Abstract
Purpose
Advanced cancers of the bile duct and gallbladder carry an ominous prognosis. Rebeccamycin analogue (RA) is a novel antitumor antibiotic where phase I trials suggested clinical efficacy in patients with biliary cancers.
Methods
The primary objective was to determine the response rate to RA in patients with advanced gallbladder and bile duct tumors. Secondary endpoints were survival and pharmacokinetic characterization. RA was given at a dose 165 mg/(m2 day) × 5 days every 3 weeks.
Results
Forty-six patients were enrolled. Nine patients were removed from study before their first planned imaging study for response. Two patients had partial responses and 16 had stable disease. On an intent-to-treat analysis the median survival was 6.3 months. A >20% drop in CA 19.9 was seen in 43% of patients with initial high levels. Grade 4 neutropenia and thrombocytopenia were seen in 35 and 5% of patients, respectively. Febrile neutropenia occurred in 16% of patients. The pharmacokinetic profile of this trial closely resembles those of prior phase I trials. Measured biliary concentrations of RA were as much as 100× greater than simultaneous plasma concentration.
Conclusion
Although RA has a response rate of 5% in advanced biliary cancers, it is associated with significant numbers of patients experiencing prolonged stable disease. Biliary concentrations of RA are significantly greater than plasma concentrations.
Keywords: Biliary cancer, Gallbladder cancer, Phase II trial
Introduction
Cancers of the bile duct and gallbladder are relatively uncommon tumors with approximately 7,480 estimated new cases in 2005 and 3,340 related deaths in the United States [1]. Complete resection is the only potentially curative therapy for cancers of the lower bile ducts. Proximal or hilar cholangiocarcinomas (Klatskin tumors) represent the greatest therapeutic challenge because of the proximity to vital structures. However, only 20–30% of patients with hilar cholangiocarcinomas are eligible for potential curative resection [2]. For unresectable disease the prognosis is poor with few survivors beyond 1 year. Advanced gallbladder cancer carries a median survival of less than 5–6 months. Perpetuo et al. [3] reviewed a 36-year experience with gall-bladder cancer from the MD Anderson Cancer Center and reported a 5-year survival of less than 5% and a median survival of 5.2 months. A review of the SEER database showed a median survival of 2 months for patients with stage IV gallbladder cancer [4]. A French review of 484 patients with gallbladder cancer showed a 1-year survival of only 9% for patients with stage IV disease [5].
Multiple chemotherapeutic agents have been tested in a limited number of patients for these cancers. Single agents generally have response rates of less than 10%. Fluorouracil has been the most extensively studied agent with response rates ranging from 0 to 24% in four small trials [6]. Despite the low response rate to fluorouracil, improvement in survival was demonstrated in a small randomized trial [7]. Paclitaxel showed no activity in 15 patients with biliary carcinomas [8], and similarly no activity has been seen for docetaxel [9]. Gemcitabine also has been evaluated as a single-agent in small phase II trials. A recent trial of fixed dose rate gemcitabine in biliary cancers showed no response [10]. Combination chemotherapy trials have been discordant with significant toxicity seen in this patient population. The combination of cisplatin and gemcitabine has been given in two small trials with higher than anticipated response rates [11, 12].
Rebeccamycin analogue (RA—NSC 655649, XL119, becatecarin) is an intercalating antitumor antibiotic which has toposiomerase II inhibitory effects. It inhibits topoisomerase II strand passing function without stabilization of the cleavable complex intermediate [13]. Studies also suggest that RA can be an inhibitor of topoisomerase I [13]. A phase I study of RA on a daily × 5 schedule performed at University Hospitals Case Medical Center determined the recommended phase II dose and schedule to be 165 mg/m2 daily × 5 repeated every 3 weeks in chemonaive patients [14]. During this phase I study antitumor activity was seen including minor responses and prolonged stable disease in four patients with carcinoma of the biliary tree (gallbladder and cholangiocarcinoma). Plasma pharmacokinetics showed evidence of enterohepatic circulation. In addition a sample of bile obtained from one patient during this study demonstrated a several fold superior concentration of RA in the bile as compared to the simultaneously measured plasma concentration during the pharmacokinetic elimination phase.
Based on the antitumor activity that we observed in biliary cancers in our phase I study as well as some pharmacokinetic rationale (enterohepatic circulation and high bile fluid concentrations of parent drug) we embarked on a phase II and pharmacokinetic multi-center study to test the efficacy of RA in patients with biliary cancers. The primary objectives of this study were to determine the response rate and toxicity to RA in this patient population. Secondary objectives included pharmacokinetic characterization and survival assessment.
