Abstract
Aim:
Interleukin-22 (IL-22) exhibits both proinflammatory and anti-inflammatory properties in various biological processes. In this study we explored the effects of exogenous recombinant IL-22 (rIL-22) on cigarette smoke (CS)-induced airway inflammation in mice.
Methods:
Male C57BL/6 mice were divided into groups: (1) CS group exposed to tobacco smoke for 3 consecutive days, (2) rIL-22 group received rIL-22 (100 mg/kg, ip), and (3) CS plus rIL-22 group, received rIL-22 (100 mg/kg, ip) before the CS exposure. The airway resistance (Rn), lung morphology, inflammatory cells in the airways, and inflammatory cytokines and CXCR3 ligands in both bronchoalveolar lavage (BAL) fluids and lung tissues were analyzed.
Results:
CS alone significantly elevated IL-22 level in the BAL fluid. Both CS and rIL-22 significantly augmented airway resistance, an influx of inflammatory cells into the airways and lung parenchyma, and significantly elevated levels of pro-inflammatory cytokines (TGFβ1 and IL-17A) and CXCR3 chemokines (particularly CXCL10) at the mRNA and/or protein levels. Furthermore, the effects of rIL-22 on airway resistance and inflammation were synergistic with those of CS, as demonstrated by a further increased Rn value, infiltration of greater numbers of inflammatory cells into the lung, higher levels of inflammatory cytokines and chemokines, and more severe pathological changes in CS plus rIL-22 group as compared to those in CS group.
Conclusion:
Exogenous rIL-22 exacerbates the airway inflammatory responses to CS exposure in part by inducing expression of several proinflammatory cytokines and CXCR3 ligands.
Keywords: cigarette smoke, lung, airway inflammation, cytokine, IL-22, IL-17A, TGFβ1, CXCR3 chemokine
Introduction
IL-22 is a member of the IL-10 cytokine family and plays an important role in the inflammatory responses associated with mucosal immunity1,2,3. Recent evidence has shown that many immune cells, such as CD4+ T cells (particularly Th17 cells), NK cells, and LTi-like cells, produce IL-224,5,6. IL-22 targets the cells of the respiratory system, skin and gastrointestinal tract. The receptor for IL-22 (IL-22R) is a heterodimeric receptor consisting of IL-22R1 and IL-10R27. IL-10R2 is ubiquitously distributed in various cells, while the expression of IL-22R1 is restricted to epithelial cells of non-hematopoietic origin, primarily those of the skin, gut, and lung. This distribution allows the cytokine to play a part in the innate immune response of the epithelia in response to various extracellular invading pathogens8,9,10.
Several studies performed in animal models have demonstrated that IL-22 exerts a protective role in the eosinophilic airway inflammation induced by allergens, ventilator-induced lung injury, and the airway inflammation and pulmonary fibrosis induced by bleomycin11,12,13,14,15,16. In human asthma studies, Pennino and colleagues demonstrated a tissue-restricted regulatory role of IL-22 in controlling the extent of the lung inflammation mediated by IFNγ17. IL-22 is a major cytokine produced by Th17 cells and is capable of inducing the release of numerous chemokines and growth factors that recruit leukocytes to the site of injury2,7,18, suggesting a proinflammatory/pathological role for IL-22. Evidence supporting the inflammatory characteristics of IL-22 includes the reduction in acanthosis and neutrophil infiltration in the inflamed skin of IL-22 knockout mice after repeated treatment with IL-23. Additionally, IL-22-secreting T cells are involved in the recruitment of inflammatory cells in an IL-22- and TNFα-dependent manner19. Recent studies have shown that IL-22 is detected at the site of allergic airway inflammation. Moreover, a higher serum concentration of circulating IL-22 is observed in patients with severe asthma compared with that in patients with milder disease or in healthy controls11,20.
Cigarette smoke (CS) is a major risk factor in the pathogenesis of chronic obstructive pulmonary disease (COPD)21. CS is a profound proinflammatory stimulus. An acute CS exposure triggers lung inflammation and the subsequent responses play crucial roles in the development of bronchospasm, airway remodeling, and lung tissue damage21. T cells are a prominent cell type found in the inflammatory environment of the lungs of COPD patients, with CD4+ and CD8+ T cells being found in both the airways and parenchyma of these patients22,23. Th1, Th17, and cytotoxic T cells (Tc) participate in lung inflammation through the release of a plethora of cytokines (including IFNγ, IL-17, and IL-22) and the induction of apoptosis in airway epithelial cells13. COPD-associated Th1 cells (known as Th17 cells) have been identified in lungs from COPD patients, indicating the important role of the Th17 cell-derived cytokines. For instance, IL-22 and IL-17A can induce the expression of MMP3 and MMP12, the latter of which is found to be increased in CS-induced emphysema24. Recent evidence from human samples has shown a significant increase in the numbers of peripheral blood IL-22+ cells in the CD4+ memory T-cell population in active smokers with COPD and an increased number of IL-22+ immunoreactive cells in both the bronchial epithelium and the bronchial submucosa in stable COPD25,26,27. A significantly elevated IL-22 concentration has been detected in the sputum and blood from COPD patients and healthy smokers compared with healthy nonsmokers25. These data might suggest the importance of IL-22 in the pathogenesis and progression of COPD. However, IL-22 can function as either an anti-inflammatory cytokine or a pro-inflammatory cytokine, depending on the context in which it is expressed11,15.
Given the multiple possible roles of IL-22 during inflammatory and protective processes, its effect on airway inflammation in the setting of CS exposure is not completely understood. The aim of this study was to investigate the in vivo effect of exogenous IL-22 on lung inflammation induced by an acute exposure to CS in an animal model. Our data suggest that the exogenous administration of IL-22 might play an inflammatory role in CS-induced airway pathology.
