One in 10 patients attends general practice with gastrointestinal symptoms.1 Irritable bowel syndrome (IBS) is typified by abdominal discomfort or pain, and bloating with disturbed defecation.2 It affects 10–20% of the general public,2 but it is difficult to define the prevalence of the disorder as it varies with different diagnostic criteria and many patients with IBS symptoms do not seek medical advice. The challenge for GPs is how to differentiate IBS from inflammatory bowel disease (IBD), which share a number of overlapping symptoms, enabling more appropriate referral to secondary care. The prevalence of IBS in general practice is far greater than IBD, which is 0.1–0.2% for ulcerative colitis and 0.05–0.1% for Crohn’s disease respectively.2
With significant demand for gastroenterology consultations and endoscopic investigations, a diagnostic pathway enabling GPs to confidently diagnose IBS will benefit both patients and secondary care gastroenterology services. The rate of colonoscopy procedures in England among primary care trusts varied from 71.6 to 194.1 per 10 000 of the population.1 The test is invasive and can be unpleasant for patients, and costs around £650 per procedure. It is estimated that the outcome of over 60% of colonoscopies performed in young patients are normal and therefore potentially avoidable. The development of alternative non-invasive tests accessible to primary care physicians is desirable and there is growing evidence that faecal calprotectin testing may fulfil this role.
Calprotectin is a calcium- and zinc-binding protein derived predominantly from neutrophils.3 Calprotectin can be measured in plasma, saliva, urine, and faeces. Elevated levels are associated with inflammation, which involves recruitment of neutrophils to the affected site. Studies have shown that raised levels of faecal calprotectin are associated with inflammation in the gut and are not influenced by extra-intestinal inflammation.3 Therefore it may be a more specific marker for intestinal inflammation than traditional markers of systemic inflammation like C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Calprotectin can be measured in a small sample of faeces. A variety of simple assays are available, most of which use enzyme-linked immunosorbent assay (ELISA) platforms. In a 2010 meta-analysis of 13 studies including 670 adults and 371 children, the pooled sensitivity and specificity of a positive faecal calprotectin result for the diagnosis of IBD was 93% and 96% respectively for adults, and 92% and 76% respectively in children and teenagers.4 Studies have shown particularly high negative predictive values (NPV); a 2011 retrospective analysis of 630 patients referred from primary to secondary care found that a normal faecal calprotectin had an NPV of 96% for the exclusion of symptomatic organic gastrointestinal disease on colonoscopy and biopsy.5 When using qualitative assays in the assessment of patients with relevant symptoms, the cutoff value for a positive test can significantly affect its sensitivity and specificity. In real terms, this equates to a tradeoff between the clinical consequences of missing a patient with IBD and the burden of unnecessary invasive endoscopic investigations. Most manufacturers currently recommend a cut-off value of 50 µg/g faeces, which is the current accepted cut-off. However, a recent study has suggested that, by reducing the cut-off to 8 µg/g faeces, a sensitivity of 100% with a specificity of 51% would mean a negative test would effectively exclude IBD.6 This requires further research but could mean that a lower cut-off than that currently recommended by the manufacturers could be used in the future.
History and clinical examination are still paramount in the diagnosis of gastrointestinal disorders. Previous recommendations for the investigation of IBS (full blood count, ESR, CRP, and coeliac serology testing) still apply, and management should be guided by the results of these tests. The National Institute for Health and Care Excellence (NICE) published its guidelines on the use of faecal calprotectin in this setting in October 2013.7 NICE guidance states that faecal calprotectin should not be used in any patient where cancer is suspected, hence it should not be part of the diagnostic work-up of those with red-flag signs or symptoms such as unexplained weight loss, anaemia and rectal bleeding, late-onset change in bowel habit, and abdominal masses.7 NICE recommends its use in the differentiation of IBS from IBD when referral to secondary care is being considered, where cancer is not suspected, where appropriate quality assurance processes are available, and where there are defined local care pathways in place for testing (Figure 1).
Figure 1.
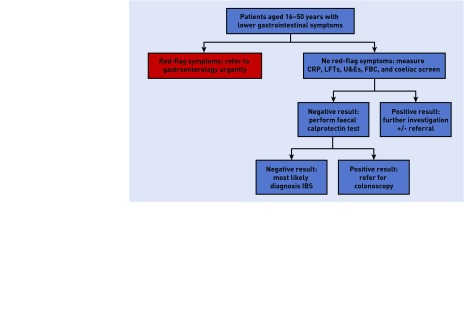
Proposed pathway for faecal calprotectin. CRP = C-reactive protein. FBC = full blood count. LFTs = liver function tests. U&Es = urea and electrolytes.
Secondary care referral to a gastroenterologist is advised where faecal calprotectin is found to be positive in an individual with lower gastrointestinal symptoms. NICE estimates a reduction in cost of £83 per patient if used this way in comparison with GPs’ current practice.7 If there is a high level of suspicion for IBD, even in the setting of a normal faecal calprotectin, for example, where there is a strong family history, secondary care referral may still be appropriate, and clinical judgement is required.
There are potential future applications for faecal calprotectin measurement in primary and secondary care. Levels of faecal calprotectin have been shown to be raised in asymptomatic family members of those with inflammatory bowel disease, which has implications for screening in primary care.3 For secondary care physicians, faecal calprotectin may have a greater role in the future management of IBD. There is a growing body of evidence that serial quantitative faecal calprotectin measurements can be used to monitor IBD activity and have been shown to have a greater predictive value for relapse than CRP and ESR. Levels of faecal calprotectin can be raised in other gastrointestinal diseases such as diverticular disease, coeliac disease, and non-steroidal anti-inflammatory drug (NSAID) enteropathy among other pathology. Stratification of calprotectin concentrations in these conditions in comparison with IBS and IBD are not well defined, and faecal calprotectin has not yet been shown to have utility in diagnosis or management of these conditions.
In conclusion, faecal calprotectin is a useful, NICE-approved adjunct in the investigation of individuals with symptoms suggestive of IBS. It will provide a significant cost benefit to GPs while reducing referrals to secondary care for both consultation and colonoscopy. It should not be used if red-flag signs or symptoms are present where urgent referral to secondary care is mandatory. The introduction of point-of-care tests for faecal calprotectin makes this an easily accessible screening tool for GPs. Further prospective evaluation of what constitutes the optimal cut-off value for the designation of a positive test will serve to enhance the performance characteristics of the test.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.NHS Atlas of Variation. Rate of colonoscopy procedures and flexisigmoidoscopy procedures per population by PCT. 2011. http://www.rightcare.nhs.uk/atlas/downloads/CancerMaps_AoV_2011.pdf (accessed 2 Oct 2014)
- 2.National Institute for Health and Care Excellence. Irritable bowel syndrome in adults Diagnosis and management of irritable bowel syndrome in primary care. London: NICE; 2008. NICE clinical guideline (CG61) [PubMed] [Google Scholar]
- 3.Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41(1):56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turvill J. High negative predictive value of a normal faecal calprotectin in patients with symptomatic intestinal disease. Frontline Gastroenterol. 2012;3:21–28. doi: 10.1136/flgastro-2011-100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A, Srinivas M, Eyre R, et al. Faecal calprotectin for differentiating between irritable bowel syndrome and inflammatory bowel disease: a useful screen in daily gastroenterology practice. Frontline Gastroenterol. doi: 10.1136/flgastro-2013-100429.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. London: NICE; 2013. NICE diagnostic guidance 11. [Google Scholar]


