Abstract
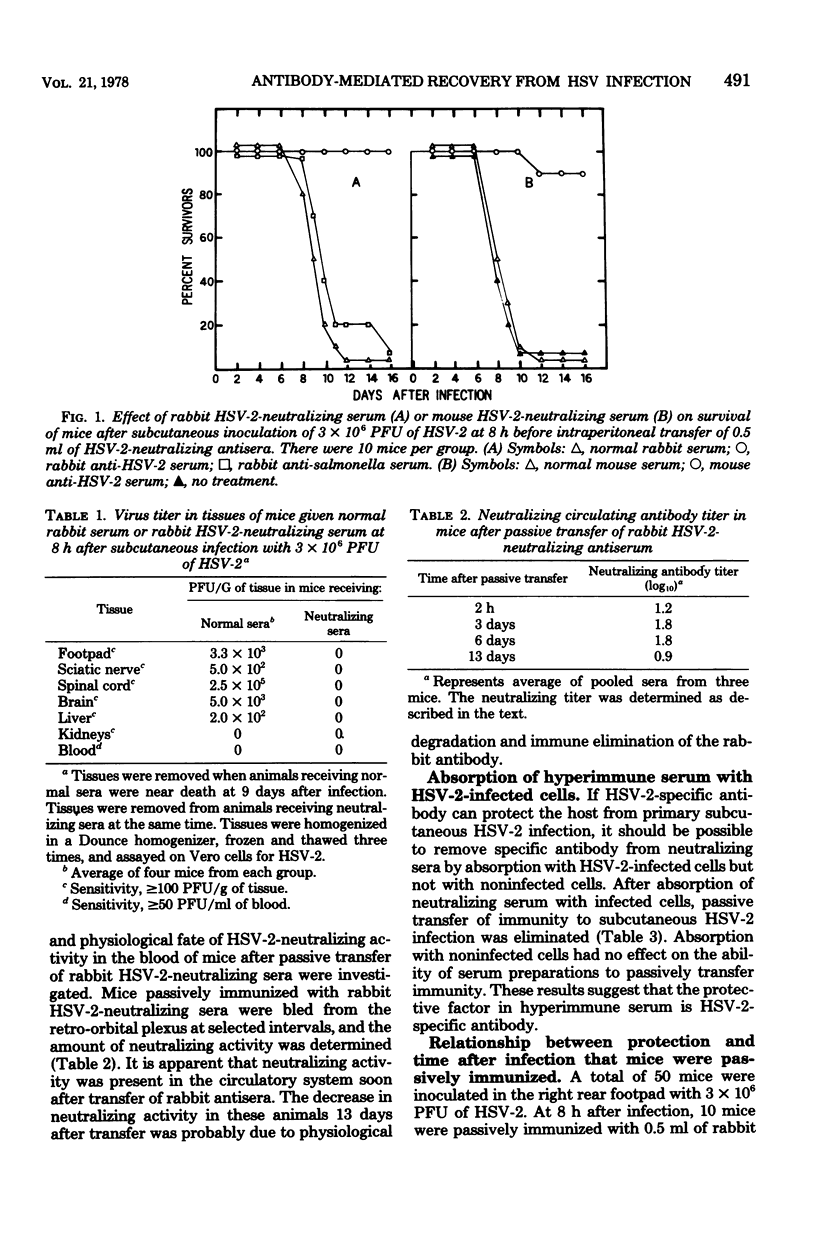
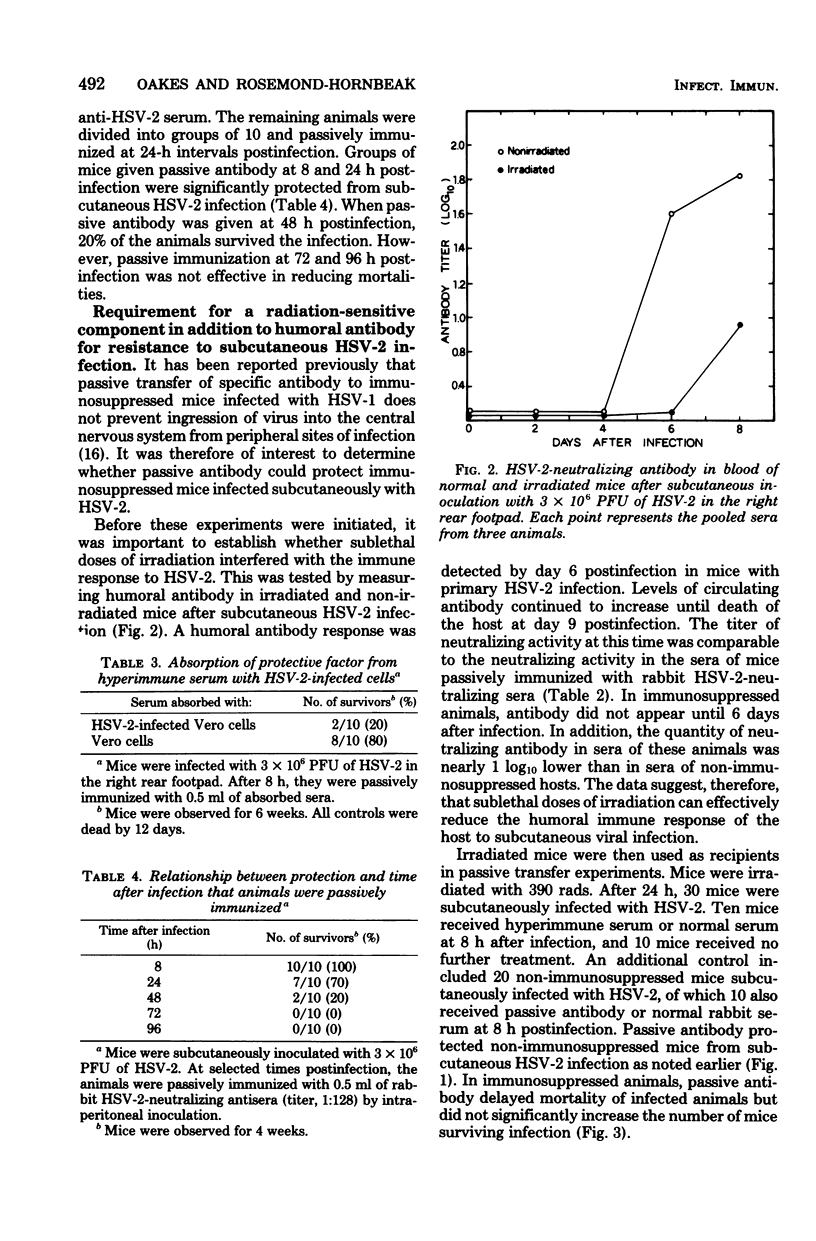
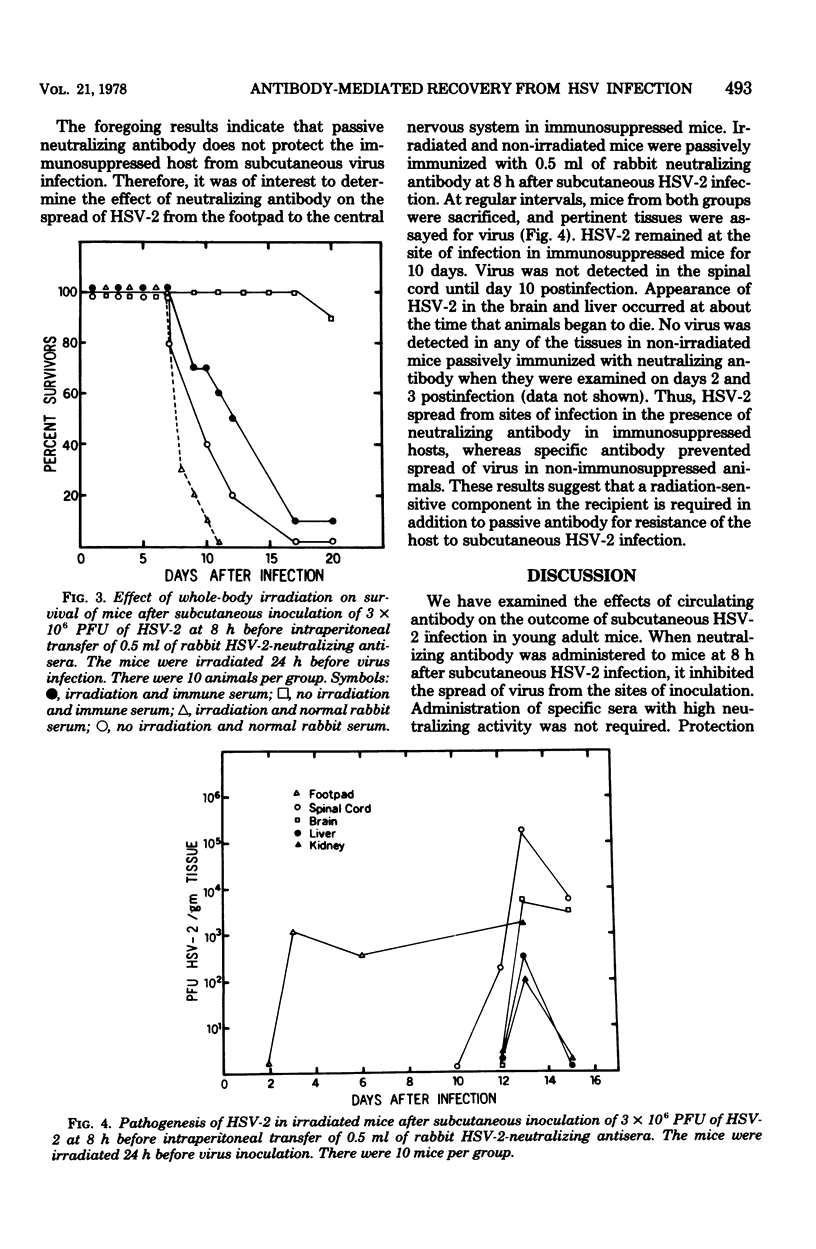
Young adult mice infected with 3 X 10(6) plaque-forming units of herpes simplex virus type 2 (HSV-2) died within 9 to 12 days after spread of the virus from the sites of infection to the spinal cord and brain. Administration of HSV-2-neutralizing antisera prepared in syngenic mice or in rabbits inhibited spread of the virus from the footpad of infected animals and prevented death. A single intraperitoneal inoculum of antiserum (virus-neutralizing titer of 1:128) was effective in protecting mice when the antiserum was given at 8 to 48 h, but not at 72 h, after virus inoculation. Immune sera absorbed with HSV-2-infected cells no longer protected mice from subcutaneous infection, whereas absorption with noninfected cells had no effect. Thus, HSV-2-specific antibodies appeared to be responsible for the protection observed. If the mice were given a sublethal dose of irradiation (390 rads) at 24 h before antibody transfer, protection was no longer obtained. This suggested that the mechanism of protection probably was not solely due to in vivo neutralization of virus, but required the participation of a radiosensitive component which has not yet