map of the BKCa channel α subunit (hSlo) tagged with Flag and GFP (Flag-hSlo-GFP). (B) Typical recordings of BKCa currents in an inside-out configuration at Vm=+40 mV and [Ca2+]i=0.1 μmol/L in HEK293 cells expressing the BKCa channel α subunit (hSlo or Flag-hSlo-GFP) alone or with the hβ1 subunit. Note that coexpression of the hβ1 subunit increased the open time duration, while Flag and GFP tags did not affect the open time duration. The red traces on the right side of (B) are expanded traces circled on the left side of (B). Such currents could not be recorded in HEK293 cells without the expression of the BKCa channel. (C) Examples of single BKCa channel currents showing the Ca2+ dependence of hSlo (inside-out patch, recorded at Vm=+40 mV). (D) Blockage of hSlo currents by 200 nmol/L IbTX (recorded from outside-out patches at Vm=+40 mV and [Ca2+]i=0.1 μmol/L). (E) Blockage of BKCa currents by 200 nmol/L IbTX (recorded from whole-cell configuration at [Ca2+]i=0.1 μmol/L). The holding potential was −80 mV, and the testing step pulses ranged from −70 mV to +70 mV in 10 mV steps, each lasting for 100 ms, followed by holding potential with −80 mV. (F) Confocal images showing the subcellular localization of α subunits in HEK293 cells transfected with Flag-hSlo-GFP. The cells were stained red with anti-Flag, representing the surface population of hSlo (upper panels) after fixation but without membrane permeabilization [P(−)], and red was also shown in the membrane and cytoplasm after fixation and membrane permeabilization [P(+)] with 0.1% Triton (lower panels). GFP (green) indicated the overall hSlo expression. The nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). The merged images show perfect colocalization of Flag- and GFP-tagged hSlo (right column) on the cell surface (upper panels) and in the whole cell (lower panels), respectively. Scale bars=50 μm.
Table 1. BKCa channel kinetic and conductance parameters showing that Flag and GFP tags did not affect the electrophysiological properties of single BKCa channel. Data shown as mean±SEM. n=cell number. aP>0.05 vs hSlo group. cP<0.01 vs hSlo group. fP<0.01 vs Flag-hSlo-GFP group.
| Cell line | n | Mean open time (ms) | Mean closed time (ms) | Conductance (pS) |
|---|---|---|---|---|
| hSlo | 17 | 1.3±0.2 | 104.4±18.9 | 201.7±3.2 |
| hSlo+hβ1 | 14 | 5.3±1.7c | 193.0±31.5c | 214.4±5.3a |
| Flag-hSlo-GFP | 15 | 1.2±0.4a | 96.6±38.2a | 203.0±3.9a |
| Flag-hSlo-GFP+hβ1 | 12 | 4.5±1.2f | 211.7±37.7f | 209.9±2.3a |
Note: Single BKCa channel currents were recorded under the inside-out configuration at [Ca2+]i=0.1 μmol/L and Vm=+40 mV.
In addition, confocal microscopy showed that anti-Flag antibody detected only the surface population of the BKCa channels (Figure 2F, the third panel of upper row), while after membrane permeabilization, anti-Flag antibody denoted the total BKCa population, as indicated by complete co-localization of Flag with GFP (Figure 2F, the fourth panel of lower row). Thus, the Flag tag was competent in examining the surface expression of the BKCa channels when without permeabilization, and Flag-hSlo-GFP was a valid and useful tool for studying BKCa channel trafficking.
Effects of DS-201 on BKCa channel currents recorded under inside-out patch and whole-cell configurations in HEK293 cells
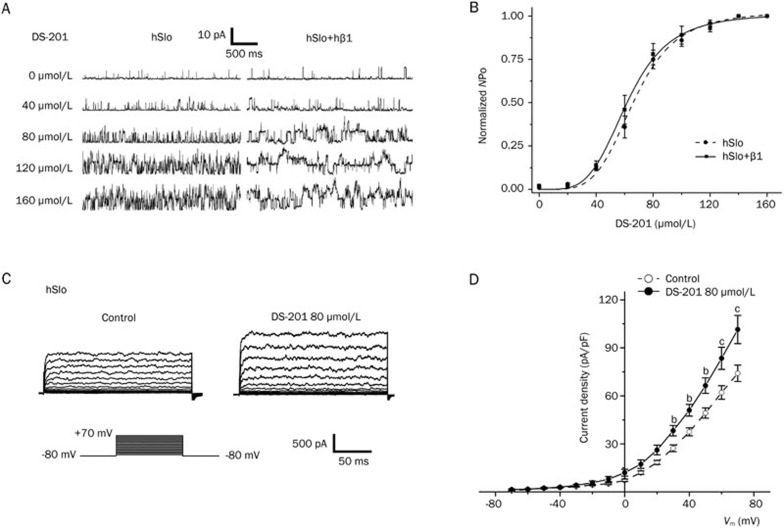
We reported that DS-201 activated the BKCa channels mainly from the intracellular side of the membrane in mouse cerebral arterial VSMCs14. Here, we further investigated the effects of DS-201 on the BKCa channels expressed in HEK293 cells under whole-cell and inside-out configurations. Figure 3A shows typical recordings of single BKCa channel currents under an inside-out configuration in HEK293 cells, with or without co-expression of the hβ1 subunit (Vm=+40 mV and [Ca2+]i=0.1 μmol/L). DS-201 activated the BKCa channels in a concentration (0–160 μmol/L)-dependent manner. Furthermore, the effects of DS-201 on BKCa channels were reversible after washout in the present study (data not shown). The curve of normalized NPo to DS-201 concentrations was fitted with Hill's equation (Figure 3B). When the [Ca2+]i level was set to 0.1 μmol/L, the half maximum activation concentration (EC50) was 62.04±1.07 μmol/L (with hβ1, n=7) or 66.64±1.54 μmol/L (without hβ1, n=6) (P>0.05). These results suggested that the β1 subunit did not affect the concentration-dependent effects of DS-201 on BKCa channels. DS-201 (80 μmol/L) also showed an agonist effect on the BKCa macroscopic currents recorded under a whole-cell configuration (Figures 3C and 3D). At serial membrane voltages (from +30 mV to +70 mV), DS-201 increased the current densities (P<0.05 or P<0.01 vs control, Figure 3D).