Materials and methods
Animals and experimental design
Male C57BL/6 mice (8–10-week old; 20–25 g body weight) were purchased commercially (Experimental Animal Research Center, Beijing, China), and maintained in a specific-pathogen free mouse facility at the Peking Union Medical College Hospital Animal Care Center. All experiments were performed in accordance with International and Institutional Guidelines for Animal Care and were approved by the Peking Union Medical College Hospital Committee on Animal Care and Use.
CS exposure was performed according to previously described procedures28,29. Briefly, the mice were placed in a closed plastic box connected to a smoke generator. The mice were subjected to whole-body exposure to the tobacco smoke of five cigarettes (Reference Cigarette 3R4F, University of Kentucky, USA) four times a day with 30-min smoke-free intervals between each smoke exposure for three consecutive days. Three additional groups of mice were included in the experimental design. One group of mice was exposed to CS and received a single intraperitoneal (ip) injection of recombinant IL-22 (rIL-22) (Generon Corporation, Shanghai, China) at a dose of 100 mg/kg body weight half an hour before the CS exposure. The second group was exposed to filtered air, but received an injection of rIL-22. The last group was exposed to filtered air. The dosage of rIL-22 used for this investigation was based on previously published articles with modifications30,31. A 100 mg/kg body weight dose of rIL-22 was found to induce sufficient airway inflammation without causing systemic effects, such as significant weight loss and fatigue, in the mice.
After the acclimatization period, the mice were randomly divided into four groups (n=6 for each group): 1, air exposure (air); 2, rIL-22 treatment (rIL-22); 3, CS exposure (CS); 4, CS exposure with rIL-22 treatment (CS+rIL-22).
The mice were sacrificed 24 h after the last of the CS exposures, and the various parameters were measured.
Airway resistance test
Airway resistance (Rn) was measured as previously described for the invasive analysis of lung mechanics using a computer-controlled small animal ventilator, the Flexivent system (Scireq, Montreal, PQ, Canada)32,33. Briefly, the mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (80 mg/kg) after 3 d of CS exposure. The trachea was exposed and a 20-gauge cannula was inserted. The mouse was mechanically ventilated at a frequency of 150 breaths/min and a tidal volume of 10 mL/kg. Two minutes after a deep inspiration at an airway pressure of 30 cmH2O, the impedance of the respiratory system was measured at a positive end-expiratory pressure (PEEP) of 2 cmH2O.
Histological analysis
After 3 d of CS exposure, the lungs were removed and inflation-fixed with 10% formalin at 25 cmH2O overnight. Then, the lungs were embedded in paraffin and sectioned at 5-μm thickness. Hematoxylin-eosin (H&E) staining was performed at the Department of Pathology, Peking Union Medical College Hospital (Beijing, China). According to a previously published method34, an index of histopathological changes was evaluated by scoring the severity and extent of the occlusion of the airway lumen, epithelial damage, goblet-cell metaplasia, squamous cell metaplasia, infiltration of inflammatory cells, and deposition of collagen around the airways. The severity of the pathological changes in each lung section was assessed as a mean score of severity on a scale of 0 to 4 (0, no disease to 4, maximal disease).
The histopathological evaluations of the airways and lung parenchyma were performed by an experienced pathologist in a blinded manner.
Masson trichrome staining
To evaluate the collagen deposition, the paraffin sections obtained from the murine lung tissues were stained using a commercial kit (MassonTrichrome Stain kit, Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instruction.
Bronchoalveolar lavage (BAL)
The mice were sacrificed, and a 20 gauge catheter was inserted into the trachea. BAL was performed twice with 1 mL of saline, and 90% of the total injected volume was recovered. The recovered BAL fluid was centrifuged, and the supernatant was collected for cytokine analysis. The cell pellets were harvested, and the red cells were lysed. The cell pellets were washed twice and resuspended in 1 mL of saline. The total cells were counted on a hemocytometer. For cell differential counting, the cells were spun onto glass slides, fixed, and stained with H&E. Four hundred cells were evaluated to differentiate the macrophages, neutrophils, and lymphocytes based on morphology.
Detection of the mRNA expression of CXCL9, CXCL10, CXCL11, IL-17A, and TGFβ1 in the lung tissue by quantitative real-time PCR (qRT-PCR)
The expression of the mRNA for CXCL9, CXCL10, CXCL11, IL17A, and TGFβ1 was determined using RT-PCR. Briefly, total RNA was isolated from lung tissues using TRIzol (Invitrogen). cDNA was synthesized according to the instructions of cDNA kit (Takara Corporation, Japan). The primers and products of the RT-PCR are listed in Table 1.
Table 1. Primer sequences and products of qRT-PCR.
| Genes | F/R | Primer sequence (5′ to 3′) | Products (bp) |
|---|---|---|---|
| GAPDH | F | TGCTGAGTATGTCGTGGAGTCT | 103 |
| R | AGAAGGGGCGGAGATGAT | ||
| CXCL9 | F | GCCTGCCTTCCTTCCTTTCTC | 147 |
| R | TTCACCCTGTCTGGCTCTGTG | ||
| CXCL10 | F | GAGTGAAGCCACGCACACAC | 80 |
| R | ACTGGGTAAAGGGGAGTGATG | ||
| CXCL11 | F | TGAGATGAACAGGAAGGTCACA | 184 |
| R | AACTTTGTCGCAGCCGTTACTC | ||
| IL-17A | F | GACTCTCCACCGCAATGAA | 204 |
| R | ACACCCACCAGCATCTTCTC | ||
| TGFβ1 | F | CCTGAGTGGCTGTCTTTTGAC | 123 |
| R | GTGGAGTTTGTTATCTTTGCTGTC |
F, forward; R, reverse.
