Abstract
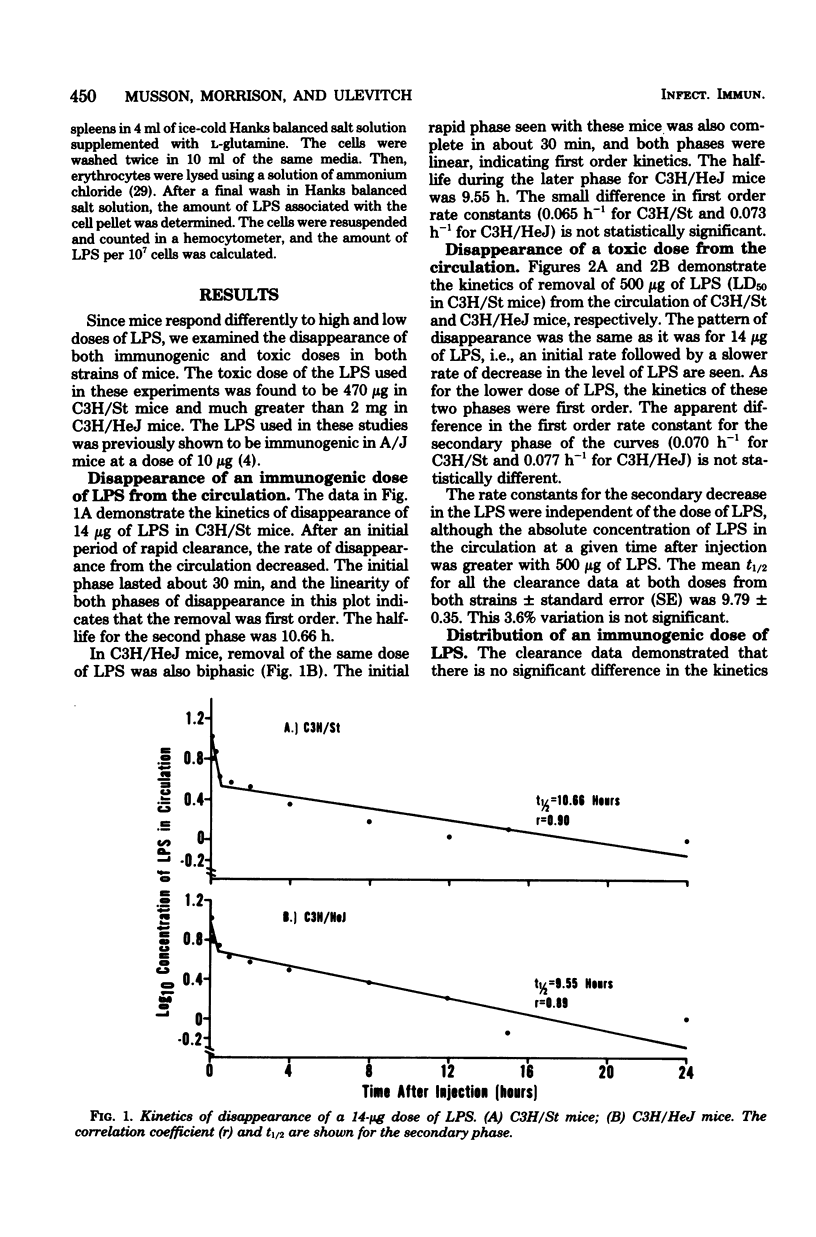
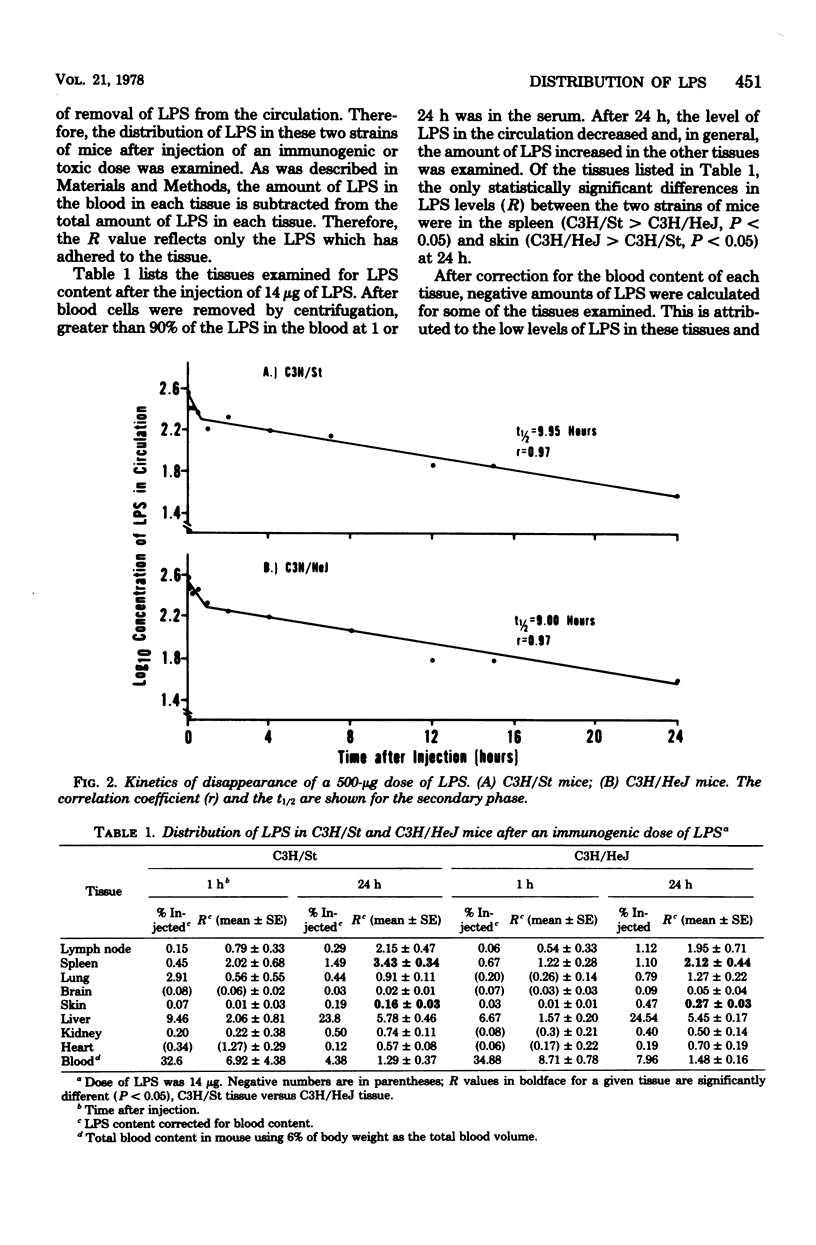
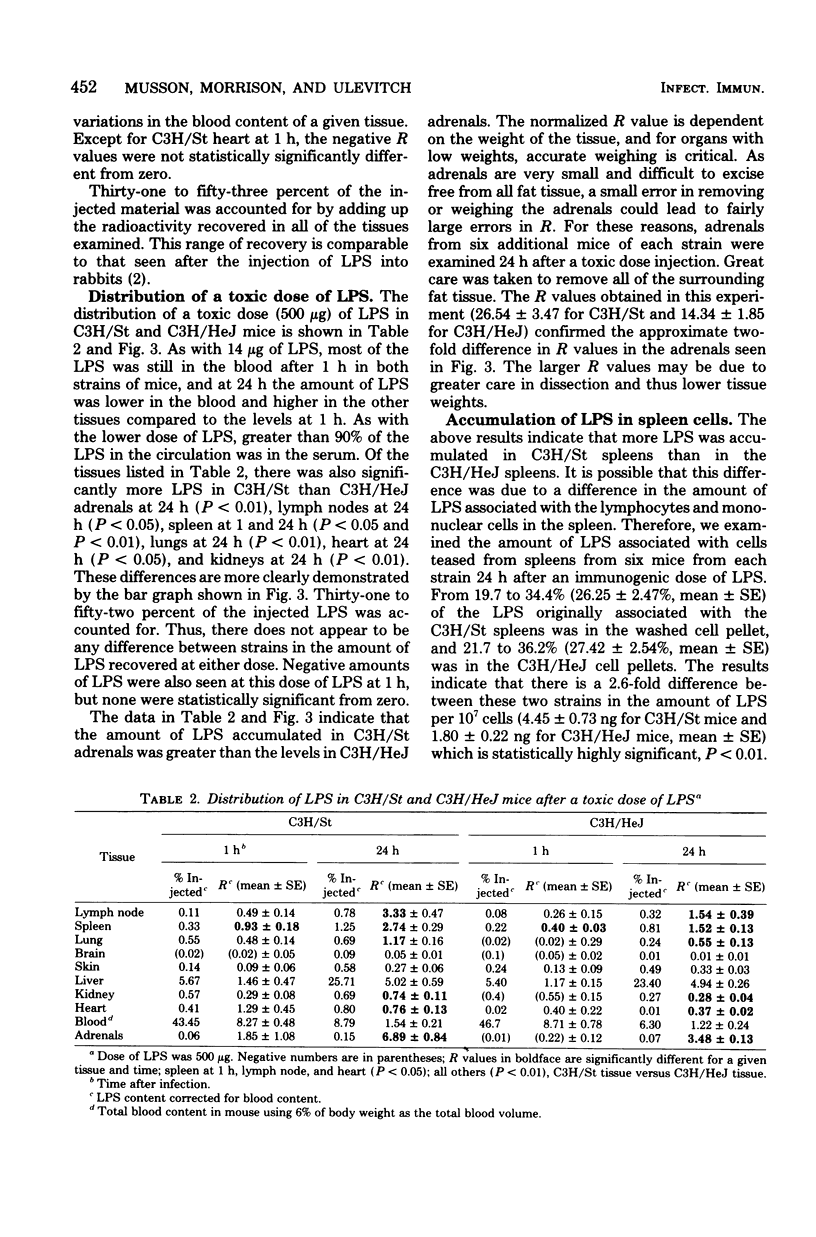
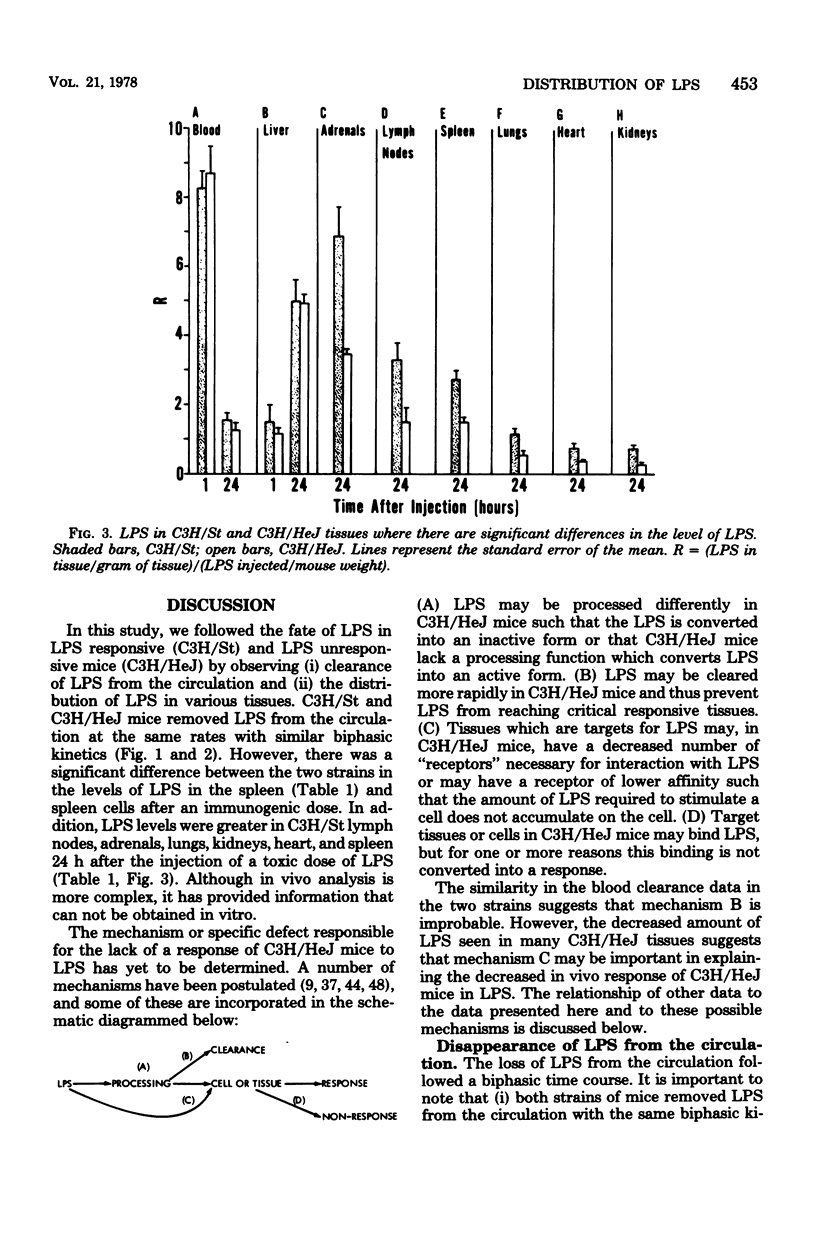
We examined the distribution of bacterial lipopolysaccharide (LPS) in LPS-responsive (C3H/St) and LPS-unresponsive (C3H/HeJ) mice. The results reported here demonstrate that the rates of removal of an immunological or a toxic dose of LPS from the circulation are the same in both strains of mice. C3H/St spleens accumulated significantly more LPS than C3H/HeJ spleens after the intravenous injection of either an immunogenic or a toxic dose of LPS. There was also a greater amount of LPS associated with cells teased from C3H/St spleens compared to those from C3H/HeJ spleens. After a toxic dose of LPS, there was more LPS in C3H/St lymph nodes, adrenals, lungs, kidneys, and heart than in the corresponding C3H/HeJ tissues. The accumulation of more LPS in tissues from C3H/St mice compared to C3H/HeJ mice suggests that these tissues are involved in the pathophysiological and, ultimately, the toxic effects of LPS. The differential accumulation of LPS in the tissues of these two strains may be the reason for the decreased responses of C3H/HeJ mice to LPS.