Figure 3.
Effects of DS-201 on BKCa currents in HEK293 cells recorded under the inside-out patch and whole-cell configurations. (A) Typical single-channel currents obtained under the inside-out configuration, showing that DS-201 dose-dependently (0–160 μmol/L) activated the BKCa(hSlo) channels of HEK293 cells with or without hβ1 coexpression. The recordings were obtained at a free [Ca2+]i level of 0.1 μmol/L and a Vm of +40 mV. (B) The concentration-response curve of DS-201 fitted by the Hill equation showing no significant difference in the EC50 between hSlo alone (n=6) and hSlo+hβ1 (n=7). (C) Representative macro-currents of control and DS-201 (80 μmol/L) treatment under a whole-cell configuration. (D) I–V curves showing that DS-201 (80 μmol/L) increased the current densities at Vm from +30 mV to +70 mV (n=8). Mean±SEM. bP<0.05 and cP<0.01 vs control.
Effects of DS-201 on the calcium and voltage dependence and on the kinetics of the BKCa channels expressed in HEK293 cells
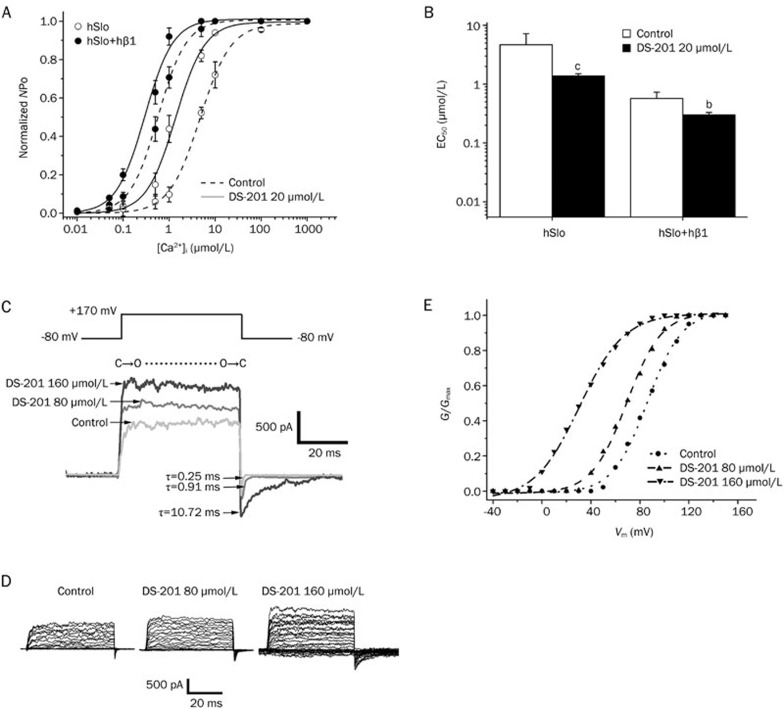
We confirmed previously that DS-201 modulated the BKCa channels of mouse cerebral arterial VSMCs by increasing the calcium and voltage dependence and by shifting the channel kinetics14. The present study further investigated whether DS-201 exerted similar acute effects on the BKCachannels expressed in HEK293 cells. Figures 4A and 4B show the effects of 20 μmol/L DS-201 on the calcium dependence of BKCa channels in an inside-out configuration (Vm=+40 mV). The open probability was normalized to the maximal NPo for each curve (Figure 4A). Coexpression of the hβ1 subunit increased the calcium dependence of BKCa channels by shifting the EC50 from 4.67±2.54 μmol/L (n=5) to 0.57±0.16 μmol/L (n=5), a result consistent with the previous report7. We reported that DS-201 at 20 μmol/L and 40 μmol/L increased the calcium dependence of BKCa channels in cerebral arterial VSMCs14. Here, we further demonstrated in HEK293 cells that 20 μmol/L DS-201, a concentration that did not activate BKCa channels significantly, shifted the EC50 of BKCa channels for calcium activation from 0.57±0.16 μmol/L (control, n=5) to 0.30±0.03 μmol/L (with hβ1, n=5) and from 4.67±2.54 μmol/L (control, n=5) to 1.38±0.12 μmol/L (without hβ1, n=5), respectively (Figures 4A and 4B). These results suggested that DS-201 increased the apparent calcium sensitivity of BKCa channels independently of the β1 subunit.