Determination of CXCL9, CXCL10, CXCL11, IL-17A, and TGFβ1 in the lung tissues by immunohistochemistry (IHC)
Immunohistochemistry (IHC) was performed as previously described35. Briefly, the tissue sections (5 μm) were deparaffinized in xylene and rehydrated in a graded series of ethanol washes. The slides were then quenched with 3% hydrogen peroxide for 10 min at room temperature, placed in a citrate buffer (pH 6.0), heated twice in a microwave oven for 5 min each, and finally blocked for 20 min with 10% normal goat serum at room temperature. The sections were next incubated with the primary antibodies in 1% BSA overnight at 4 °C. The detection was carried out using biotinylated goat anti-rabbit secondary antibodies (Dakocytomation), streptavidin-avidin-biotin complex/horseradish peroxidase (HRP) (Vectastain EliteABC; Vector Laboratories) and diaminobenzidine tetrahydrochloride (Sigma-Aldrich).
ELISA analysis of cytokines or chemokines
The concentrations of CXCL9, CXCL10, CXCL11, IL-6, IL-8, IL-22, TNFα, and TGFβ1 in the BAL fluid were measured using ELISA kits (R&D Systems) according to the manufacturer's instruction.
Statistical analysis
The data are expressed as the mean±SD. As appropriate, comparisons between two groups were carried out using analysis of variance (ANOVA) and the non-parametric two-tailed t test using GraphPad PRISM software (Version 6.0 for Windows; GraphGrad, San Diego, CA, USA). P<0.05 was considered significant.
Results
Effect of rIL-22 and/or acute CS exposure on airway resistance (Rn)
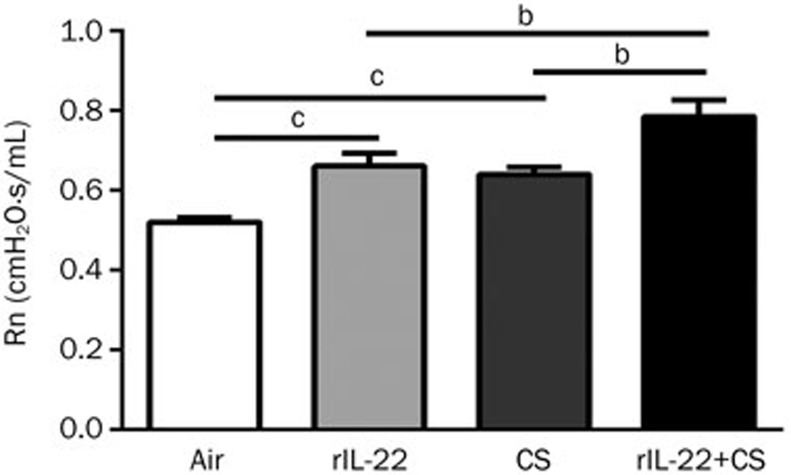
First, we evaluated the effect of the acute CS exposure in the presence and absence of rIL-22 on the airway resistance in the mice after 3 d of CS exposure. We used the small animal ventilator (FlexiVent, Montreal, Canada) to measure the airway resistance as reflected by Rn. The Rn value was significantly higher in the mice treated with rIL-22 or exposed to CS than that in the air-exposed mice (all P<0.01). Notably, the mice treated with CS+rIL-22 demonstrated a significant further elevation of the Rn value compared with the mice exposed only to CS or with rIL-22 (all P<0.05), suggesting a synergistic effect of the exogenous rIL-22 and CS exposures on the airway resistance (Figure 1).
Figure 1.
Effect of CS exposure with or without rIL-22 on the airway resistance (Rn). The measurement of Rn was performed after 3 d of CS exposure. The airway resistance (Rn) of the mice was significantly increased after 3 d of CS or exogenous rIL-22 exposure and was further significantly elevated in the CS+rIL-22 group. n=6 mice per group from two independent experiments. bP<0.05, cP<0.01.
Effect of rIL-22 and/or acute CS exposure on airway inflammation
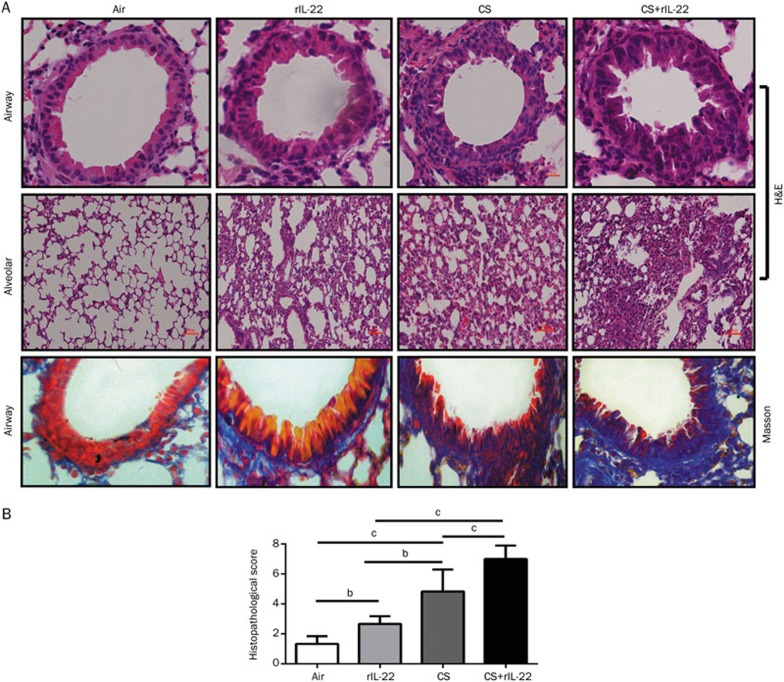
We next examined the histological changes in the mice exposed to CS in the absence or presence of rIL-22. The mice pretreated with rIL-22 showed a slightly increased infiltration of inflammatory cells in the lungs, with mild squamous metaplasia and collagen deposition. The epithelial cell damage, inflammatory cell infiltration around the airways and lung parenchyma, squamous metaplasia, and collagen deposition were more obvious in the CS-exposed mice. Importantly, these histopathological changes were more severe in the CS+rIL-22-treated mice (Figure 2A). To quantify the degree of inflammation, we used a semi-quantitative scoring system as described in the Materials and methods34. Compared with the air-exposed mice, the mice pretreated with rIL-22 or exposed to CS showed significantly higher scores (P<0.05 and P<0.01, respectively). Furthermore, the mice exposed to CS showed significantly higher histopathological scores than those treated with rIL-22 alone (P<0.05). The highest pathological scores were observed in the mice pretreated with rIL-22 and exposed to CS (all P<0.01 vs the CS group and the rIL-22 group) (Figure 2B).
