Abstract
This study examines the barriers and facilitators of retention among patients receiving buprenorphine/naloxone at eight community-based opioid treatment programs across the United States. Participants (n=105) were recruited up to three-and-a-half years after having participated in a randomized clinical trial comparing the effect of buprenorphine/naloxone and methadone on liver function. Semi-structured interviews were conducted with 67 patients provided with buprenorphine/naloxone who had terminated early and 38 patients who had completed at least 24 weeks of the trial. Qualitative data were analyzed using the constant comparison method. Barriers to buprenorphine/naloxone retention that emerged included factors associated with: (1) the design of the clinical trial, (2) negative medication or treatment experience, and (3) personal circumstances. The facilitators comprised: (1) positive experience with the medication, (2) personal determination and commitment to complete, and (3) staff encouragement and support. The themes drawn from interviews highlight the importance of considering patients’ prior experience with buprenorphine/naloxone and methadone, medication preference, personal circumstances, and motivation to abstain from illicit use or misuse of opioids, as these may influence retention. Ongoing education of patients and staff regarding buprenorphine/naloxone, especially in comparison to methadone, and support from staff and peers are essential.
Keywords: qualitative, buprenorphine, retention, patient perspectives
BACKGROUND
Opioid dependence is often a chronic relapsing condition associated with negative consequences in multiple life domains, including high mortality rates (Kimber et al. 2010; Hser et al. 2001), overdose death (Binswanger et al. 2007), HIV and hepatitis infection (Friedman, Newton & Klein 2003; Ronald, Robertson & Elton 1994; Specter 1994), and criminal involvement (Skinner et al. 2011; Hser 2007). Fortunately, pharmacotherapy with either methadone or buprenorphine has been shown in clinical trials to be effective in reducing opioid use (Mattick et al. 2008).
Methadone
Extensive research on multiple continents since the 1970’s has shown that methadone treatment is effective in reducing opioid use (Mattick et al. 2003). It has been associated with reduced: criminal behavior (Ball & Ross, et al., 1991); mortality (Caplehorn et al., 1996); and HIV infection (Metzger et al., 1993); and to improve quality of life (Xiao et al. 2010; Ponizovsky & Grinshpoon 2007). However, despite the benefits of methadone, this medication is subject to control through its required on-site dose administration early in treatment and for unstable patients who must access care through carefully regulated Opioid Treatment Programs (OTPs).
Buprenorphine, a relatively new treatment for opioid dependence in the United States
Buprenorphine was approved for use in the treatment of opioid dependence by the Food & Drug Administration (FDA) in 2002 (Center for Substance Abuse Treatment 2004). Due to its partial µ-opioid agonist properties, it is less likely to cause respiratory depression and overdose death than full opioid agonists such as methadone. In addition to the sublingual buprenorphine monoproduct tablet, buprenorphine/naloxone combination sublingual tablets (in generic form) and film (Suboxone) are available in the US. Buprenorphine/naloxone contains the opioid antagonist naloxone, which is not well absorbed sublingually, but precipitates opioid withdrawal upon injection in opioid tolerant individuals, thereby discouraging its intravenous misuse. In the US, both preparations can be prescribed by specially-licensed physicians and dispensed as a prescription for the treatment of opioid dependence outside of OTPs.
Buprenorphine can also be provided through OTPs with the same regulatory structure as methadone, including required bundled services (counseling, urine testing). Constraints in permitted take home doses of buprenorphine were the same as those for methadone until 2012, when the federal regulations for OTPs permitted patients to receive buprenorphine take homes on par with patients receiving buprenorphine by prescription (United States Department of Health and Human Services 2012). While buprenorphine has been shown to be safe and effective in treating opioid dependence (Kamien, Branstetter & Amass 2008; Fudala et al. 2003; Ling et al. 1998; Strain et al. 1996; Kosten 1994), there are a number of barriers to this treatment, foremost its cost and availability (Bazazi et al. 2011; Ducharme & Abraham 2008).
Treatment retention
Retention in drug treatment is associated with better outcomes (Zhang, Friedmann & Gerstein 2003; Hubbard et al. 1997; Ball & Ross 1991). Studies in the US, United Kingdom, and Australia have shown a relationship between treatment retention and outcomes, such as decreased drug use or reduced criminal involvement (Teesson et al. 2008; Gossop et al. 2003; Hubbard 2003; Simpson et al. 1997; Simpson 1981).
However, treatment retention is influenced by a number of factors, including type of medication and previous treatment experience (Kelly et al. 2011; Deck & Carlson 2005; Booth, Corsi & Mikulich-Gilbertson 2004; Koester, Anderson & Hoffer 1999; Magura, Nwakeze & Demsky 1998; Rhoades et al. 1998; Simpson et al. 1997; Saxon et al. 1996). Although some studies have found similar retention rates for buprenorphine and methadone (Johnson et al. 2000; Strain et al. 1994), others have shown poorer retention for buprenorphine when compared to methadone (Bell et al. 2009; Connock et al. 2007; Kristensen et al. 2005; Fischer et al. 1999). A recent review of clinical trials comparing the effectiveness of buprenorphine to methadone and placebo reported that buprenorphine, provided in a flexible dosing schedule, was less likely than methadone to retain patients in treatment; a fixed dosing schedule was less or equally as likely to retain patients than low dose methadone; and medium dose buprenorphine was less likely to retain more patients than low dose methadone (Mattick et al. 2008).