Figure 4.
Effects of DS-201 on the calcium and voltage dependence and the kinetics of BKCa channels expressed in HEK293 cells. (A) DS-201 at 20 μmol/L increased the calcium dependence of BKCa channels with or without hβ1 subunit expression in HEK293 cells in inside-out patch and Vm=+40 mV. The currents were normalized with maximal NPo for each curve. (B) Statistical bar graphs from (A). The EC50 of Ca2+-induced activation was decreased by 20 μmol/L DS-201 from control 0.57±0.16 μmol/L (n=5) to 0.30±0.03 μmol/L (n=5) with the hβ1 subunit and from control 4.67±2.54 μmol/L (n=5) to 1.38±0.12 μmol/L (n=5) without the hβ1 subunit, respectively. (C) Typical BKCa currents and tail currents showing the effects of 80 μmol/L and 160 μmol/L DS-201 on the kinetics of BKCa channels (without hβ1). “C” and “O” indicate the closed state and open state, respectively. (D) Typical macroscopic currents recorded in the inside-out macro-patches, showing the effects of DS-201 (80 and 160 μmol/L) on hSlo BKCa channels (without hβ1) with step pulses. (E) Effects of 80 and 160 μmol/L DS-201 on the G/Gmax–Vm curves. Mean±SEM. bP< 0.05, cP< 0.01 vs control.
In addition, we examined the effects of DS-201 on the voltage dependence and kinetics of the BKCa channels (hSlo, without the hβ1 subunit) in the inside-out macro-patches ([Ca2+]i=0.1 μmol/L). Figure 4D shows that DS-201 (80 and 160 μmol/L) increased the current amplitude, consistent with Figure 3C. In addition, DS-201 shifted the G/Gmax–Vm curve (fitted from Figure 4D) leftward (Figure 4E), suggesting that DS-201 could activate BKCa channels at relatively more negative membrane potentials.
It is known that the current transition from a holding potential to a test pulse represents the shift of the BKCa channel from closed state to open state, and the tail current traces represent the transition from an open state to closed state. We found that 80 μmol/L and 160 μmol/L DS-201 did not affect the transition from closed state to open state (Figure 4C). The time constants of activation were 0.35 ms (control), 0.26 ms (80 μmol/L DS-201), and 0.31 ms (160 μmol/L DS-201). However, the same DS-201 concentrations (80 and 160 μmol/L) significantly slowed the transition from open state to closed state (Figure 4C). The time constants of deactivation were 0.25 ms (control), 0.91 ms (80 μmol/L DS-201), and 10.72 ms (160 μmol/L DS-201). These data were consistent with those observation in mouse cerebral arterial VSMCs in our previous report14, suggesting that DS-201 could bind to the BKCa channel α subunit directly and could retain it in the open state, thus inhibiting the transition from open to closed state.
DS-201 enhanced the expressions of the α subunit, but not of the β1 subunit, of BKCa channels in cultured VSMCs and HEK293 cells
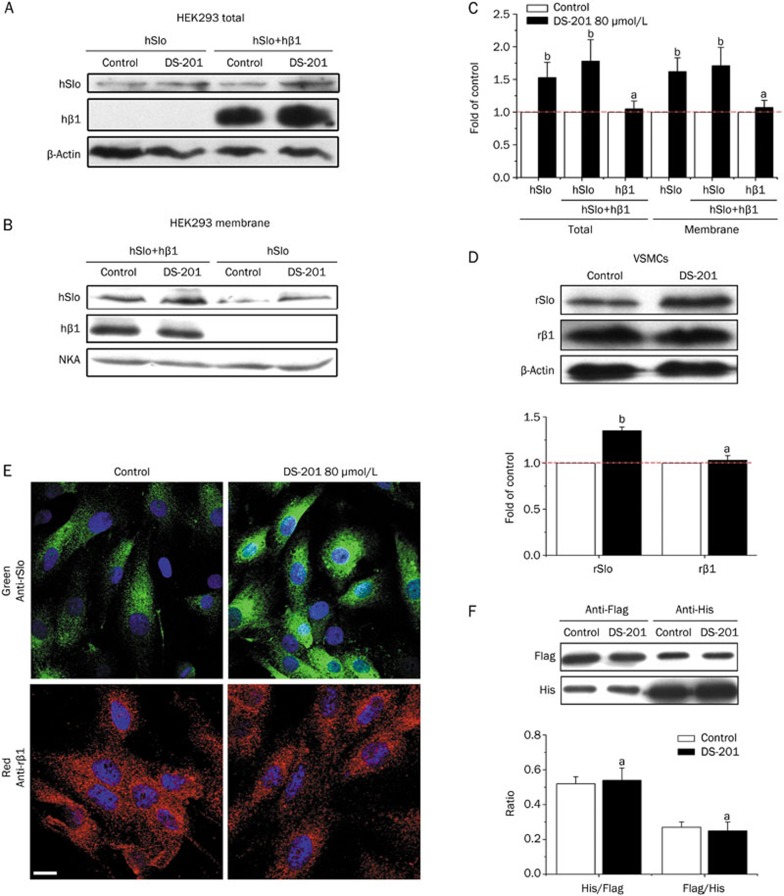
It is well recognized that the β1 subunit increases the expression of the α subunit of BKCa channels in VSMCs16. Therefore, in addition to observing the “acute” effects of DS-201 on channel electrophysiological properties, we further investigated whether prolonged use of DS-201 would affect the expression of BKCa channel subunits both in cultured VSMCs and in HEK293 cells expressing the α and β1 subunits. After incubation of cells with DS-201 for 12 h, Western blotting was performed. As shown in Figures 5A and 5B, DS-201 at 80 μmol/L increased the total and membranous expression levels of hSlo in HEK293 cells. However, DS-201 at the same concentration did not affect β1 subunit expression. A statistical summary of the subunit expression with or without DS-201 treatment is shown in Figure 5C. In cultured VSMCs, DS-201 at 80 μmol/L also increased the total protein expression level of the α subunit but did not affect the expression of the β1 subunit (Figure 5D), a result consistent with that observed in HEK293 cells. Confocal images further proved that 80 μmol/L DS-201 promoted the expression of the α subunit (green) and did not affect the expression of the β1 subunit (red) in cultured VSMCs (Figure 5E).