Figure 2.
Effect of CS exposure with or without rIL-22 on the pulmonary inflammation and collagen deposition around airways. The mice were killed after 3 d of CS exposure. The lungs were removed and inflated with 1 mL of 10% formalin. Histological analysis of the lungs of the mice exposed to CS with or without rIL-22 was performed. Compared with the mice treated with rIL-22 or short-term CS exposure alone, there was a greater accumulation of leukocytes, distortion of alveolar architecture, and collagen deposition around airways of the mice treated with CS exposure and rIL-22. (A) H&E and Masson's trichrome staining. (B) Semi-quantitative assessment was performed in mice after 3 d of CS exposure using a previously reported scoring method. A significantly higher score was observed in mice treated with CS+rIL-22 relative to the CS-exposed mice. n=6 mice per group from two independent experiments. bP<0.05, cP<0.01. Original magnification ×400 (H&E and Masson).
Effects of rIL-22 and/or acute CS exposure on the infiltration of inflammatory cells into the airways
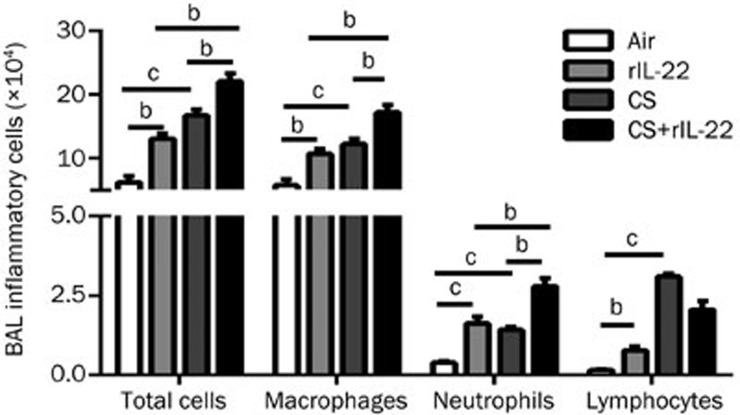
The numbers of total inflammatory cells and the differential subpopulations in the recovered BAL fluid were remarkably greater in the mice treated with rIL-22 or exposed to CS compared with the air-exposed group (all P<0.01) (Figure 3). There were no statistically significant differences between the CS group and the rIL-22 group for the total and differential numbers of inflammatory cells. Notably, the CS+rIL-22 group had the highest numbers of inflammatory cells and differential subpopulations in the BAL fluid, except for lymphocytes (all P<0.05 vs the CS group and the rIL-22 group) (Figure 3).
Figure 3.
Effect of CS exposure with or without rIL-22 on the influx of inflammatory cells into the airways. The total inflammatory cells and differential subpopulations were recovered from the BAL fluid after 3 d of CS exposure in mice treated with or without rIL-22. The results are expressed as the mean±SD. n=6 animals per group from two independent experiments. bP<0.05, cP<0.01.
