Abstract
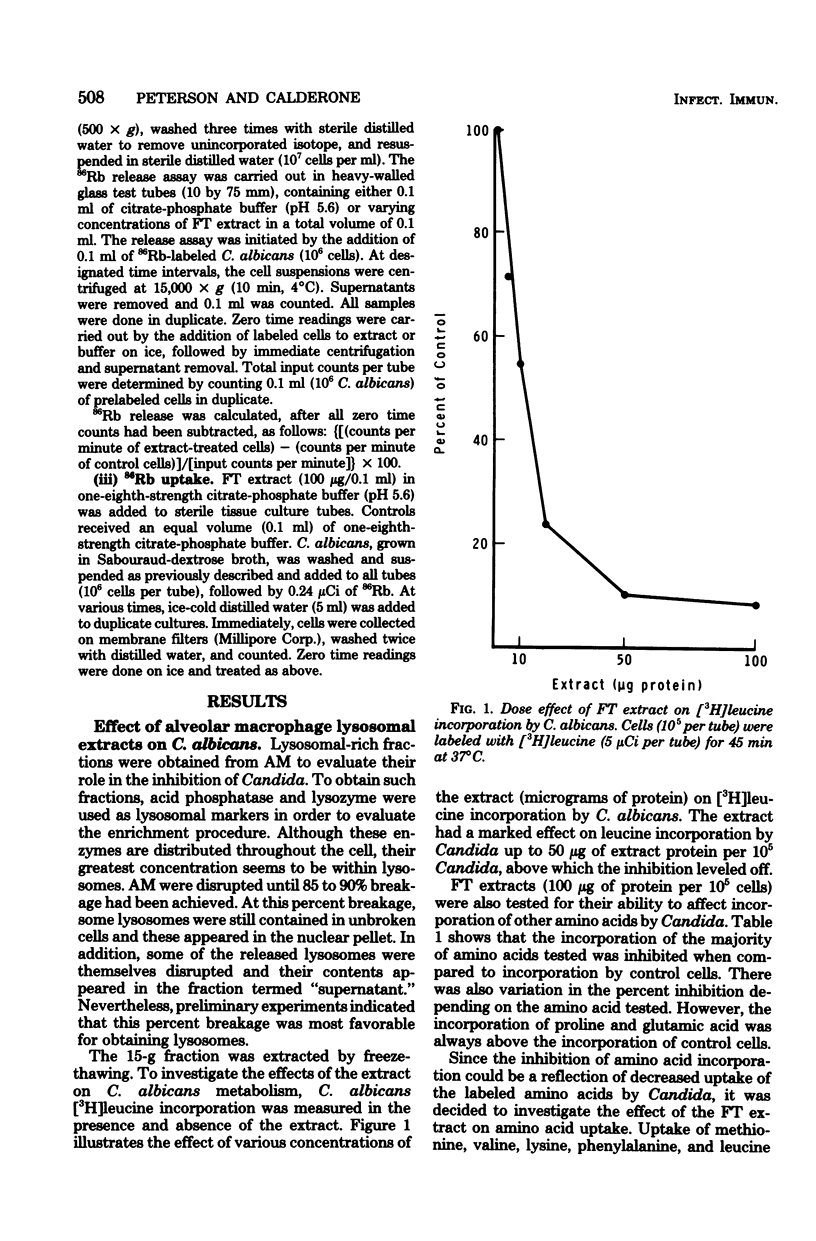
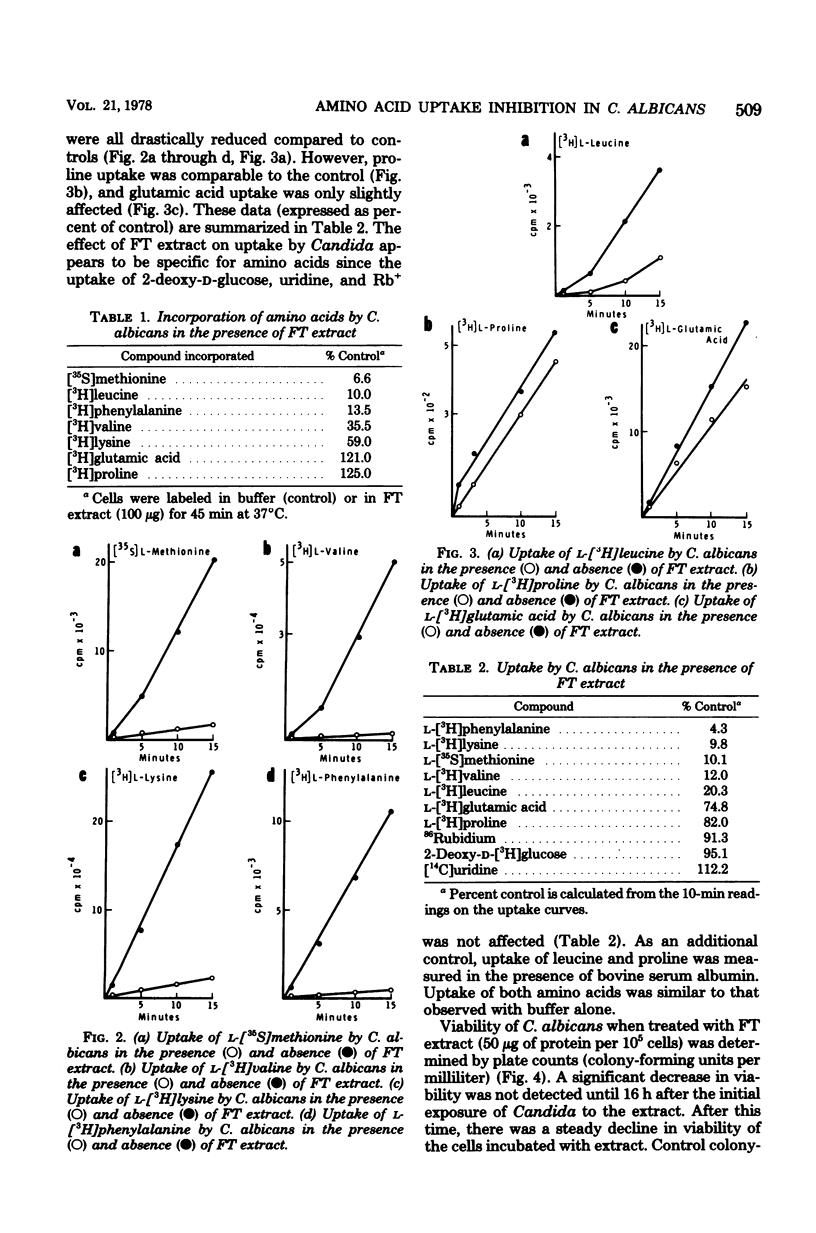
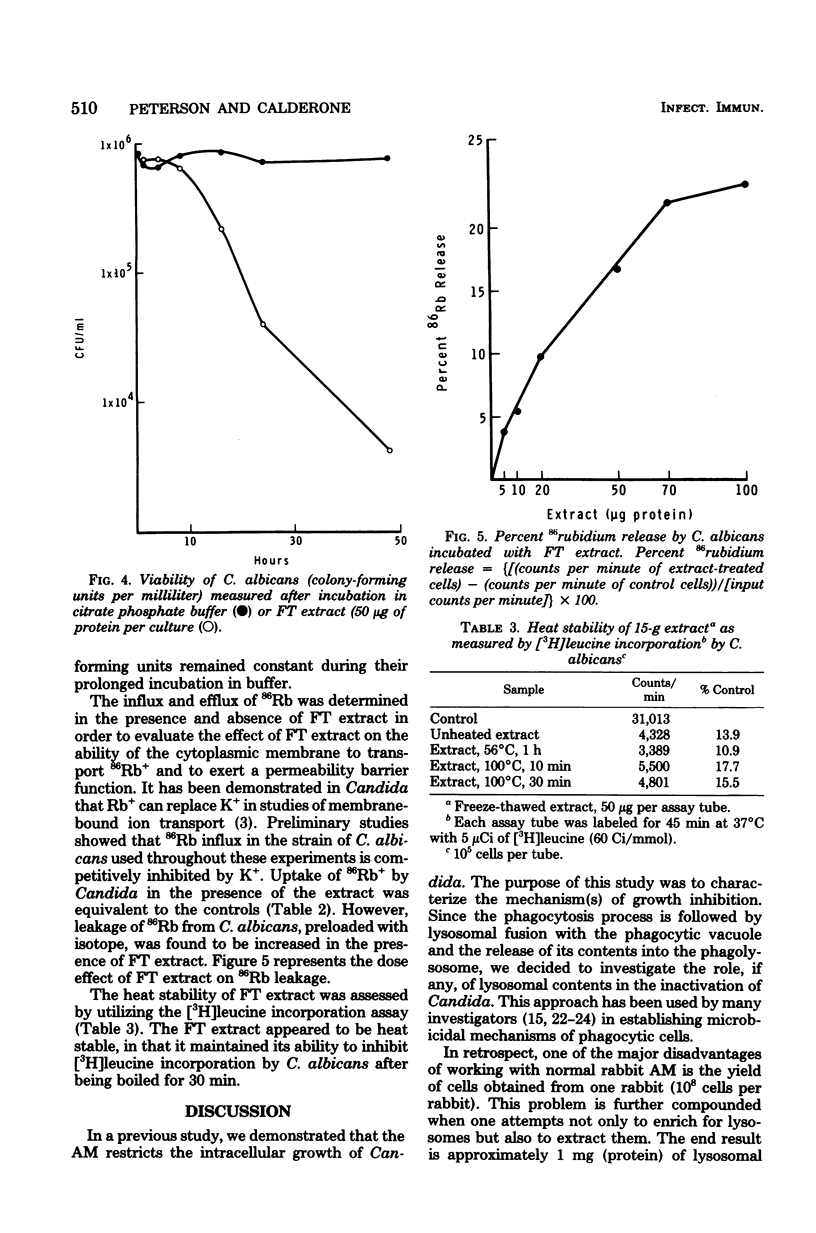
Lysosomal-rich fractions, obtained from normal rabbit alveolar macrophages, were extracted and tested for their effects on Candida albicans. The uptake and incorporation of various compounds (amino acids, uridine, 2-deoxy-D-glucose, and Rb+) by C. albicans were measured in the presence and absence of extract. These studies demonstrated that the extract had a specific effect on the uptake of certain amino acids by C. albicans. Of the amino acids tested, the uptake of methionine valine, lysine, phenylalanine, and leucine was drastically reduced in the presence of extract, whereas proline and glutamic acid uptake was unaffected. Those amino acids whose uptake was inhibited have been shown to be transported in other yeasts by a general amino acid permease. The existence of a general amino acid permease in C. albicans is compatible with our data. Additionally, the