Figure 5.
DS-201 upregulated the expression of the BKCa channel α subunit but did not affect the expression of the β1 subunit. (A and B) Western blotting showing the effects of 80 μmol/L DS-201 on the total and membranous protein expression levels of the α and β1 subunits in HEK293 cells. Endogenous β-actin and Na+/K+-ATPase (NKA) served as loading controls, respectively. (C) Statistical results showing the enhancing effects of 80 μmol/L DS-201 on the membranous and total expressions of the α subunit but not of the β1 subunit in HEK293 cells. The signal intensity of each band was normalized to the band of β-actin or NKA. The effects of DS-201 were expressed as fold changes compared with control. (D) Effects of 80 μmol/L DS-201 on the total protein expression levels of the α and β subunits in cultured VSMCs. (E) Confocal images showing that DS-201 enhanced the expression of the BKCa α subunit (upper, green) but did not affect the expression of the β1 subunit (lower, red) in cultured VSMCs. Scale bar=50 μm. (F) Co-IP assay showing the effects of DS-201 on the interaction between the α (Flag-tagged) and β1 (His-tagged) subunits in HEK293 cells. The statistical results (lower) showed that DS-201 did not affect the ratio of hβ1 to hSlo or of hSlo to hβ1, when either anti-Flag or anti-His was used as the bait. Mean±SEM. aP>0.05, bP<0.05 vs control.
Furthermore, we investigated using co-IP assay whether DS-201 affected the expression of hSlo by accelerating the interaction of hSlo with hβ1. The anti-Flag (for hSlo) and anti-His (for hβ1) antibodies were used as the baits for immunoprecipitation. We calculated the portion of hβ1 co-immunoprecipitated by hSlo, and vice versa. DS-201 did not change the ratio of hSlo to hβ1 when either the anti-Flag or the anti-His antibody was used (Figure 5F). These results suggested that DS-201 accelerated the expression of hSlo, but not by affecting the interaction between hSlo and hβ1.
DS-201 accelerated the trafficking of the α subunit independently of the β1 subunit of BKCa channels in HEK293 cells
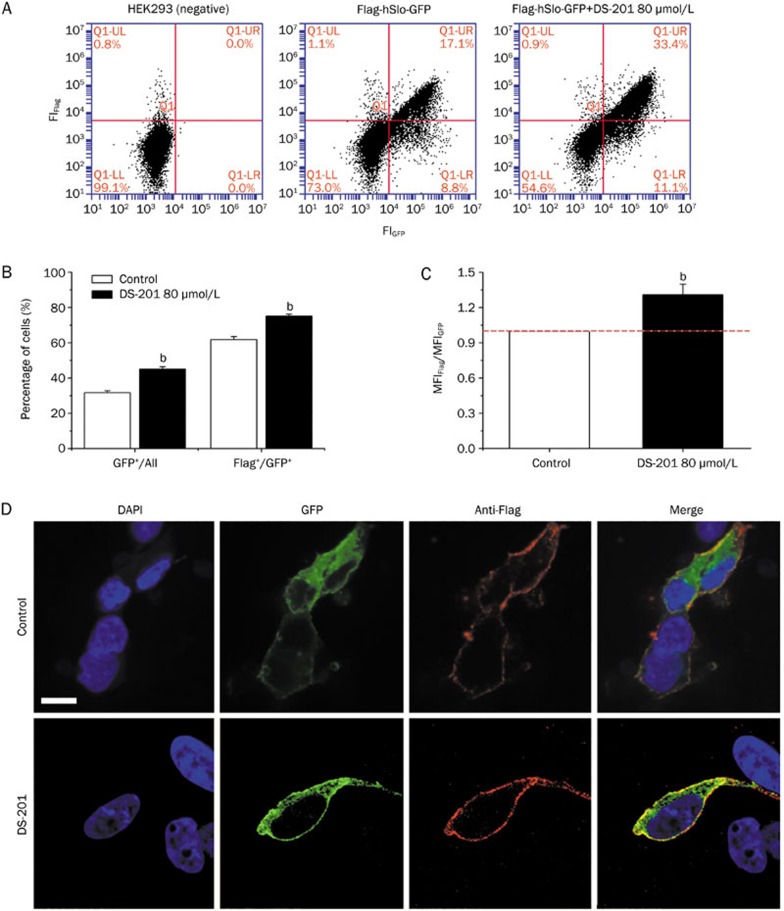
Many ion channels function only when their subunits are transferred from the Golgi apparatus to the plasma membrane. Thus, investigation of channel trafficking is necessary for identifying the action of a drug on ion channels. Whether DS-201 promotes the trafficking of the pore-forming α subunit of BKCa channels is unknown. To address this question, plasmid Flag-hSlo-GFP was transfected to HEK293 cells to detect the subcellular localization of the α subunit, with the help of FCM and confocal microscopy. Incubation of the transfected HEK293 cells with DS-201 (80 μmol/L) for 12 h increased the membranous level of the α subunit (Figure 6). DS-201 at 80 μmol/L increased the percentage of GFP-positive cells from 31.7%±1.1% to 41.1%±1.3% (n=8, P<0.05) (Figures 6A and 6B), indicating again that DS-201 increased the global expression level of the α subunit. In addition, within the GFP-positive