Effects of rIL-22 and/or acute CS exposure on the expression of CXCR3 ligands and cytokines at the mRNA level
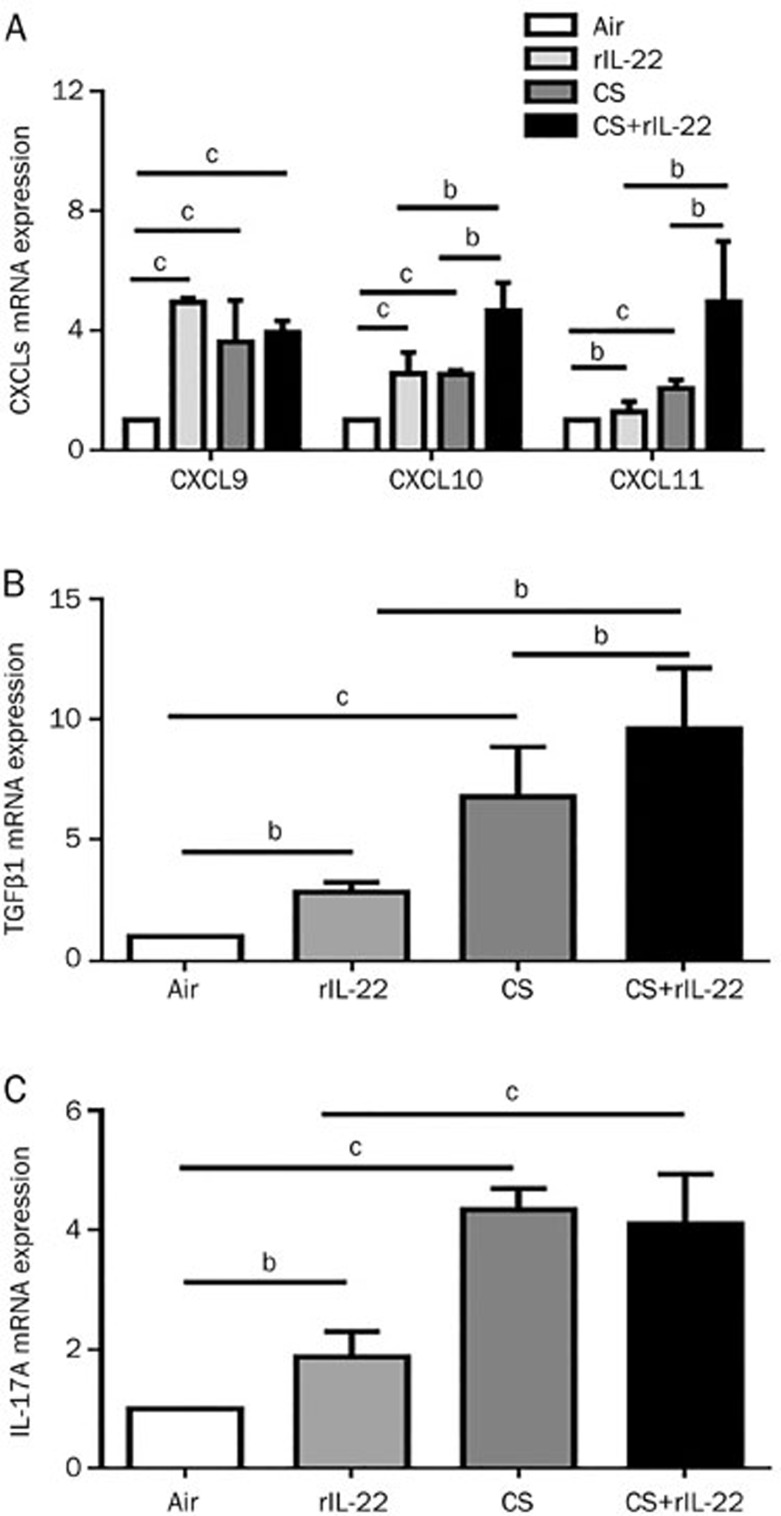
As shown in Figure 4, the expression of the mRNA for the CXCR3 ligands was significantly greater in the rIL-22 and CS exposure groups than that in the air-exposed group. However, there were no significant difference in these chemokines between the CS and rIL-22 groups at mRNA level. The expression levels of the mRNA for CXCL10, CXCL11, and TGFβ1 were greatest in the CS+rIL-22 group (Figures 4A and 4B). The administration of exogenous rIL-22 alone induced the slightly elevated expression of the IL-17A mRNA in the air-exposed mice, whereas CS exposure induced a significant elevation of the IL-17A mRNA in lung tissue (Figure 4C). No further increase in the IL-17A mRNA expression was observed in the mice challenged with both CS and rIL-22.
Figure 4.
Effect of CS exposure with or without rIL-22 on the mRNA expression of cytokines and CXCR3 ligands in the lung tissue. The mRNA expression after 3 d of CS exposure was determined by real-time quantitative RT-PCR. (A) mRNA expression of CXCR3 ligands. (B) mRNA expression of TGFβ1. (C) mRNA expression of IL-17A. The results are expressed as the mean±SD. n=6 mice per group from at least two independent experiments. bP<0.05, cP<0.01.
Effects of rIL-22 and/or acute CS exposure on the expression of CXCR3 ligands and cytokines at the protein level
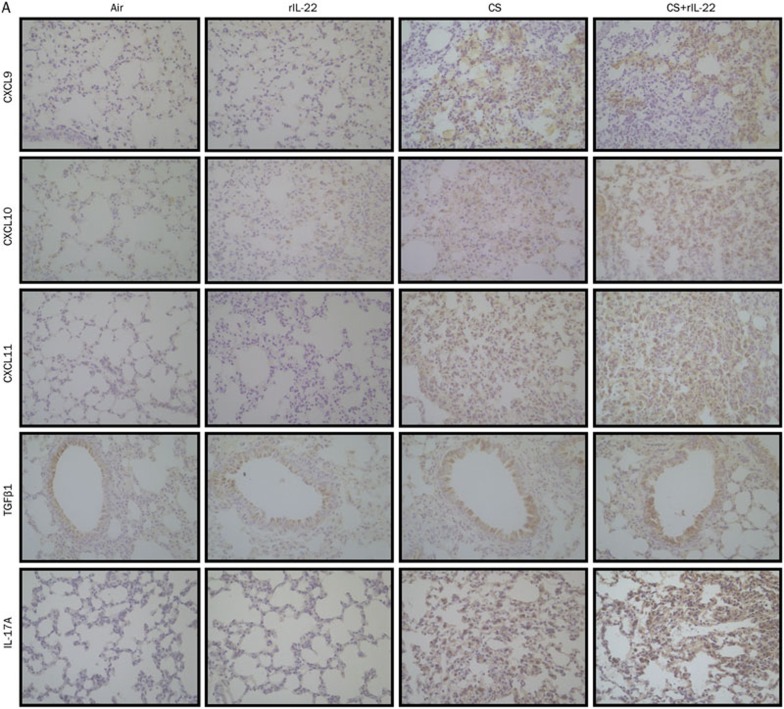
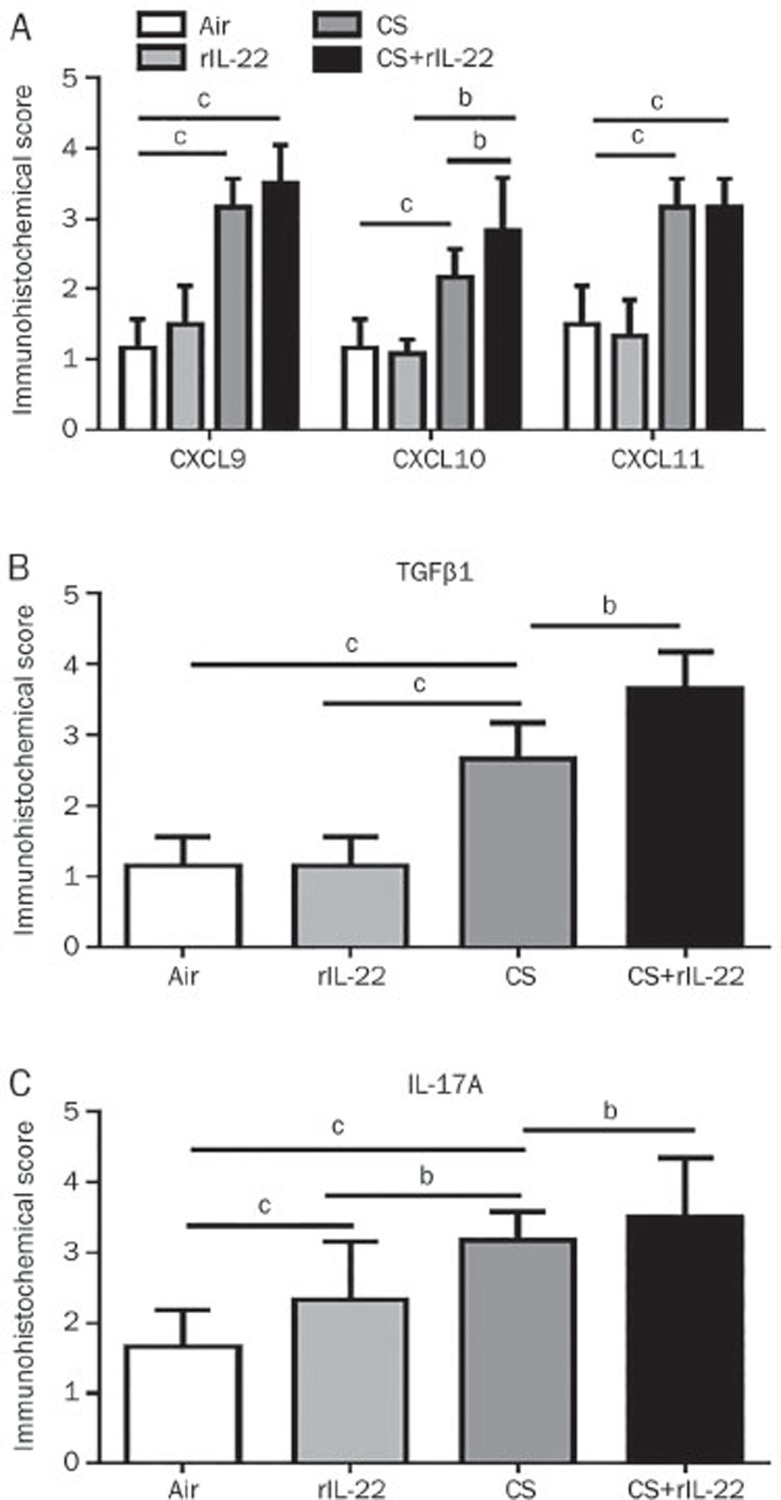
To determine the expression of the chemokines and cytokines at the protein level, we performed IHC staining of the lung tissue sections. Significant increases in CXCL9, CXCL10, and CXCL11 were observed in the mice exposed to CS, with or without rIL-22, but not in the air-exposed mice pretreated with rIL-22 (Figure 5). Among the CXCR3 