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAREY F. J., BRAUDE A. I., ZALESKY M. Studies with radioactive endotoxin. III. The effect of tolerance on the distribution of radioactivity after intravenous injection of Escherichia coli endotoxin labeled with Cr51. J Clin Invest. 1958 Mar;37(3):441–457. doi: 10.1172/JCI103624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Sultzer B. M. Genetic control of B-cell responses. I. Selective unresponsiveness to lipopolysaccharide. Scand J Immunol. 1975;4(2):139–143. doi: 10.1111/j.1365-3083.1975.tb02610.x. [DOI] [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Scher I., Osborne B., Rosenstreich D. L. Cellular mechanism of endotoxin unresponsiveness in C3H/HeJ mice. J Immunol. 1976 Feb;116(2):454–461. [PubMed] [Google Scholar]
- HERRING W. B., HERION J. C., WALKER R. I., PALMER J. G. Distribution and clearance of circulating endotoxin. J Clin Invest. 1963 Jan;42:79–87. doi: 10.1172/JCI104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., ROWLEY D., WARDLAW A. C. Investigations on the mechanism of stimulation of non-specific immunity by bacterial lipopolysaccharides. Immunology. 1958 Jul;1(3):181–203. [PMC free article] [PubMed] [Google Scholar]
- Husberg B., Linell F., Nilsson T., Fritz H. Renal cortical necrosis induced in rabbits by local perfusion followed by systemic administration of endotoxin. Eur Surg Res. 1975;7(4-5):230–241. doi: 10.1159/000127808. [DOI] [PubMed] [Google Scholar]
- Kabir S., Rosenstreich D. L. Binding of bacterial endotoxin to murine spleen lymphocytes. Infect Immun. 1977 Jan;15(1):156–164. doi: 10.1128/iai.15.1.156-164.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Hoffmann M. K., Thomas L. Induction of phenotypic lymphocyte differentiation in LPS unresponsive mice by an LPS-induced serum factor and by lipid A-associated protein. J Immunol. 1977 May;118(5):1910–1911. [PubMed] [Google Scholar]
- Landy M., Baker P. J. Cytodynamics of the distinctive immune response produced in regional lymph nodes by Salmonella somatic polysaccharide. J Immunol. 1966 Nov;97(5):670–679. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Moeller G. R., Terry L., Snyderman R. The inflammatory response and resistance to endotoxin in mice. J Immunol. 1978 Jan;120(1):116–123. [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- OROSZLAN S. I., MORA P. T., SHEAR M. J. REVERSIBLE INACTIVATION OF AN ENDOTOXIN BY INTRACELLULAR PROTEIN. Biochem Pharmacol. 1963 Oct;12:1131–1146. doi: 10.1016/0006-2952(63)90087-x. [DOI] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L. Inheritance of lipopolysaccharide-enhanced nonspecific resistance to infection and of susceptibility to endotoxic shock in lipopolysaccharide low-responder mice. Infect Immun. 