cells, DS-201 (80 μmol/L) also increased the percentage of Flag-positive cells from 61.8%±1.7% to 75.2%±1.0% (Figure 6B) and shifted the MFIFlag/GFP (an indicator of the membranous α subunit relative to the total α subunit pool) from 0.13 to 0.18 (Figure 6C), suggesting that DS-201 accelerated the trafficking of the α subunit toward the cell membrane. The confocal images further provided intuitive evidence that DS-201 (80 μmol/L) increased the membranous level of the α subunit in HEK293 cells (Figure 6D).
Figure 6.
Effects of DS-201 on the subcellular localization of BKCa channel α subunits in HEK293 cells. (A) Original FCM recording showing that 80 μmol/L DS-201 increased the percentage of GFP-positive (the first plus the fourth quadrant) and Flag-positive cells (the first quadrant) in HEK293 cells expressing the α subunit. Untransfected HEK293 cells served as negative controls. (B) Statistical results from (A). GFP+, GFP-positive cells. Flag+, Flag-positive cells (n=8). (C) The statistical results showing the effects of 80 μmol/L DS-201 on the MFIFlag/MFIGFP ratio, representing the membranous level of the α subunit (n=8). (D) Confocal images showing the membranous and intracellular distributions of hSlo. GFP (green) and anti-Flag (red) represent the global and membranous hSlo, respectively. Note that DS-201-treated cells show increased membranous hSlo, compared with control cells. Scale bar=50 μm. bP<0.05 vs control.
DS-201 stabilized the membranous retention of BKCa channel proteins in HEK293 cells
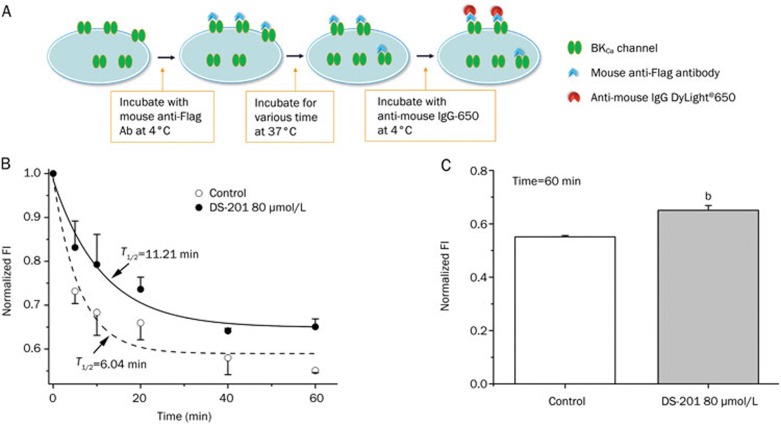
Endocytosis is an important mechanism for downregulating the functional expression of channels in the cell membrane. Therefore, the increase in membranous BKCa channels shown above might partially have been caused by decreased internalization (endocytosis) when DS-201 was administered. If this relationship was true, the BKCa channels might remain in the plasma membrane for a longer time. Here, we used flow cytometry to confirm this possibility. Surface BKCa channels were initially labeled with the anti-Flag antibody at 4 °C, and then the cell samples were switched to 37 °C to allow the channel protein to internalize for different lengths of time. After these procedures, some channels were supposed to be internalized, but others were not. The channels that were not internalized were stained with DyLight®650-conjugated donkey anti-mouse IgG antibody and were detected by flow cytometry (Figure 7A). Figure 7B shows the single exponential curves indicating the channel internalization dynamics over time. The curves revealed that DS-201 decreased the internalization speed, with a shift of the t1/2 from 6.04 min (control, n=5) to 11.21 min (DS-201, n=5) (P<0.05). Figure 7C shows the fluorescence intensity (FI) 60 min after DS-201 was administered: DS-201 reduced the decay of FI compared with the control, suggesting that DS-201 stabilized the anchoring of the BKCa channel in the plasma membrane to some extent.