Patients and methods
Patients with advanced biliary carcinoma (gallbladder cancer, cholangiocarcinoma, ampullary cancer) not amenable to conventional surgical approach were eligible. No prior chemotherapy was allowed. Patients had to have a good performance status (ECOG PS 0, 1, 2) with adequate hematologic parameters (WBC ≥ 3,000/μL, platelets ≥ 100,000/μL; hemoglobin ≥ 10 g/dL) and renal function (serum creatinine within institutional normal range or calculated creatinine clearance ≥ 60 ml/min/1.73 m2). Measurable disease was required and all patients gave informed consent. From a hepatic standpoint bilirubin less than or equal to 1.5 mg/dL and AST levels less than or equal to 2.5 × upper limit of normal were required. A parallel dose escalation trial in patients with mild to moderate hepatic dysfunction was being done simultaneously, and results of that trial will be reported separately.
Rebeccamycin analogue was given as a daily 1-h infusion for five consecutive days. RA was diluted in 0.5–1 L of NS or D5 W. Treatment was repeated every 3 weeks. A central venous access device was required for all patients given the risk of painful superficial phlebitis observed during phase I trials. Patients were treated until disease progression or development of unacceptable toxicity. A 25% dose reduction for subsequent cycles was allowed if grade 4 hematologic toxicities occurred. Delays in dosing were allowed in increments of 1 week for hematologic recovery to baseline. Delays of greater than 2 weeks resulted in removal of patients from the trial.
All patients must have had imaging studies to define measurable disease ≤4 weeks prior to starting treatment. Patients were re-evaluated every 2 cycles for response. Toxicity was graded according to CTC version 2. Patients were considered evaluable for response if they were able to complete at least one cycle of therapy and were followed for 3 weeks. Standard RECIST criteria were used for response evaluation and confirmation [15]. Although not required by protocol, patients with elevated baseline CA 19-9 levels had their tumor marker measured prior to each cycle.
Sample size calculation based on a two-stage accrual design as described by Simon [16] was employed. Initially 12 evaluable patients would be accrued. If ≥1 response was seen the second stage of accrual would begin for final total of 37 evaluable patients. If at least 4 responses (11%) are seen in the 37 evaluable patients RA would be declared worthy of further investigation. This design has a 90% power of detecting a response rate of at least 20% in the final 37 patients.
The pharmacokinetic plasma sampling protocol involved sample collection before infusion and immediately before infusion termination on each treatment day. This sampling schedule was meant to reveal net accumulation in plasma of drug during successive days of treatment. The protocol also included collection of specimens at intervals of 1, 2, 4, 8, 12, and 24 h after infusion termination after the first and last treatment days of the cycle to define the drugs’ plasma elimination pharmacokinetics. We collected additional samples at nominal study times of 36, 48, 72, 96, and 120 h after termination of infusion on the fifth treatment day to study late elimination pharmacokinetics for cycle one only. Blood was drawn at the above time points into a purple-topped plasma separation tube containing EDTA. Methodology for the assay has been previously described [14]. A unique part of this trial was the performance of upper endoscopy or ERCP in ten of the patients enrolled at Case Western Reserve University in order to obtain samples for pharmacokinetic measurements on the bile. The computer programs Scientist and PK Analyst (Micromath, Salt Lake City, UT) were used for nonlinear least squares fitting of equations to plasma RA concentration versus time data sets and calculation of pharmacokinetics parameters. A four exponential terms, repeated dose model was selected on the basis of better calculated Model Selection Criterion (MSC) values [MSC, derived from Akaike Information Criterion (AIC) values] [17] in relation to MSCs calculated for a similar three term model in a representative sample of data sets.
Results
Patients
From September 2000 to September 2003 a total of 46 patients were enrolled at 3 institutions. One patient never received treatment and therefore 45 patients were eligible for evaluation on an intent-to-treat basis. Thirty-seven patients had imaging studies performed at the first determined time point (pre-cycle 3). Reasons for removal from study within the first two cycles include: three patients were removed from study due to declining performance status (all during cycle 1); early death in two patients during cycle 1 (autopsy not performed); one patient had bowel perforation on day 15 during cycle 1 due to disease; one patient had a bone fracture and did not continue therapy; one patient received one cycle of therapy and was removed from study for worsening signs of dementia. Patient characteristics are depicted in Table 1. The majority of patients had cholangiocarcinomas with good performance status. A total of 176 cycles were administered (range 1–20 cycles per patient).