chemokines, only the immunohistological score for CXCL10 was further significantly increased in the mice exposed to CS+rIL-22 pretreatment compared with the CS-exposed mice and the rIL-22-treated mice (all P<0.05), suggesting a synergistic effect of CS and rIL-22 on induction of CXCL10. Similarly, the expression of TGFβ1 and IL-17A was significantly elevated in the CS+rIL-22 group compared with the CS group (Figure 5).
Figure 5A.
Effect of CS exposure with or without rIL-22 on the local lung expression of CXCR3 ligands and cytokines. The expression of these cytokines and chemokines at the protein level was evaluated by immunohistochemical staining in the lungs of the mice after 3 d of CS exposure. (A) Immunohistochemical staining for identification of chemokines and cytokines.
Figure 5B.
(B) A semi-quantitative assessment was performed in mice exposed for 3 d to CS using a previously reported method. A significantly higher score was observed in mice treated with CS+rIL-22 compared with the CS-exposed mice. n=5–8 mice per group from at least two independent experiments. bP<0.05, cP<0.01. Original magnification ×400.
We also determined the concentrations of the CXCR3 chemokines and cytokines in the BAL fluid. As shown in Table 2, there were significant increases in the concentrations of CXCL9, CXCL10, and CXCL11 in the CS-exposed mice. rIL-22 further augmented the effect of the CS exposure for the production of CXCL9 and CXCL10, but not CXCL11. The levels of the measured cytokines, including IL-6, IL-8, IL-22, TNFα, and TGFβ1, were significantly increased in the CS-exposed mice, but not in the rIL-22 pretreated mice. Furthermore, the CS exposure in combination with the rIL-22 pretreatment induced further increases in the secretion of these cytokines, with the exception of IL-6, in the BAL fluid. However, the concentrations of these cytokines and chemokines in the BAL fluids of the rIL-22 group were not significantly greater than those of the air-exposed group.