One limitation of most clinical trials is the lack of qualitative data to examine participants’ reasons for discontinuing treatment. Few studies have examined patient perspectives, attitudes, and reasons for leaving methadone (Al-Tayyib & Koester 2011; Reisinger et al. 2009; Schwartz et al. 2008) and buprenorphine treatment (Egan et al. 2011; Winstock, Lintzeris & Lea 2011; Awgu, Magura & Rosenblum 2010; Wallen, Lorman & Gosciniak 2006). Patients’ perspectives are useful in understanding potential barriers to and facilitators of retention and can inform strategies for improving treatment with buprenorphine.
The present study (hereafter referred to as the Retention Study) was designed to explore the reasons for the lower treatment retention rates in buprenorphine/naloxone compared to methadone treatment (46% versus 74%, respectively) found among participants enrolled in the clinical trial entitled “Starting Treatment with Agonist Replacement Therapies” (START). The Retention Study was initiated to better understand, from the patient perspective, the barriers to and facilitators of retention among patients who received buprenorphine/naloxone.
Starting Treatment with Agonist Replacement Therapies (START)
START was a Phase 4 study to assess liver function in participants randomized to either open-label buprenorphine/naloxone or methadone (See Saxon et al. 2013 for details). Individuals were recruited between May 2006 and October 2009 at nine federally licensed OTPs across the US. Due to higher dropout in the buprenorphine/ naloxone condition, the initial randomization scheme of 1:1 (buprenorphine/naloxone to methadone) was changed to 2:1, 18 months after study initiation.
Participants were inducted on medication after being instructed to abstain from opioids for 12–24 hours to present in mild to moderate opioid withdrawal, or as deemed appropriate by the study physician. Participants were to attend the clinic daily for observed medication administration except Sundays and holidays or when take-home medications were permitted by local regulations, were titrated to an appropriate medication dose, and were tapered off medication starting after 24 weeks or were transitioned to non-study medication. Assessments included urine drug screens, adverse event monitoring (weekly), and self-reported drug use (every four weeks). Participants who missed 14 or more consecutive days of study medication were terminated from the study. At study completion (by Week 24), participants were referred for clinical treatment and/or to local treatment resources by Week 32.
Buprenorphine was provided as a combination sublingual tablet containing both buprenorphine and naloxone. The induction period included the first three days of dosing, with the first dose ranging from 2–8 mg. Study staff observed participants during dosing and a second Day 1 dose was provided if deemed appropriate (up to a total Day 1 maximum dose of 16 mg). The remaining induction dosing schedule was determined by the study physician’s clinical judgment, with a maximum daily dose of 32 mg. For the remainder of the stabilization phase, flexible dosing was employed according to clinical impression and the participant’s clinical need. Investigators were encouraged to dose adequately to decrease craving and to obtain negative urine toxicology specimens. Recommended dose changes were made in 2–8 mg increments.
METHODS
The present study used background survey questionnaire, semi-structured interview, and medication dosing data. Participants were interviewed during September 2009 - September 2010, which was up to three-and-a-half years after their participation ended in START.
Sampling and recruitment
Interviews were conducted at eight of the nine OTPs that participated in START (three in California, two in Connecticut, one in Oregon, one in Pennsylvania, and one in Washington). One site, which discontinued participation in the START study, was not included. Retention Study staff provided site research staff with START participant identification numbers and postage-stamped envelopes containing invitation letters for a health study, which included a toll free number. Site research staff distributed Retention Study recruitment materials to their START participants. When individuals called the toll free number, Retention Study staff provided information about the study, answered questions, and scheduled participants for an interview.
Data collection
Three of the co-authors (Hasson, Teruya, Thomas) conducted the interviews. Participants provided written informed consent and were asked to authorize release of their START data (e.g., medication, duration of participation). The interview guide included questions covering patients’ experience in START (e.g., prior experience with buprenorphine and methadone, medication preference, dosing, opioid use, reasons for leaving START early, what kept them in START). START participants also completed a background questionnaire (e.g., demographics, treatment history). Audiotaped interviews lasted approximately one hour, were conducted in private rooms at the OTPs, and participants were paid for their participation. The present study was approved by the South General Institutional Review Board (IRB) at the University of California, Los Angeles, and each site’s IRB of record.
Data analysis
Analyses sought to identify and describe barriers to and facilitators of retention in START among two subsets of the Retention Study sample, namely the buprenorphine/naloxone early terminators and completers, and explore themes and patterns that emerged from the data across the eight sites. This report focuses on the buprenorphine/naloxone participants because they had higher rates of drop-out than methadone patients enrolled in the parent study. In addition, analyses of barriers to retention among the methadone participants interviewed were common to both medication groups and reasons for premature termination of methadone treatment have been well-examined (Reisinger et al. 2009; Joe et al. 1998; Magura et al. 1998).