Table 1.
Patient characteristics (n = 45)
| Characteristic | |
| Age (median with range) | 61 (range 45–78) |
| ECOG performance status 0/1/2 | 13/28/4 |
| Gender (male/female) | 23/22 |
| Pathology | |
| Cholangiocarcinoma | 32 |
| Gallbladder carcinoma | 9 |
| Ampullary carcinoma | 24 |
Response
Responses and stable disease were verified by an independent advisory board convened by the NCI/CTEP. Two patients (5%) had a partial response. One patient had advanced gallbladder cancer with retroperitoneal involvement, and one patient had cholangiocarcinoma with metastases to the lung. A further 16 of the 45 patients (35%) had stable disease (patients receiving at least 4 cycles, stable disease range 3–22 months). The proportion of patients with stable disease and partial response was 40%.
Tumor markers
If patients had an initial elevated baseline CA 19.9 levels, then this was followed serially; 21 had an elevated baseline CA19.9 level. Median pretreatment CA 19.9 levels was 575 μ/mL (range 51–12,163) and pre-cycle 2 was 326 μ/ mL (range 12–67,925). Nine of these 21 patients (43%) showed more than 20% reduction in CA19.9 level within two cycles. Of those with a drop in CA19.9, 2/9 patients had progressive disease on first imaging evaluation while 7/ 12 without a drop in CA19.9 had progressive disease as their best response.
Survival
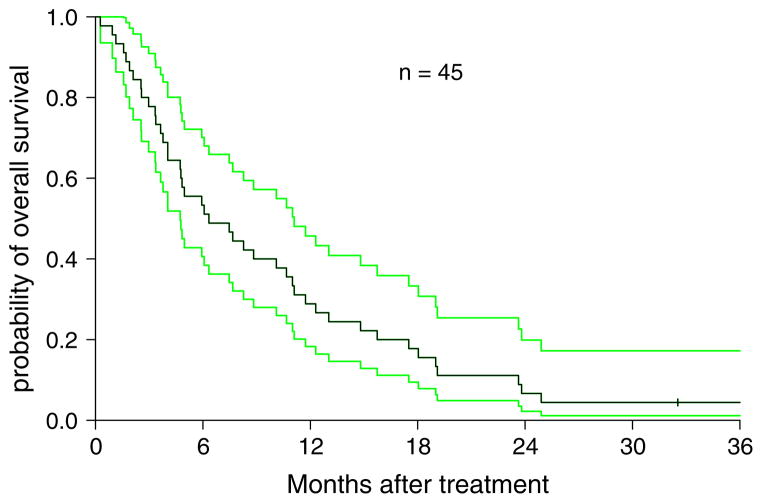
Survival function was estimated using Kaplan-Meier method and difference between groups was tested using logrank test. For all 46 patients who met eligibility (intent-to-treat analysis) the overall survival was 6.3 months (95% CI: 4.7–10.7) with a 6 month and 1 year survival rate of 53 and 29%, respectively (Fig. 1). In the 37 completing 2 cycles of therapy, the overall median survival was 8.8 months (95% CI 5.9–12.3 months) and the 6 month, and 1-year survivals were 65 and 35%, respectively. Patients with clinical benefit (stable disease or partial response) had a significantly higher survival (median 12.3 months) compared to those with progressive disease (median 4.8 months) (P = 0.0004). Subgroup analysis showed no difference in survival comparing male to female; ECOG PS 0 versus 1–2; or age <60 or ≥60.
Fig. 1.
Kaplan–Meier estimation of overall survival on an intent-to-treat analysis (n = 45) with 95% confidence interval
Toxicity
Toxicity is shown in Table 2. All toxicities listed as possibly related to the study drug are shown. Grade 3 or 4 hematologic toxicity was the predominant side effect. Grade 4 neutropenia and thrombocytopenia were seen in 35 and 5% of patients, respectively. Febrile neutropenia occurred in 16% of patients. Non-hematologic toxicities occurred at only low frequencies. Grades 1 and 2 nausea was common; however, the trial did not require the use of 5HT3 antagonists. Eleven percent of patients had one or more episodes of asymptomatic febrile reaction on the day of RA administration. Sixteen patients (43%) required dose reductions during treatment. Fourteen required one dose reduction while two patients had two reductions. Two patients died within 30 days of starting treatment.