Table 2. Cytokine concentrations in BAL fluid in mice after 3 d of CS exposure. Results are expressed as mean±SD. n=6 mice per group from at least two independent experiments. bP<0.05 vs CS-exposed mice. fP<0.01 vs air-exposed mice and rIL-22 pretreated mice.
| CXCL9 (ng/L) | CXCL10 (pg/mL) | CXCL11 (ng/L) | IL-22 (pg/mL) | IL-8 (pg/mL) | IL-6 (ng/L) | TGFβ1 (ng/L) | TNFα (ng/L) | |
|---|---|---|---|---|---|---|---|---|
| Air | 66.33±5.40 | 101.15±4.86 | 44.46±4.15 | 74.53±0.73 | 58.39±7.79 | 87.74±6.96 | 127.06±5.32 | 343.78±29.95 |
| IL-22 | 70.00±4.75 | 125.59±2.39 | 44.05±2.54 | 75.29±0.33 | 61.42±6.24 | 90.92±6.40 | 133.74±7.28 | 332.83±22.29 |
| CS | 75.13±8.63f | 143.86±5.43f | 53.67±5.26f | 91.52±5.09f | 77.05±4.14f | 109.78±6.97f | 173.63±4.53f | 407.83±20.69f |
| CS+IL-22 | 85.04±9.70b | 152.90±9.34b | 56.38±3.46 | 95.45±3.75b | 92.41±5.22b | 112.91±9.67 | 187.29±3.38b | 423.54±43.74b |
Discussion
CS-induced airway inflammation is known to be associated with the development of several lung diseases, including asthma, chronic bronchitis and emphysema21,36. In the present study, we showed that CS exposure induces IL-22 and that exogenous administration of rIL-22 induced slight infiltration of the inflammatory cells into the airways and pulmonary parenchyma, airway epithelial damages, and mild collagen deposition around the airways. Furthermore, we demonstrated that exogenous rIL-22, synergistically with the CS insult, significantly augmented the airway inflammation, epithelial damage, and airway aberrant repair as indicated by the much greater levels of collagen deposition around the airways. These data indicate that IL-22 might be acting in a proinflammatory manner to exacerbate the local airway inflammation induced by an acute CS exposure.
IL-22, acting cooperatively with co-expressed IL-17A, has been demonstrated to have a proinflammatory/pathological role in airway inflammation2,37. Besnard and colleagues showed increased levels of IL-22 in the sera of asthmatic patients. These authors also demonstrated a proinflammatory role of IL-22, via regulation of the expression of IL-17A, in the onset of experimental allergic asthma11. Sonnenberg and colleagues demonstrated the production of IL-22 and IL-17A by CD4+Th17 cells in the lung in response to bleomycin-induced acute tissue damage and airway inflammation in a mouse model. These authors confirmed that the proinflammatory effect of IL-22 occurs only in the presence of IL-17A15. Consistent with these results, our current data showed that the 3-d exposure to CS in combination with rIL-22 pretreatment significantly increased the IL-22 level in BAL fluid (Table 2) and the IL-17A expression in the lung tissue (Figure 5). We also observed an increased level of expression of IL-17A in the lung tissue by IHC staining in the rIL-22 and CS+rIL-22 groups, although IL-17A mRNA was not further induced by CS and rIL-22. One possible explanation might be that IL-22 and IL-17A are produced by activated Th17 cells upon persistent CS insult38. It is possible that exogenous IL-22 might facilitate the effect of CS on the airway inflammation via induction of IL-17A.
However, CS contains many toxic chemicals, including dioxins. Most of the effects of the toxins are mediated by the aryl hydrocarbon receptor (AHR)39. AHR has recently been shown to specifically regulate IL-22 production33. Recent findings in both mice25 and humans40 have demonstrated that CS extracts promote T-cells to undergo Th17 differentiation and augment the percentage of IL-22-producing cells in an AHR-dependent manner. Consistent with these observations, our data showed that acute CS exposure induced significant secretion of IL-22 into the BAL fluid. A possible explanation for this observation is that IL-22 in the microenvironment might be heavily influenced by CS exposure. The target cells through which IL-22 fulfills its pro-inflammatory functions are the airway and pulmonary alveolar epithelial cells. We further detected the expression of chemokines and cytokines, focusing on CXCL9, CXCL10, CXCL11, IL-6, IL-8, and TNFα, in both lung tissues and BAL fluid. Our results showed that all these cytokines and chemokines increased robustly after CS exposure at either the mRNA or protein level. CXCL9, CXCL10, and CXCL11 are ligands for CXCR3. They interact with CXCR3 receptor exclusively and promote the recruitment of inflammatory cells, including neutrophils, macrophages and lymphocytes, into the lung tissues. CXCR3 is mainly expressed on the surfaces of CD4+T cells, CD8+T cells, NK cells and dendritic cells41. It has been shown that CXCR3 and its ligands are upregulated in a CS-exposed animal model28,29 or CS-exposed COPD patients42,43. Th17 cells also express the CXCR3 receptor1,2. Thus, it is reasonable to suspect that CXCR3 receptor/ligand interaction recruits Th17 cells into the inflamed lung tissue upon CS exposure. Th17 cells are then stimulated and produce Th17-associated cytokines, particularly IL-17A and IL-2244. This might explain the increases in IL-17A and IL-22 after the CS exposure in our study. The elevation of IL-8 in the BAL fluid after CS exposure might be due to the effect of pulmonary IL-17A because IL-17 is capable of stimulating the production of IL-8 in the airway epithelial cells45. Of interest was the inconsistency in the expression of cytokines and chemokines measured by mRNA and the IHC scores as demonstrated by our study. This might reflect the complexity of airway inflammation by CS exposure in vivo, and the acute effect of IL-22 in facilitating the CS-induced pulmonary pathology. Liang and colleagues reported that the acute response of IL-22 occurred within 6 h30. By contrast, we tested the parameters 3 d after a single injection of IL-22.
Despite the short period of the CS exposures in our study, we observed a significant increase in the airway resistance (Rn) and a robust deposition of collagen around the airways. Our data showed that TGFβ1, which is closely associated with collagen deposition, was upregulated by the rIL-22. This upregulation was more significant when rIL-22 was combined with exposure to CS. Recent data have demonstrated a contributing role of IL-22 in the TGFβ1-mediated airway epithelial-mesenchymal transition in the pathogenesis of asthma46. However, there are no data regarding the relationship between IL-22 and TGFβ1 under the conditions of CS exposure.