Qualitative data analyses were conducted on transcribed audio recordings of the interviews. Transcripts were reviewed against the audio recordings for accuracy and completeness, edited, and uploaded into Atlas.ti for coding. Analyses were conducted simultaneously with data collection and data interpretation in an iterative process according to established procedures for qualitative research (Creswell 2003; Huberman & Miles 1994; Glaser & Strauss 1967). The process involved the repeated reading of the transcripts, development of a code list, coding of the data to identify emerging patterns relevant to study objectives, and use of the constant comparison method (Demetrovics et al. 2009; Glaser & Strauss 1967). Development of the preliminary code list was guided by the interview topics (e.g., medication preference, prior experience with buprenorphine and methadone, reasons for discontinuing START). Inductive codes that emerged from the data were added (e.g., comparisons of buprenorphine and methadone), and code lists were adjusted and refined.
The major thematic categories emerged from the data as coded text and transcripts were read and discussed among the co-authors. The barriers were finally re-conceptualized in the following categories: design of the clinical trial, effects of the buprenorphine/naloxone, and personal circumstances. For the facilitators of completion, major themes included: medication “worked” – felt “normal,” personal determination and commitment, and staff encouragement and support.
Several methods were used to ensure the rigor of the qualitative work. Portions of coded transcripts were randomly and independently coded by at least two researchers to ensure that the codes were being applied consistently and had acceptable levels of agreement indicating good reliability (Boyatzis 1998). Triangulation (Patton 1990) involved multiple members of the Retention Study team and co-authors reviewing and discussing analytic findings, and cross-checking interview data with other information collected (e.g., dosing records, background questionnaire data). Descriptive statistics were calculated from the quantitative data collected via the participant questionnaire to provide insight into the backgrounds of participants by barrier and facilitator thematic categories.
RESULTS
Sample characteristics
The sample for the present analyses is comprised of 105 participants who were randomly assigned to receive buprenorphine/naloxone, of whom 67 participated in START for less than 24 weeks (17% of the total buprenorphine/naloxone early terminators in START), and 38 of whom completed at least 24 weeks of the study (11% of the total buprenorphine/naloxone completers in START). Table 1 shows that the groups were similar in that the majority of the participants were male, White, had injected opioids sometime in their lives, and had prior methadone treatment. About one quarter had prior buprenorphine treatment, about half were unemployed, and almost half were receiving welfare benefits. The mean longest period of consecutive years of opioid use was 10.4 for the early terminators and 13.8 for the completers. However, the participants receiving buprenorphine/naloxone who completed START were significantly older (p =0.0059) than the group who terminated early from START (45.5 years vs. 39.2 years old, respectively). The Retention Study buprenorphine/naloxone sample was similar to the START subsample that were randomized to buprenorphine/naloxone in terms of gender (68% male), and race/ethnicity, but was significantly older (41.5 years vs. 37.5 years old, respectively; p =0.0007).
Table 1.
Sample characteristics of participants in the Retention Study and START
| Characteristics | Retention Study | START | ||
|---|---|---|---|---|
| Terminated Early* (n=67) |
Completed* (n=38) |
Total (n=105) |
Total (n=740) |
|
| Male % | 66 | 66 | 66 | 68 |
| Race/ethnicity % | ||||
| White | 58 | 69 | 62 | 69 |
| Black | 6 | 13 | 9 | 9 |
| Hispanic | 21 | 13 | 18 | 13 |
| Other | 15 | 5 | 11 | 9 |
| Mean age (SD)1,2 | 39.2 (11.4) | 45.5 (10.1) | 41.5 (11.31) | 37.5 (11.24) |
| Ever injected opiates % | 78 | 66 | -- | -- |
| Mean longest period in years using opiates (SD) | 10.4 (8.48) | 13.8 (11.37) | -- | -- |
| Prior buprenorphine treatment % | 28 | 24 | -- | -- |
| Prior methadone treatment % | 73 | 66 | -- | -- |
| Employment % | ||||
| Full or part-time | 22 | 29 | -- | -- |
| Student | 13 | 5 | -- | -- |
| Retired/disabled | 13 | 24 | -- | -- |
| Unemployed | 52 | 42 | -- | -- |
| On welfare % | 48 | 47 | -- | -- |
Significant difference at p=0.0059 between Terminated Early and Completed groups
Significant difference at p=0.0007 between buprenorphine/naloxone participants in Retention Study sample and total START sample
SD = Standard Deviation
-- Data not available
Status in START
Thematic findings
Buprenorphine/naloxone patient perspectives on barriers to retention in START
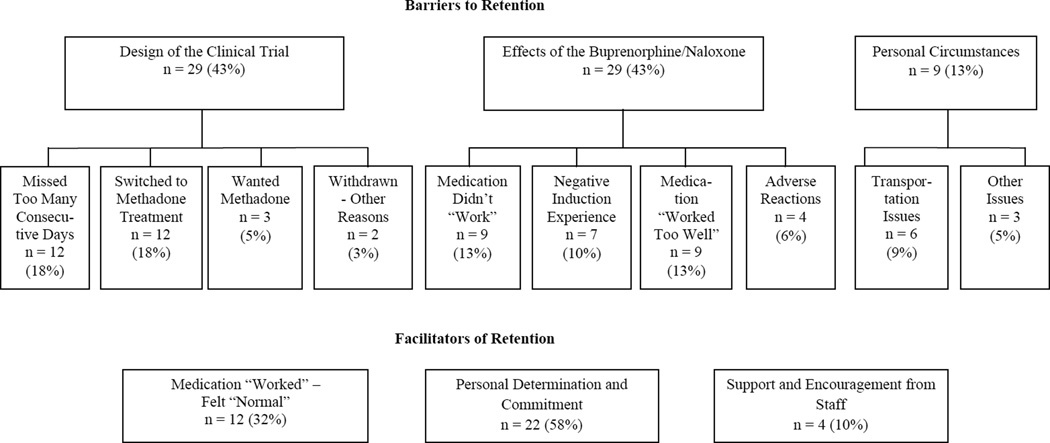
Although participants who received buprenorphine/naloxone described a range of factors that impeded their ability or influenced their desire to complete START, they typically were able to articulate a main reason for their non-completion. Figure 1 shows the major thematic categories of the main barriers. Quotations from participants are provided to illustrate the themes and subthemes.