Table 2.
Toxicity with possible, probable or definitive attribution to RA
| Highest NCI toxicity grade (% patients with event)
|
||||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | ||||
| Neutropenia | 0 | 2 (5) | 6 (16) | 13 (35) |
| Platelets | 0 | 2 (5) | 9 (24) | 2 (5) |
| Anemia | 6 (16) | 10 (27) | 5 (14) | 0 |
| Non-hematological | ||||
| Febrile neutropenia | 0 | 0 | 6 (16) | 0 |
| Infection | 1 (3) | 0 | 0 | 0 |
| Fever (drug-related) | 4 (11) | 0 | 0 | 0 |
| Chills/rigors | 2 (5) | 0 | 0 | 0 |
| Flushing | 4 (11) | 1 (3) | 0 | 0 |
| Fatigue | 7 (19) | 14 (38) | 1 (3) | 0 |
| AST | 3 (8) | 0 | 2 (5) | 0 |
| ALT | 0 | 1 (3) | 3 (8) | 0 |
| Bilirubin | 1 (3) | 1 (3) | 1 (3) | 0 |
| Alkaline phosphatase | 1 (3) | 2 (5) | 0 | 0 |
| Constipation | 3 (8) | 4 (11) | 1 (3) | 0 |
| Nausea | 19 (51) | 4 (11) | 1 (3) | 0 |
| Vomiting | 5 (14) | 4 (11) | 1 (3) | 0 |
| Stomatitis | 3 (8) | 3 (8) | 1 (3) | 0 |
| Taste | 6 (16) | 0 | 0 | 0 |
| Anorexia | 7 (19) | 0 | 1 (3) | 0 |
| Headache | 3 (8) | 0 | 0 | 0 |
| Neuro-sensory | 2 (5) | 0 | 0 | 0 |
| Alopecia | 2 (5) | 0 | 0 | 0 |
| Rash | 0 | 1 (3) | 0 | 0 |
| Dyspnea | 0 | 1 (3) | 1 (3) | 0 |
| Hyponatremia | 0 | 0 | 1 (3) | 0 |
| Hypercalcemia | 1 (3) | 0 | 0 | 1 (3) |
| Hypomagnesemia | 1 (3) | 0 | 0 | 0 |
Pharmacokinetics
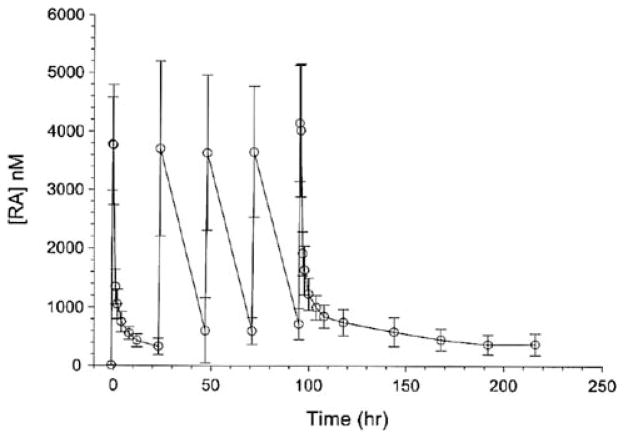
Significant quantities of RA accumulated in plasma with dose repetition at intervals of 24 h (Fig. 2). The data sets were well described by a four exponential term model when the data were weighted as [concentration]−2. The first of these terms describes the infusion and simultaneous distribution of the drug. The remaining three terms describe elimination from plasma. The three discernible elimination processes have apparent half-lives of about 0.4 ± 0.15, 7.9 ± 3.9, and 123 ± 78 h (mean ± SD). This model and data weighting did not predict end-of-infusion plasma maximum concentrations of drug with good accuracy in some of the data sets. This model and data weighting scheme predicted quite accurately the minimum plasma concentrations of drug observed between administered doses and the protracted terminal elimination of RA from plasma.
Fig. 2.
RA plasma concentration versus time
Trapezoidal areas under the RA concentration versus time curves from time zero to infinity was 33,594 ± 9,331 nM h (mean ± SD). Clearance calculated as Dose/AUC0–∞ (7.9 ± 2.2 L h−1 m−2, n = 29) did not vary with dose over the range of doses administered in this study. Apparent volumes of distribution at steady state calculated as (Dose × AUMC)/AUC2 (for the first dose) were large (377 ± 88 L m−1). There was no statistically significant difference detectable in t test of means of comparisons of female versus male patients or patients <65 versus ≥65 years of age. Five patients had bile samples available for RA measurements. Table 3 depicts the results and compares them to plasma RA concentrations drawn near the same time point. Measured biliary concentrations of drug were as much as 100× greater that nearly simultaneous plasma concentration.