To our knowledge, this is the first descriptive study specifically focusing on the effect of exogenous rIL-22 on CS-induced airway inflammation. Our data showed that exogenous rIL-22 and CS synergistically exacerbate the infiltration of inflammatory cells into the airways and lung parenchyma. This synergy further triggers the release of inflammatory cytokines and chemokines into the lung in this animal model. Our work suggest that IL-22 functions as a proinflammatory cytokine in the presence of other pro-inflammatory cytokines or chemokines, such as IL-17A and CXCL10, in the context of a short-term CS exposure. Further studies with conditional IL-22 knockout mice and IL-22 knock-in mice are needed to address the role of IL-22 in the airway inflammation induced by CS.
Author contribution
Jiu-rong LI performed all the procedures of the experiments; Wei-xun ZHOU carried out the pathological analysis; Ke-wu HUANG facilitated and helped to perform the physiological experiments; Yang JIN helped with designing the experiments and drafted the manuscript; Jin-ming GAO designed and supervised the experiment and drafted the manuscript.
Acknowledgments
This work is partly supported by National Natural Science Foundation of China (81170040 and 81470229), and National Science and Technology Pillar Program during the Twelfth Five-year Period 2012BAI05B00.
We are grateful for Prof Guan-liang SHAN's kind help in facilitating the whole experiment, and we thank the technicians at the Department of Pathology, Peking Union Medical College Hospital for help in generating the pathological slides and the staff of the Animal Center, Peking Union Medical College Hospital for caring for the animals. We thank Dr Xi-qiang YAN for his generosity in providing recombinant IL-22 and his helpful comments.
References
- Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–63. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Valdez P. IL-22 in mucosal immunity. Mucosal Immunol. 2008;1:335–8. doi: 10.1038/mi.2008.26. [DOI] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–32. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–29. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–61. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A. 2013;110:12768–73. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–63. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–76. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Alcorn JF. TH17 cells in asthma and inflammation. Biochim Biophys Acta. 2011;1810:1066–79. doi: 10.1016/j.bbagen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, et al. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis. Mediators Inflamm. 2013;2013:209179. doi: 10.1155/2013/209179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol. 2011;128:1067–76. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, Quaranta M, et al. IL-22 suppresses IFN-gamma-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol. 2013;131:562–70. doi: 10.1016/j.jaci.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Muhl H. Pro-inflammatory signaling by IL-10 and IL-22: Bad habit stirred up by interferons. Front Immunol. 2013;4:18. doi: 10.3389/fimmu.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Hirose K, Takahashi K, Nakajima H. Roles of IL-22 in allergic airway inflammation. J Allergy (Cairo) 2013;2013:260518. doi: 10.1155/2013/260518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Smyth LJ, Starkey C, Vestbo J, Singh D. CD4-regulatory cells in COPD patients. Chest. 2007;132:156–63. doi: 10.1378/chest.07-0083. [DOI] [PubMed] [Google Scholar]
- Smyth LJ, Starkey C, Gordon FS, Vestbo J, Singh D. CD8 chemokine receptors in chronic obstructive pulmonary disease. Clin Exp Immunol. 2008;154:56–63. doi: 10.1111/j.1365-2249.2008.03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–26. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD. 2013;10:459–65. doi: 10.3109/15412555.2013.770456. [DOI] [PubMed] [Google Scholar]
- Paats MS, Bergen IM, Hoogsteden HC, van der Eerden MM, Hendriks RW. Systemic CD4+ and CD8+ T-cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J. 2012;40:330–7. doi: 10.1183/09031936.00079611. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–24. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Xiang R, Zhou W, Lu B, Cheng D, Gao J. Attenuation of acute lung inflammation induced by cigarette smoke in CXCR3 knockout mice. Respir Res. 2008;9:82. doi: 10.1186/1465-9921-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Xiang RL, Liu Y, Zhou WX, Jiang L, Lu B, et al. Acute pulmonary inflammation is inhibited in CXCR3 knockout mice after short-term cigarette smoke exposure. Acta Pharmacol Sin. 2008;29:1432–9. doi: 10.1111/j.1745-7254.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, et al. IL-22 induces an acute-phase response. J Immunol. 2010;185:5531–8. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–76. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, et al. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir Res. 2010;11:34. doi: 10.1186/1465-9921-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–71. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298:1277–81. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- Jia YT, Wei W, Ma B, Xu Y, Liu WJ, Wang Y, et al. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J Immunol. 2007;179:7808–19. doi: 10.4049/jimmunol.179.11.7808. [DOI] [PubMed] [Google Scholar]
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, et al. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013;182:1286–96. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252:184–94. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Torii K, Saito C, Furuhashi T, Nishioka A, Shintani Y, Kawashima K, et al. Tobacco smoke is related to Th17 generation with clinical implications for psoriasis patients. Exp Dermatol. 2011;20:371–3. doi: 10.1111/j.1600-0625.2010.01224.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–75. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–9. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- Kelsen SG, Aksoy MO, Georgy M, Hershman R, Ji R, Li X, et al. Lymphoid follicle cells in chronic obstructive pulmonary disease overexpress the chemokine receptor CXCR3. Am J Respir Crit Care Med. 2009;179:799–805. doi: 10.1164/rccm.200807-1089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, O'Shea JJ, Watford WT. Interleukin-22: a sheep in wolf's clothing. Nat Med. 2008;14:247–9. doi: 10.1038/nm0308-247. [DOI] [PubMed] [Google Scholar]
- Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–53. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Nishioka M, Chakir J, Risse PA, Almaghlouth I, Bazarbashi AN, et al. IL-22 contributes to TGF-beta1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res. 2013;14:118. doi: 10.1186/1465-9921-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]