Figure 1.
Thematic categories of main barriers to and facilitators of retention in START among buprenorphine/naloxone patients interviewed
Design of the clinical trial
Some participants reported that aspects of the study protocol (e.g., dosing requirements, randomization to either buprenorphine/naloxone or methadone) resulted in their being withdrawn by the investigators or in their dropping out.
Missed too many consecutive days
Twelve participants reported that they were withdrawn from the study due to missing 14 or more consecutive days of study medication (e.g., incarcerated, family emergency, surgery). Many of the participants emphasized that had they not been told that they were discontinued from START, they would have returned once they were able. The following quotation illustrates the desire and intentions of one participant to return to START: “You could only miss 14 days in a row…to stay on it [START]. And I came back like the 15th day. So they told me I was no longer eligible for the study.”
Switched to methadone treatment
When asked why they left START early, 12 participants responded that they were presented with an opportunity to switch or transfer to methadone treatment outside of the study. Once they switched medications, they were withdrawn from START. While a number of them indicated that they had a preference for methadone and/or were experiencing some discomfort with the buprenorphine/naloxone (e.g., “jittery,” “restless legs”, “headaches,” “didn’t hold me completely”), a few left because they were approved for publicly funded treatment with methadone that continued beyond the study period. One participant recalled, “I had told ‘em that I was waiting for…my SSI [Supplemental Security Income] so I could get back on the methadone.”
Many of these participants were able to transfer to methadone at the study site to continue their treatment. Several indicated that they were surprised that they could no longer continue in the study because of their decision to switch, as methadone was one of the study medications. A few participants acknowledged that they would have stayed in START had they not had other treatment options.
Wanted methadone
Although START participants were informed that they would be randomly assigned to the study medication, many had an initial preference for one or the other. Three of the participants assigned to the buprenorphine/naloxone group expressed that they left START early because of their preference for methadone. After learning that they would be receiving buprenorphine/naloxone, two participants left START without taking their first dose. One, who was not familiar with buprenorphine/naloxone, said “They told me…I couldn’t get methadone, I was gonna get Suboxone, and I left. I walked out the door.” The second individual was “apprehensive” about taking the sublingual tablet while already taking 12 pills for a medical condition. The third participant in this group who had had success with methadone prior to enrollment in START, had hoped to be assigned to methadone.
Withdrawn for other reasons
Two participants perceived that they were withdrawn from START, one after becoming pregnant, and the other for using prescribed opioids while on the study, apparently without informing the research staff.
Effects of the buprenorphine/naloxone
Although some participants had experience with buprenorphine, including the combination buprenorphine/naloxone, in general, participants were not as familiar with buprenorphine as they were with methadone, especially at the beginning of START. Thus, when speaking about their experiences with buprenorphine/ naloxone during START, participants who had previously taken methadone often made comparisons in terms of physical and/or mental reactions to the medication. Several participants expressed skepticism or apprehension about the relatively new medication, while others may have had unrealistically high expectations about it. Finally, a number of participants voluntarily dropped out of START, whereas others were withdrawn due to adverse reactions.
Medication didn’t “work.”
The nine participants in this group described the medication as not “working” for them. Participants seemed to have expectations about how buprenorphine/naloxone should “work” (e.g., “miracle drug,” similar to methadone) or expressed concern about not knowing what to expect, and these perceptions seemed to present a barrier to completing START. When asked what “really didn’t work” meant, one participant replied, “Like when I would take it, like I would bust out in the sweats and feel like sick…It would make me feel like dope-sick, you know.”
Reported negative effects of the medication included headaches, nervousness, anxiety, stomach cramps, diarrhea, and withdrawal symptoms (e.g., cold sweats, nausea, sleeplessness). Several participants reported they had opioids in their systems at the time of induction onto buprenorphine/naloxone. Consequently, they did not experience relief from withdrawal symptoms upon taking the first few doses of medication, as they would have had, had they refrained from opioid use for the prescribed period of time prior to induction. This may not have been a problem, had they been inducted on methadone.
According to some participants, the withdrawal symptoms or negative reactions continued beyond the induction period, despite dosage adjustments. When a few participants who were at the maximum dose of 32 mg continued to feel sick, they seemed to conclude that the medication was not going to be effective for them and decided to leave the study. The following quotation is illustrative:
"It didn't work at all…I took the medication…It wasn't doing what I thought it was gonna do, like take care of the withdrawals…But I can understand because they don't wanna start you out at a huge dose…I had gone up to the maximum dose, and so I couldn't go any higher. And so, at that point, it still wasn't working for me."