1977 May;16(2):432–438. doi: 10.1128/iai.16.2.432-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Rudbach J. A., Johnson A. G. Alteration and restoration of endotoxin activity after complexing with plasma proteins. J Bacteriol. 1966 Oct;92(4):892–898. doi: 10.1128/jb.92.4.892-898.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A. Molecular immunogenicity of bacterial lipopolysaccharide antigens: establishing a quantitative system. J Immunol. 1971 Apr;106(4):993–1001. [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., McAdam K. P. Genetic non-responsiveness of murine fibroblasts to bacterial endotoxin. Nature. 1977 Sep 8;269(5624):153–155. doi: 10.1038/269153a0. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). IV. Cellular basis of the unresponsiveness of C3H/HeJ mouse spleen cells to LPS-induced mitogenesis. J Immunol. 1977 Jan;118(1):274–281. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of host responses to endotoxin. Infect Immun. 1972 Jan;5(1):107–113. doi: 10.1128/iai.5.1.107-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic factors in leucocyte responses to endotoxin: further studies in mice. J Immunol. 1969 Jul;103(1):32–38. [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- THOMAS L., GOOD R. A. Studies on the generalized Shwartzman reaction: I. General observations concerning the phenomenon. J Exp Med. 1952 Dec;96(6):605–624. doi: 10.1084/jem.96.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffa-Bachi P., Kaplan J. G., Bona C. The mitogenic effect of lipopolysaccharide. Metabolic processing of lipopolysaccharide by mouse lymphocytes. Cell Immunol. 1977 Apr;30(1):1–11. doi: 10.1016/0008-8749(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Watson J. Differentiation of B lymphocytes in C3H/HeJ mice: the induction of Ia antigens by lipopolysaccharide. J Immunol. 1977 Mar;118(3):1103–1108. [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]
- Watson J., Trenkner E., Cohn M. The use of bacterial lipopolysaccharides to show that two signals are required for the induction of antibody synthesis. J Exp Med. 1973 Sep 1;138(3):699–714. doi: 10.1084/jem.138.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlydaszyk J. C., Moon R. J. Fate of 51Cr-labeled lipopolysaccharide in tissue culture cells and livers of normal mice. Infect Immun. 1976 Jul;14(1):100–105. doi: 10.1128/iai.14.1.100-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