Figure 7.
DS-201 stabilized the membranous retention of BKCa channel proteins in HEK293 cells. (A) Schematic illustration showing the protocol for investigating BKCa channel internalization. Cell surface BKCa channels were first stained with anti-Flag antibody and then the α subunits were allowed to internalize for different time (0, 5, 10, 20, 40, and 60 min) at 37 °C. The remaining channels in the membrane were labeled by DyLight®650-conjugated donkey anti-mouse IgG antibody and were detected by flow cytometry. (B) Normalized fluorescence intensity indicating the effects of DS-201 (80 μmol/L) on the membranous anchoring of BKCa channels (n=5). The time course of internalization was fitted by a single exponential equation: y=A1×exp(-x/t1)+y0. (C) Statistical results showing the normalized fluorescence intensity (FI) at 60 min of internalization (n=5). Note that DS-201 stabilized the membranous retention of BKCa channel α subunits. Mean±SEM. bP<0.05 vs control.
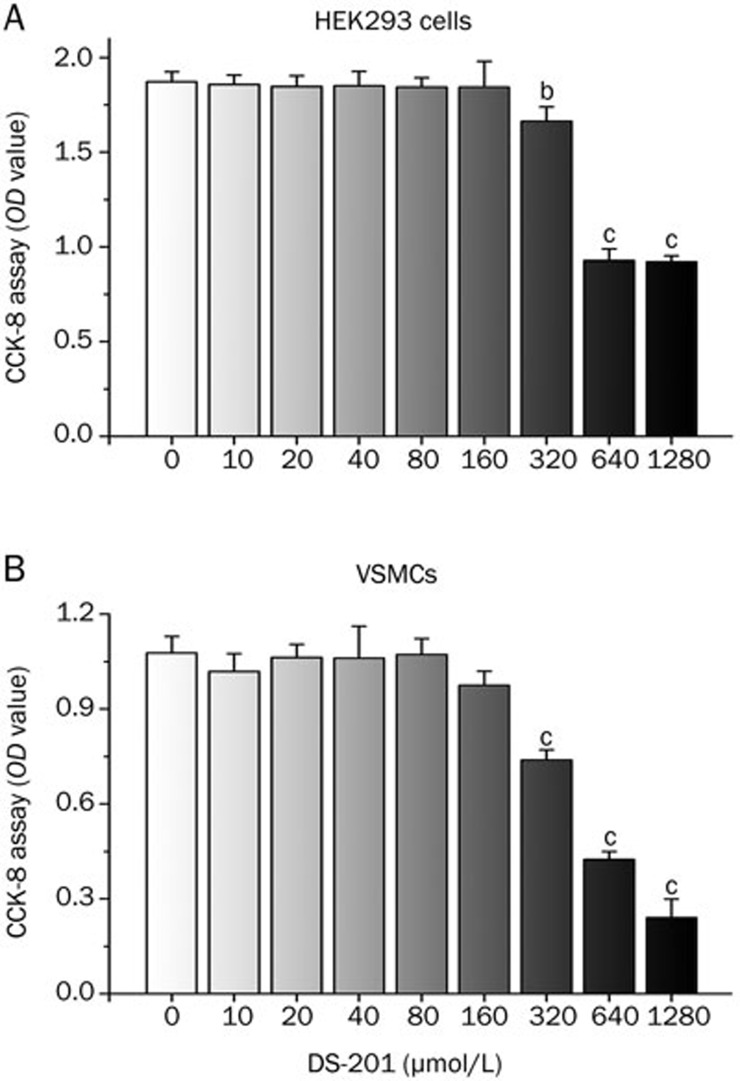
DS-201 induced cell death only at very high concentrations
The potential cytotoxicity of DS-201 on HEK293 cells and VSMCs was evaluated by CCK-8 assay. DS-201 exposure for 12 h at relatively lower concentrations (10, 20, 40, 80, and 160 μmol/L) did not significantly affect cell viability. However, DS-201 at 320 μmol/L or higher concentration induced cell death and decreased cell viability in a dose-dependent manner (Figure 8). Therefore, the concentrations of DS-201 used in most of the experiments in this study (160 μmol/L or lower) were safe to cells and did not exert significant cytotoxic effects on HEK293 cells or VSMCs.
Figure 8.
CCK-8 assay showing the concentration-dependent cytotoxicity of DS-201 on HEK293 cells (A) and VSMCs (B) exposed to DS-201 for 12 h. Note that DS-201 induced cell death and reduced the cell viability of both types of cells only at concentrations greater than 320 μmol/L. Mean±SEM. bP<0.05, cP<0.01 vs respective control (DS-201 at 0 μmol/L).
Discussion
It is well known that the native BKCa channel consists of four α subunits and four β subunits (β1-β4). The α subunit, encoded by Slo or the KCNMA1 gene, is a pore-forming portion of the channel. Slo is the only gene encoding the α-subunit, and KCNMB1 was the gene encoding the β1 subunit in VSMCs2,4,17. The present study first revealed that DS-201 modulated BKCa channels in a novel manner, independent of the β1 subunit. DS-201 directly modulated the pore-forming α subunit by increasing the calcium and voltage dependency and shifting the channel kinetics. These might be considered the acute electrophysiological effects of DS-201 on BKCa channels. In addition, DS-201 promoted the expression, membranous retention and trafficking of the α subunit when DS-201 acted for a longer period of time (12 h).