While some participants had difficulties with the medication early on, a few explained that their initial experiences were positive, but they later began to experience unpleasant physical symptoms. For example, one participant shared:
“Everything was fine for, really, almost a month…First it was just diarrhea, you know, and I said, ‘Ah, that’ll go away’…and it did…I started having stomach cramps as well…I talked to the doctor and talked to people here, and we all agreed that we should give it more time, that if it is caused by the Suboxone,…my body will probably adjust to it and the symptoms will either go away altogether…or lessen greatly…But it turned out it just kept getting worse and worse. And two or three months’ worth of diarrhea and intense stomach cramping, I had finally just made the decision that I was not gonna take it anymore.”
While some participants reported abstaining from opioids during START, others described using opioids in an attempt to obtain relief from their withdrawal symptoms, and ultimately decided to drop out of START. One participant said, “Well, I left because the Suboxone wasn’t working for me anymore…and I got nervous on it. And I started using again, so I left the study.”
Medication “worked too well.”
Nine participants indicated that they dropped out of START because they wanted to feel the euphoric effects when they used opioids, but were not able to do so while taking buprenorphine/naloxone. They described being disappointed that they could not feel the opioid effect despite using increasing quantities of heroin, or could only use opioids, including heroin, after some of the buprenorphine/naloxone was out of their systems. As one participant put it,“[It] took away my choice of whether to use or not…It made it to where I didn’t have a choice. I couldn’t use because I wasn’t gonna feel nothin’ anyway…It worked too well for my purpose.” Some participants talked about missing doses so they could “get high.” A participant commented:
“For a while, you know, I was like back and forth, you know, I would use a little bit. But if you use on this stuff, you know, you can’t really feel it that good. So like on the weekends, I wouldn’t take it or whatever.”
Some participants may not have been ready to abstain from opioids. One individual said, “I wasn’t ready to quit…I wasn’t ready to be clean-clean,” and “the more loaded you are, the less you have to deal with.”
Negative induction experience
While most of the participants stayed beyond the initial induction period, others described their experiences on the first few days of receiving buprenorphine/naloxone, using words and phrases like “nightmare,” “awful,” “hell,” and “thought I was gonna die.” It is notable that these same participants disclosed that they had methadone or some other opioid in their systems when inducted. They recalled experiencing, for example, hallucinations, body cramps, diarrhea, lack of energy, fever, chills, headaches, vomiting, and/or convulsions.
Several individuals said that they were not given specific information about preparing for induction with the possibility of receiving buprenorphine/naloxone. One participant explained,
“They didn’t tell me that if I had methadone in my system I’d go into, you know, complete withdrawal. I thought if I was just adding the methadone…I would just feel okay.”
However, others said that even though START study staff specifically “warned” them about having opioids in their systems prior to induction, they were reluctant to stop using because either they did not wish to experience withdrawal symptoms or actually thought they were in withdrawal when they presented for randomization and induction. One participant said:
“I was doing about 6 grams a day. And they told me not to…have any heroin in my system 24 hours prior to the date that was preset. It’s pretty hard when you’re a heroin addict, and telling you not to do any heroin 24 hours before that.”
The majority of participants in this group remembered that they did not return to the study after the first day of dosing.
Adverse reactions
Although most of the participants who expressed having terminated from the study early due to the effects of buprenorphine/naloxone dropped out on their own accord, four participants said they had to “switch to methadone” following serious reactions to the buprenorphine/naloxone. One participant had been on buprenorphine/naloxone for approximately three months before experiencing seizures, whereas another said, “The Suboxone actually put me into rapid cycling and I ended up hospitalized.” A third participant explained, “My hepatic panel, my liver enzymes, went through the roof…So that’s why they took me off the Suboxone,” and the fourth had an allergic reaction to the medication.
Personal circumstances
One of the major barriers to completing START involved personal circumstances and choices not primarily due to the design of START or the buprenorphine/naloxone itself. These involved transportation issues, competing priorities, and issues with clinic policies/staff that prompted participants to drop out of the study. Six participants communicated that they left START due to transportation issues (e.g., automobile problems, cost of bus fare or gasoline) and distance to the clinic (e.g., 45-minute drive). Two participants conveyed that having weighed competing priorities for their time (e.g., work, time with family), participation in START took lower priority, particularly given the inconvenience of the clinic location or the schedule of the mandatory meetings (e.g., during the day). Notably, both individuals expressed that they also felt they no longer needed the medication.
Participant characteristics and main barriers to retention
Table 2 displays characteristics of the participants according to the thematic categories and subcategories. Within the group that terminated START early due to aspects of the “design of the clinical trial,” the participants that discussed the theme “switched to methadone” were generally in their mid thirties; the majority had injected opioids and reported prior experience with both buprenorphine and methadone. While half of them reported having a preference for buprenorphine/naloxone, slightly less than that had no medication preference at enrollment. Participants in this group typically stayed in START for about one month, and the average maximum dose they received (27 mg, with a range of 10–32 mg) was at the higher end of the range allowable in the trial (2–32 mg). In contrast, participants in the “wanted methadone” group were, on average, in their fifties, had injected opioids, had prior experience with methadone, and left START relatively soon after randomization, which accounts for the lower average maximum dose (4 mg, with a range of 1–12 mg). All participants in this group had a preference for methadone upon entering START.