been defined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston D. L., Cohen A., Spindler M. A. Herpesvirus hominis infection in patients with myeloproliferative and lymphoproliferative disorders. Br Med J. 1972 Nov 25;4(5838):462–465. doi: 10.1136/bmj.4.5838.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell P. A., Ross A., Miller L. H., Kuo B. Prevention of varicella by zoster immune globulin. N Engl J Med. 1969 May 29;280(22):1191–1194. doi: 10.1056/NEJM196905292802201. [DOI] [PubMed] [Google Scholar]
- Costa J., Rabson A. S., Yee C., Tralka T. S. Immunoglobulin binding to herpes virus-induced Fc receptors inhibits virus growth. Nature. 1977 Sep 15;269(5625):251–252. doi: 10.1038/269251a0. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against herpes simplex virus. II. Protection conferred by sensitized spleen cells. J Infect Dis. 1973 Jun;127(6):632–638. doi: 10.1093/infdis/127.6.632. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Steinberg S., Brunell P. A. Zoster immune globulin. A further assessment. N Engl J Med. 1974 Jan 31;290(5):243–245. doi: 10.1056/NEJM197401312900503. [DOI] [PubMed] [Google Scholar]
- Godfrey R. C. Resistance to intracerebral challenge in mice immunized against herpes simplex virus. Br J Exp Pathol. 1972 Oct;53(5):529–539. [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Hyman R. W., Rapp F. Genome location of polyadenylated transcripts of herpes simplex virus type 1 and type 2 DNA. Virology. 1976 Nov;75(1):145–154. doi: 10.1016/0042-6822(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan P. R., Ennis F. A. The elimination of herpes simplex plaques by antibody and the emergence of resistant strains. J Immunol. 1977 Jun;118(6):2167–2175. [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Rager-Zisman B., Bloom B. R. Immunological destruction of herpes simplex virus I infected cells. Nature. 1974 Oct 11;251(5475):542–543. doi: 10.1038/251542a0. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Melewicz F. M., Gordon D. S. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. I. Identification of the population of effector cells. J Immunol. 1977 Feb;118(2):558–566. [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Walz M. A., Yamamoto H., Notkins A. L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976 Dec 9;264(5586):554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]
- Wheeler C. E., Jr Pathogenesis of recurrent herpes simplex infections. J Invest Dermatol. 1975 Oct;65(4):341–346. doi: 10.1111/1523-1747.ep12607603. [DOI] [PubMed] [Google Scholar]