extract had no effect on the uptake of uridine, 2-deoxy-D-glucose, and Rb+. Leakage of 86Rb by C. albicans was detected in the presence of the extract. Viability of Candida, as measured by colony-forming ability, decreased after a 16-h incubation of C. albicans with the extract.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dabrowa N., Howard D. H. Uptake of L-proline by Histoplasma capsulatum. Can J Microbiol. 1976 Aug;22(8):1188–1190. doi: 10.1139/m76-173. [DOI] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Fungicidal properties of a chymotrypsin-like cationic protein from human neutrophils: adsorption to Candida parapsilosis. Infect Immun. 1977 Aug;17(2):382–388. doi: 10.1128/iai.17.2.382-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Rubidium release: a rapid and sensitive assay for amphotericin B. J Infect Dis. 1976 Sep;134(3):238–244. doi: 10.1093/infdis/134.3.238. [DOI] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Further studies on preparation and properties of phagocytin. J Exp Med. 1960 Mar 1;111:323–337. doi: 10.1084/jem.111.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Schwencke J. A proline transport system in Saccharomyces chevalieri. Biochim Biophys Acta. 1969 Mar 11;173(2):313–323. doi: 10.1016/0005-2736(69)90114-x. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Bactericidal activity of specific and azurophil granules from human neutrophils: studies with outer-membrane mutants of Salmonella typhimurium LT-2. Infect Immun. 1978 Jan;19(1):131–137. doi: 10.1128/iai.19.1.131-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Larimore F., Levy J. S. Inhibition of amino acid transport by ammonium ion in Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):325–331. doi: 10.1128/jb.124.1.325-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Characterization of cationic protein-bearing granules of polymorphonuclear leukocytes. Lab Invest. 1971 Mar;24(3):229–236. [PubMed] [Google Scholar]