Table 2.
Characteristics of buprenorphine/naloxone participants who terminated early from START by perceived main barrier to retention (n = 67)
| Characteristics | Barriers to Retention in Treatment with Buprenorphine/naloxone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Design of the Clinical Trial (n=29) |
Effects of the Buprenorphine/naloxone (n=29) |
Personal Circumstances (n=9) |
||||||||
| Missed Too Many Consecutiv e Days (n=12) |
Switched to Methadon e Treatment 1 (n=12) |
Wanted Methadone 2 (n=3) |
Withdraw n - Other Reasons (n=2) |
Medicatio n Didn't "Work" (n=9) |
Medicatio n "Worked Too Well"3 (n=9) |
Negative Induction Experienc e (n=7) |
Adverse Reactions (n=4) |
Transportatio n Issues (n=6) |
Other Issues (n=3) |
|
| Age, average (range) | 40 (27–60) | 34 (22–59) | 51 (35–54) | 33 (26–39) | 35 (19–47) | 36 (22–54) | 50 (37–57) | 37 (25–51) | 45 (35–56) | 39 (29–45) |
| Male, No. (%) | 9 (75) | 7 (58) | 2 (67) | 1 (50) | 4 (44) | 8 (89) | 6 (86) | 1 (25) | 4 (67) | 2 (67) |
| Ever injected opioids, No. (%) | 12 (100) | 9 (75) | 3 (100) | 1 (50) | 5 (56) | 7 (78) | 7 (100) | 3 (75) | 3 (50) | 3 (100) |
| Average longest period using opioids, years (range) | 11 (5–35) | 8 (1–23) | 5 (4–5)4 | 7 (3–10) | 7 (4–21) | 8 (2–26) | 17 (2–36) | 13 (6–36) | 14 (3–42) | 8 (4–11) |
| Prior buprenorphine experience5, No. (%) | 6 (50) | 8 (75) | 1 (33) | 1 (50) | 4 (44) | 7 (78) | 1 (14) | 1 (25) | 3 (50) | 2 (67) |
| Prior methadone experience5, No. (%) | 12 (100) | 10 (83) | 3 (100) | 2 (100) | 8 (89) | 9 (100) | 7 (100) | 3 (75) | 5 (83) | 3 (100) |
| Medication preference at enrollment, No. (%) | ||||||||||
| Buprenorphine/naloxone | 8 (67) | 6 (50) | 0 | 2 (100) | 4 (44) | 3 (33) | 2 (29) | 3 (75) | 3 (50) | 2 (67) |
| Methadone | 3 (25) | 1 (8) | 3 (100) | 0 | 5 (56) | 6 (67) | 5 (71) | 0 | 2 (33) | 0 |
| None | 1 (8) | 5 (42) | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (17) | 1 (33) |
| Days in START, average (range)6 | 44 (3–130) | 29 (3–57) | 4 (1–9) | 59 (36–82) | 49 (16–117) | 44 (9–138) | 2 (1–4) | 30 (4–56) | 67 (18–99) | 88 (21–144) |
| Maximum dose in START, average, mg (range) | 21 (8–32) | 27 (10–32) | 4 (0–12) | 28 (24–32) | 24 (16–32) | 18 (4–32) | 15 (8–32) | 24 (16–32) | 24 (12–32) | 24 (16–32) |
| Race/ethnicity, No. (%) | ||||||||||
| White (non-Hispanic) | 7 (58) | 7 (58) | 1 (33) | 2 (100) | 6 (67) | 5 (56) | 3 (57) | 3 (75) | 3 (50) | 1 (33) |
| Hispanic | 2 (17) | 2 (17) | 1 (33) | 0 | 1 (11) | 3 (33) | 1 (14) | 0 | 2 (33) | 2 (67) |
Participants took advantage of the opportunity to switch to methadone treatment outside START which resulted in their termination from START.
Participants had a strong preference for methadone, but were assigned to receive buprenorphine/naloxone.
Participants reported that they were not able to feel the effects of opioids they desired (e.g., euphoria, sedation) while on buprenorphine/naloxone, thus as one of them put it, the medication "worked too well."
Average based on 2 of 3 respondents.
Prior experience includes detoxification and maintenance treatment, and/or illicit use of buprenorphine and/or methadone.
Days in START was calculated by subtracting the date of last dose from the randomization date, then adding one (randomization day) to the difference.
Among the participants who left START early due to the perceived negative effects of the buprenorphine/naloxone, the majority who ascribed to the theme “medication didn’t ‘work’” had prior experience with methadone, whereas less than half had prior experience with buprenorphine; the participants were almost evenly divided in terms of the medication they preferred; the average length of stay in START was slightly less than two months, and the average maximum dose was relatively high (24 mg, with a range of 16–32 mg).
Participants in the group that described that the “medication ‘worked too well’” were generally in their mid thirties; all had injected opioids and had prior methadone experience; the majoritypreferred methadone at enrollment, their average length of stay was slightly over one month, and their average maximum dose (18 mg, with a range of 4–32 mg) was in the allowable mid-range.
In comparison, the participants in the group that expressed that they had a “negative induction experience” were typically middle-aged; all had injected opioids and had prior experience with methadone, but only a few had prior experience with buprenorphine. The majority of participants reported having had a preference for methadone at enrollment. This group’s longest period of opioid use averaged about two decades; participants in this group typically left START within a few days of randomization, and the average maximum dose (15 mg, with a range of 8–32 mg) was near the middle of the allowable range.