Table 3.
Comparison of plasma versus biliary concentrations of RA in five individual patients
| Patient | Concentration (pmol/mL)
|
|
|---|---|---|
| Bile | Plasma | |
| 5 | 58,077 | 1,539 |
| 10 | 114,644 | 1,015 |
| 11 | 31,060 | 1,451 |
| 17 | 21,330 | Not available |
| 22 | 73,498 | 1,560 |
Endoscopy was performed on selected patients on day 5 within 6–8 h of RA administration. Plasma samples were obtained within 2 h of biliary samples
Discussion
Carcinoma of the gallbladder and bile ducts accounts for 4% of all malignancies involving the gastrointestinal system. Advanced cancers of the biliary tract including gallbladder cancer remain a therapeutic challenge. No standard therapy exists and response rates to chemotherapy remain low. Most studies reported to date have included only a small number of patients with variable results reported for the same agent. Combination chemotherapy has been associated with a slightly higher response rate, but significantly greater toxicity in the patient population. Treatment of patients with advanced biliary cancer remains difficult in part due to biliary complications and frequent need for biliary drainage. Most studies show overall survival for advanced disease of less than 4–6 months. Gallbladder cancer patients have a slightly better survival as compared to bile duct tumors.
Our phase I trial data suggested efficacy in biliary tract tumors where 4 out 5 patients enrolled had prolonged stable disease including a patient with gallbladder cancer who had a near partial remission. In addition we found significantly higher amount of RA in the bile sampled for a patient with external biliary drainage on this trial compared to simultaneous plasma levels. Furthermore, pharmacokinetic analysis showed hepatobiliary recirculation of drug. Given these observations there was significant impetus to perform a phase II trial of RA in advanced biliary cancer.
A response rate of less than 10% was seen in our phase II trial. In addition in our trial a further 35% of the patients had stable disease. The overall clinical benefit rate of 40% (partial response + stable disease) was encouraging in a disease which carries a poor prognosis. Although not an endpoint in this trial, many patients noted improvements in symptomatology with therapy. Furthermore, although only half the patients with bile duct tumors on our trial had elevated baseline CA19.9 level, 43% of these patients showed a > 20% reduction in their tumor marker within 1–2 cycles with RA therapy. The clinical benefit and tumor marker reduction rate with this agent led to the development of a phase III trial of RA compared to fluorouracil and leucovorin in advanced biliary cancers.
From a toxicity standpoint, as anticipated from phase I trials, hematologic toxicity was the most significant side effect, the majority of patients required one dose reduction for either grade 4 neutropenia and thrombocytopenia or dose delays. Given the frequent dose reductions (43% of patients) and delays, the current ongoing phase III trial of RA in biliary cancers is using a lower dose of this agent (i.e., 140 mg/m2 × 5 days repeated every 4 weeks). Of importance is the absence of alopecia and any other significant non-hematologic toxicity. Although a significant amount of grade 1–2 nausea was seen, anti-emetics were not mandated by this trial and left to the investigator. Patients treated with 5HT3 antagonists had significant reduction in their nausea.
The pharmacokinetic profile of this trial closely resembles those of our phase I trial [14]. Initial concern was that given that RA is hepatically metabolized, in the setting of biliary cancers, we may see higher rates of toxicities. Furthermore, we had shown a pharmacokinetic-pharmacodynamic association between AUC and hematologic toxicity. This study, however, showed that pharmacokinetic disposition of RA is similar in biliary cancer patients with normal liver function tests as compared to patients with other solid tumors. To our knowledge, this is the first study demonstrating the feasibility of endoscopic biliary sampling to measure drug in bile. All of these samples showed a higher biliary concentration of RA as compared to plasma RA drawn around the same time. These data substantiate the single biliary RA concentration measurement performed in our earlier study [14]. Although it is not clear if such a phenomenon is seen with other chemotherapy agents, this finding is intriguing; its association with response is unclear.