The role of the β subunit in BKCa channels has been extensively investigated using co-expression of different β subunits with the α subunit, including β1 to β418,19. As a whole, the β subunit shifts the voltage-dependent characteristics of the channel activity to a direction of more negative membrane potential by increasing Ca2+ sensitivity. The second role of the β subunit is that it increases the membrane expression and trafficking of BKCa channels16. Recently, we reported for the first time that, in Han Chinese patients with primary hypertension, the whole-cell current and Ca2+ sensitivity of BKCa channels were reduced in VSMCs due to downregulation of the β1 subunit but not of the α subunit9. These results suggested that the α and β subunits of BKCa channels were rather independently regulated by different molecular mechanisms. The basic function of BKCa channels can be expressed by the α subunit alone, and the β subunit has only supplementary action on BKCa channel function. There have been reports showing that some chemical molecules modified BKCa channel function by interacting with the β subunit20, whereas other molecules regulated the channel function by direct interaction with the α subunit21.
Here, we investigated the effects of DS-201 on BKCa channels, including the electrophysiological properties, channel protein expression and trafficking, with the convenience of HEK293 cell line, which allows for heterologous expression of the α subunit with or without the β1 subunit. Cultured VSMCs were also used as native vascular cells in this study. We first found that DS-201 activated BKCa channels reversibly in a dose-dependent manner, and the β1 subunit was not involved in this effect. The EC50 of DS-201 was 62.04 μmol/L with the β1 subunit and 66.64 μmol/L without it. These EC50 values were similar to our previous observation (68.5 μmol/L) in mouse cerebral arterial VSMCs14. Based on these observations, we used 80 μmol/L DS-201 in most of the experiments. At this concentration, DS-201 increased the whole-cell BKCa currents significantly. We further observed previously that 20 and 40 μmol/L DS-201 increased the apparent calcium sensitivity of BKCa channels in mouse cerebral arterial VSMCs14. The present study also chose a low concentration (20 μmol/L) of DS-201, which did not activate the BKCa channels but could increase the apparent calcium sensitivity of the channel, to test the effects of DS-201 on the calcium dependence of this channel in HEK293 cells expressing either the α subunit alone or with the β1 subunit. The results showed that 20 μmol/L DS-201 enhanced the apparent calcium sensitivity of the channel in HEK293 cells, and this effect was independent of the β1 subunit. However, it is interesting that DS-201, at lower concentrations (20 and 40 μmol/L), did not shift the voltage dependence and open/closed kinetics of the channel. The reason for this finding could be that BKCa channels are more sensitive to calcium than to voltage, or other mechanisms were involved in the phenomenon. As such, we used higher DS-201 concentrations (80 and 160 μmol/L) to avoid potential disputes in explaining the mechanisms. The data revealed that DS-201 increased the voltage dependence and inhibited the transition of the channel from the open state to the closed state in HEK293 cells. These results were quite consistent with those observed in the native BKCa channels of mouse cerebral VSMCs14. We further showed that DS-201 increased the protein expression of the α subunit but did not affect the expression of the β1 subunit, either in HEK293 cells or in cultured VSMCs. DS-201 also did not affect the interaction of the α and β1 subunits, as shown by the co-IP assay. Taken together, these data reveal that the β1 subunit did not contribute to the effects of DS-201 on BKCa function, including those effects on channel open probability, calcium and voltage dependence, shifting of channel kinetics, and channel α subunit expression. These effects of DS-201 are unique and quite different from those of other substances, compared even with many other Chinese medicinal herbs with vasorelaxing effects, such as Puerarin. The latter could induce vasodilation, and the β1 subunit was involved in this effect22.
As mentioned above, channel proteins must be transported and inserted to the cell membrane to function. Therefore, controlling channel trafficking dynamics with drugs could serve as a new pharmacological approach in treating diseases. We reported that BKCa currents were decreased in hypertension, together with the downregulation of β1 subunit expression but not α subunit expression9. Functional defects of the β1 subunit could lead to a reduction in BKCa α subunit trafficking, and the α subunit would be retained in the cytoplasm and could not be targeted to the cell membrane efficiently.