Buprenorphine/naloxone patient perspectives on facilitators of retention in START
When participants were asked what kept them in START (at least 24 weeks), three major themes that emerged (see Figure 1).
The majority (71%) of the participants with buprenorphine/naloxone who completed START (n=38) described having a preference for buprenorphine/naloxone at enrollment, only about a third of them (37%) acknowledged that they had previous experience with buprenorphine, and among these participants, almost a third (29%) spoke favorably about the experience. In contrast, the majority (89%) of participants had prior experience with methadone, and among this group, 42% described their experiences using mixed (both positive and negative) or neutral language, 29% commented unfavorably about the experiences, and 18% remarked favorably about their experiences. The average maximum dose for this group was 24 mg, with a range of 8–32 mg.
Medication “worked” – felt “normal”
Twelve participants communicated satisfaction with buprenorphine/naloxone in terms of how they responded, overall, to the medication and how well they were able to function and conduct their lives. A word used repeatedly was “normal,” with some comparing their physical and/or mental state while on the medication to their pre-opioid-using periods. For example:
“Once it was steady [dose]…I didn't feel any high at all. I mean, I just felt normal. I felt like how I remember myself when I was 13 or 14 before I used opiates. You know, just - I didn't think about 'em and I didn't notice I was on anything…I felt normal, neutral.”
Some individuals expressed that they responded to the buprenorphine/naloxone as they expected; others seemed surprised with the results, with one of them saying:
“It made me actually feel better. I wasn’t sick. You know, my stomach wasn’t bothering me. My muscles weren’t aching like they were going through detox…I was like, ‘Well, it actually does work.’ For a new drug, it works; it’s doing what it’s supposed to do.”
Some of the participants who spoke favorably about buprenorphine/naloxone compared their experiences with prior methadone treatment (e.g., less sedation, longer suppression of withdrawal symptoms). For example, one participant recalled, “You take the methadone, you know, you can feel it hit you…You kinda go up and down. But with the Suboxone, it was a nice steady…on a very even keel.”
However, although some participants seemed pleased with their general reaction to the buprenorphine/naloxone compared to methadone, others pointed out that there were also some downsides to the medication. One individual described the experience saying, “There’s a big difference with Suboxone…Methadone cuts off the…rough edges of life. Suboxone doesn’t. The rough edges are still there. It helps you physically, but not emotionally.”
Many of the participants described feeling more “comfortable” with the medication, once dosage adjustments were made. For example, one person shared:
“[It] took a couple of days, almost a week, before we got the right doses for my body, my weight…And it did what it’s supposed to have done…to keep me from not being sick, and I wasn’t nodding and all that, when I adjusted to it about two to three weeks.”
Besides relieving withdrawal symptoms and cravings for opioids, some participants described additional benefits they received from taking the buprenorphine/naloxone, including relief from pain and ability to focus.
Although participants who completed START with buprenorphine/naloxone portrayed overall positive experiences with the medication, some indicated that they also struggled with negative effects of the medication (e.g., headaches, cravings, muscle cramps, slightly sedated), and/or did not receive immediate relief from withdrawal symptoms. One participant explained:
“At first there was like some physical reactions that I never had before…Like joints locking up…Then it went away…I think my body was adjusting…to the withdrawing and it was finding out what my physical status was also, too, from not being under the influence of heroin all the, most of the time.”
Personal determination and commitment
Twenty-two participants attributed their successful completion of START to their conscious decision, willpower, and commitment to finish something they started and/or to stop using opioids. For a few, being told that they could switch to methadone at the clinic if they completed START and the participant compensation were additional incentives. Others recognized that they needed to continue taking the medication to stop using opioids.
“I didn’t wanna go back to drugs, so I was doing what I had to do to stay clean. That’s what kept me coming back ‘cause I know that was something that I had to meet halfway…So there’s no rain or sleet or fog that would stop me ‘cause I was coming ‘cause I knew it was benefitting me and my health.”
Some participants interviewed considered buprenorphine/naloxone treatment in START as a means to achieve their personal goals, such as not using opioids of any kind (“free of it”), being involved in the life of a granddaughter, and acquiring a new sense of achievement.
Support and encouragement from staff
In describing what kept them in START, four participants mentioned research and other clinic staff (e.g., counselors) who “worked with” them (e.g., scheduling visits, calling them on the phone when they missed appointments). Participants used words such as “nice,” “caring,” “respectful” in describing particular staff members and how important the support and encouragement provided was to their success in START. One participant said, “They [staff] showed me that there’s a light at the end of that tunnel. There’s hope. You hear that? There’s hope!”
Participant characteristics and main facilitators of retention
Table 3 displays characteristics of the participants according to the three thematic categories considered by participants as facilitators of retention (as indicated in Figure 1). Characteristics in common across all three categories included an average age of mid-forties, having a history of injecting opioids and prior methadone experience. The participants who discussed “methadone worked – felt normal” and “personal determination and commitment” as important facilitators of retention were more likely to be male, whereas participants who were female were more likely to report “support and encouragement from staff” as important. Finally, those reporting personal determination and commitment had used opioids for more years on average than those in the other two categories.