In conclusion, RA has a response rate of 5% in advanced biliary cancers but it is associated with significant numbers of patients experiencing prolonged stable disease. Therapy results in a decline of CA19.9 in 43% of patients in whom an elevated level of tumor marker is detected at diagnosis. Pharmacokinetic studies show similar disposition of RA in patients with adequate liver function compared to patients in prior studies with other solid tumors. Biliary concentrations of RA are significantly greater than plasma concentrations. This trial also shows the feasibility of performing multi-institutional trials of systemic therapy in biliary cancers.
Acknowledgments
Supported in part by grants U01 CA62502, MO1-RR-00080, 5K23 CA109348-01 (to AD) and TRF 23XS087A (to AD) from the National Institutes of Health to Case Western Reserve University and by grants P30CA47904 and M01-RR-00056 from the National Institutes of Health to the University of Pittsburgh Cancer Institute and Medical Center.
Contributor Information
Afshin Dowlati, Email: axd44@po.cwru.edu, afshin.dowlati@case.edu, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
James Posey, University of Alabama, Birmingham, AL, USA.
Ramesh K. Ramanathan, Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh Medical Center and Cancer Institute, Pittsburgh, PA, USA
Linda Rath, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Pingfu Fu, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Amitabh Chak, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Smitha Krishnamurthi, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Joanna Brell, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Stephen Ingalls, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA.
Charles L. Hoppel, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA
Percy Ivy, National Cancer Institute, Bethesda, MD, USA.
Scot C. Remick, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA. Case Western Reserve University, Cleveland, OH, USA
References
- 1.American Cancer Society. Cancer facts and figures. American Cancer Society; Atlanta: 2005. [Google Scholar]
- 2.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Perpetuo MD, Valdivieso M, Heilbrun LK, et al. Natural history study of gallbladder cancer: a review of 36 years experience at M.D. Anderson Hospital and Tumor Institute. Cancer. 1978;42:330–335. doi: 10.1002/1097-0142(197807)42:1<330::aid-cncr2820420150>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder, histologic types, stage of disease, grade and survival rates. Cancer. 1992;70:1493–1497. doi: 10.1002/1097-0142(19920915)70:6<1493::aid-cncr2820700608>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi S, Benhamiche AM, Isambert N, et al. Trends in incidence and management of gallbladder carcinoma: a population-based study in France. Cancer. 2000;89:757–762. doi: 10.1002/1097-0142(20000815)89:4<757::aid-cncr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Daines WP, Rajagopalan V, Grossbard ML, et al. Gallbladder and biliary tract carcinoma: a comprehensive update, Part 2. Oncology (Williston Park) 2004;18:1049–1059. [discussion 1060, 1065–1066, 1068 (review)] [PubMed] [Google Scholar]
- 7.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 8.Jones DV, Jr, Lozano R, Hoque A, et al. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol. 1996;14:2306–2310. doi: 10.1200/JCO.1996.14.8.2306. [DOI] [PubMed] [Google Scholar]
- 9.Papakostas P, Kouroussis C, Andoulakis N, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract. A multicenter phase II study. Eur J Cancer. 2001;37:1833–1838. doi: 10.1016/s0959-8049(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 10.Eng C, Ramamathan RK, Wong MK, et al. A Phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol. 2004;27:565–569. doi: 10.1097/01.coc.0000135924.94955.16. [DOI] [PubMed] [Google Scholar]
- 11.Malik IA, Aziz Z, Zaidi SH, et al. Gemcitabine and cisplatin is a highly effective combination chemotherapy in patients with advanced cancer of the gallbladder. Am J Clin Oncol. 2003;26:174–177. doi: 10.1097/00000421-200304000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Carraro S, Servienti PJ, Bruno MF. Gemcitabine and cis-platin in locally advanced or metastatic gallbladder and bile duct adenocarcinoms. ASCO. 2001;20:abstract 2333. [Google Scholar]
- 13.National Cancer Institute, Division of Cancer Treatment, Investigators Brochure. Rebeccamycin Analog NSC 655649. Bethesda, Maryland: 1999. [Google Scholar]
- 14.Dowlati A, Hoppel CL, Ingalls ST, et al. Phase I clinical and pharmacokinetic study of rebeccamycin analog NSC 655649 given daily for five consecutive days. J Clin Oncol. 2001;19:2309–2318. doi: 10.1200/JCO.2001.19.8.2309. [DOI] [PubMed] [Google Scholar]
- 15.Therase P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 17.Akaike H. An information criterion (AIC) Math Sci. 1976;14:5–9. [Google Scholar]