The trafficking processes of proteins include forward trafficking (toward the plasma membrane), internalization (endocytosis) and recycling to the membrane. Any change in one or more of these processes will affect the expression levels of channels in the plasma membrane. The present study first found that DS-201 increased the gene expression of the BKCa α subunit without affecting the β1 subunit, and, furthermore, DS-201 enhanced the trafficking of the BKCa channel α subunit again independently of the β1 subunit. However, we could not accurately evaluate whether one or more steps were involved in the effects of DS-201, because the three steps were dynamic and connected each other and were difficult to distinguish. We found that DS-201 slowed the internalization and stabilized the α subunit's anchoring in the plasma membrane. It is also possible that the recycling process was involved in the actions of DS-201, and this possibility might have affected the interpretations of the results. However, a study by McEwen et al23 using similar methods, indicated that the amounts of recycling from internalization of the Kv1.5 channel were small (less than 10% for recycling and approximately 30% for internalization), and the recycling kinetics were slower than those of internalization. The present study showed that DS-201 increased the membranous levels of BKCa channels (an indicator of the comprehensive trafficking processes) by approximately 38.5%, as indicated by the MFIFlag/GFP ratio (Figure 6C). However, in an experiment that examined only the internalization and potential recycling processes (Figure 7), the difference in membranous BKCa levels before and after DS-201 treatment was less than 20% (Figure 7C). Therefore, we inferred that DS-201 could accelerate forward trafficking. Certainly, it was a limitation of this study that we did not quantitatively examine the forward trafficking. If taken together, the electrophysiological results and the expression and trafficking results of this study demonstrated that DS-201 potentiated the function of the BKCa channel and did not require the presence of the β1 subunit. Therefore, DS-201 had complementary effects in diseases with β1 subunit deficiency, such as hypertension. These extraordinary effects of DS-201 on BKCa channels favored more widespread use of Danshen in cardiovascular medicine.
The data presented here suggested an interaction between DS-201 and the hSlo subunit, and this action could lead to enhancement of BKCa channel activity. Considering the amino acid compositions of the β1 subunit, there were 118 amino acid residues in the extracellular loop, while only 18 residues and 13 residues were located in the N- and C-terminals, respectively. However, DS-201 modulated the BKCa channels mainly from the cytoplasmic side of the membrane, so there was little opportunity for DS-201 to bind to the cytoplasmic N- or C-terminal of the β1 subunit. We infer that DS-201 could bind to the α-subunit directly, but the exact binding site is unknown and requires further study. Because BKCa channel activity in VSMCs generated outward currents that drive the membrane potential in the negative direction, eventually counteracting vascular contraction, DS-201-induced activation of BKCa could be an underlying mechanism, or at least contributing to the Danshen-induced relaxation of VSMCs.
Author contribution
Xiao-qiu TAN, Xiu-li CHENG, Xiao-rong ZENG, and Ji-min CAO designed the research; Xiao-qiu TAN, Xiu-li CHENG, Yan YANG, Li YAN, Jing-li, GU, and Hui LI performed the experiments; Xiao-qiu TAN, Xiu-li CHENG, and Ji-min CAO analyzed the data; Xiao-qiu TAN, Xiu-li CHENG, and Ji-min CAO wrote the paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31300948 to Xiao-qiu TAN, 81173661 to Yan YANG, 81300139 to Jing-li GU, and 31171088 to Ji-min CAO). We thank Prof Isao INOUE for his helpful comments during the manuscript preparation.
References
- Rusch NJ. BK channels in cardiovascular disease: a complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297:H1580–2. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Wu SN. Large-conductance Ca2+-activated K+ channels: physiological role and pharmacology. Curr Med Chem. 2003;10:649–61. doi: 10.2174/0929867033457863. [DOI] [PubMed] [Google Scholar]
- Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–56. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha+beta subunit complexes. J Physiol. 1997;502:545–57. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–6. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, et al. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61:519–25. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- Wu T, Ni J, Wu J. Danshen (Chinese medicinal herb) preparations for acute myocardial infarction. Cochrane Database Syst Rev. 2008;(2):CD004465. doi: 10.1002/14651858.CD004465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Chen JM, Peng Y, Wu Q, Xiao PG. Investigation of Danshen and related medicinal plants in China. J Ethnopharmacol. 2008;120:419–26. doi: 10.1016/j.jep.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Cheng TO. Danshen: a popular chinese cardiac herbal drug. J Am Coll Cardiol. 2006;47:1498. doi: 10.1016/j.jacc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cai F, Li PY, Li ML, Chen J, Chen GL, et al. Activation of high conductance Ca2+-activated K+ channels by sodium tanshinoneII-A sulfonate (DS-201) in porcine coronary artery smooth muscle cells. Eur J Pharmacol. 2008;598:9–15. doi: 10.1016/j.ejphar.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Tan X, Yang Y, Cheng J, Li P, Inoue I, Zeng X. Unique action of sodium tanshinone II-A sulfonate (DS-201) on the Ca2+ dependent BKCa activation in mouse cerebral arterial smooth muscle cells. Eur J Pharmacol. 2011;656:27–32. doi: 10.1016/j.ejphar.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem. 2004;279:48231–7. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol. 2007;97:3508–16. doi: 10.1152/jn.00009.2007. [DOI] [PubMed] [Google Scholar]
- Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- Orio P, Latorre R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J Gen Physiol. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–31. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- Dopico AM. Ethanol sensitivity of BKCa channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am J Physiol Cell Physiol. 2003;284:C1468–80. doi: 10.1152/ajpcell.00421.2002. [DOI] [PubMed] [Google Scholar]
- Sun XH, Ding JP, Li H, Pan N, Gan L, Yang XL, et al. Activation of large-conductance calcium-activated potassium channels by puerarin: the underlying mechanism of puerarin-mediated vasodilation. J Pharmacol Exp Ther. 2007;323:391–7. doi: 10.1124/jpet.107.125567. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, et al. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–20. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]