Table 3.
Characteristics of buprenorphine/naloxone participants who completed START by perceived facilitator of retention (n = 38)
| Characteristics | Facilitators of Retention in Treatment with Buprenorphine/naloxone | ||
|---|---|---|---|
| Medication “Worked” – Felt “Normal” (n=12) |
Personal Determination and Commitment (n=22) |
Support and Encouragement from Staff (n=4) |
|
| Age, average (range) | 45(23–59) | 46(24–65) | 44 (29–54) |
| Male, No. (%) | 10(83) | 14(64) | 1(25) |
| Ever injected opioids, No. (%) | 8(67) | 14(64) | 3(75) |
| Average longest period using opioids, years (range) | 10(1–24) | 16(2–49) | 10 (4–20) |
| Prior buprenorphine experience1, No. (%) | 5(42) | 6(27) | 3(75) |
| Prior methadone experience1, No. (%) | 11(92) | 19(86) | 4(10)0 |
| Medication preference at enrollment, No. (%) | |||
| Buprenorphine/naloxone | 8(67) | 16(73) | 3(75) |
| Methadone | 4(33) | 6(27) | 1(25) |
| None | 0(0) | 0(0) | 0(0) |
| Maximum dose in START, average, mg (range) | 23(12–32) | 23(8–32) | 28 (24–32) |
| Race/ethnicity, No. (%) | |||
| White (non-Hispanic) | 9(75) | 13(59) | 4(100) |
| Hispanic | 1(8) | 4(18) | 0(0) |
Prior experience includes detoxification and maintenance treatment, and/or illicit use of buprenorphine and/or methadone.
DISCUSSION
This study explored barriers and facilitators of buprenorphine/naloxone retention from the perspectives of participants in a clinical trial comparing the impact of methadone vs. buprenorphine/naloxone on liver function. Although the 46% retention rate at 24 weeks for the entire sample of participants randomly assigned to buprenorphine/naloxone was similar to the retention rates reported in other trials with buprenorphine (Mitchell et al. 2012; Soyka et al. 2008; Kristensen et al. 2005; Johnson & McCagh 2000; Schottenfeld et al. 1997; Ling et al. 1996), it remained lower than that of the participants randomized to methadone.
Study Context
Participants indicated that aspects of the design of the clinical trial contributed to their premature discontinuation on the study medication. In the context of this particular trial, some reasons for early termination from START related to the research protocol were unavoidable. For example, participant preference for a particular medication could not be accommodated within the study protocol. However, in clinical settings, patient preferences can be considered in conjunction with provider recommendations. Further, providing information on treatment options and engaging patients in making decisions about their own treatment is consistent with the patient-centered approach to care (Bechtel & Ness 2010; Institute of Medicine 2001) and is supported by our finding that the majority of participants who completed START preferred buprenorphine/naloxone at enrollment. The START protocol also required participants to take the medication daily, thus missing 14 or more consecutive days of medication necessitated study discontinuation, whereas clients in treatment programs may be allowed to return.
Notably, the primary goal of START was to compare methadone and buprenorphine/naloxone on liver function. It is important to point out the discrepancy between the protocol for buprenorphine/naloxone treatment in START (e.g., daily dosing, weekly assessments, observed dosing, randomized assignment to medication) and the more typical experience of office-based buprenorphine/naloxone treatment, for which the program requirements are minimal (see CSAT 2004). Thus, retention may be improved if some of the protocol-related issues are eliminated, particularly barriers associated with personal circumstances (e.g., daily dosing requirement).
Implications
Two reasons for study discontinuation (hospitalization and travel) are potentially avoidable during community treatment, if medical staff is contacted to continue treatment in the hospital and if patients are made aware of their options to be temporarily medicated in another OTP near their travel site. Special take-home doses can be approved for travel by the Center for Substance Abuse Treatment (CSAT) at the request of the treating physician, or arrangements can be made for the patient to receive buprenorphine through a physician at another site. Federal regulations were recently revised to permit OTP patients receiving buprenorphine to obtain take home doses outside the OTP regulation constraints for time in treatment that remain in effect for methadone patients (United States Department of Health and Human Services 2012), which address some of thebarriers reported by participants and may improve retention.
Financial factors such as availability of a subsidized treatment slot also contributed to attrition in the START buprenorphine/naloxone group. This has been reported in other trials with opioid agonists (Booth, Corsi & Mikulich-Gilbertson 2004) and in community treatment (Reisinger et al. 2009). Federal legislation that provides parity for insurance coverage for substance abuse treatment with primary care, and the Affordable Care Act, will make opioid treatment more accessible in states that opt to expand Medicaid access.
That many participants lacked accurate information about, and experience with, buprenorphine/naloxone treatment prior to START may have contributed to participant preference for methadone, especially early in the trial. Patients presenting for induction in START, many of whom had prior experience with methadone, may have been hoping to be assigned to methadone and were ready to begin treatment with this medication, but were poorly prepared for buprenorphine/naloxone, or expected that induction on to buprenorphine/naloxone would be similar to methadone. As some of the participants may not have been at an acceptable level of opioid withdrawal when presenting for induction, the first dose of buprenorphine/naloxone may have precipitated withdrawal symptoms. Due to the pharmacologic properties of buprenorphine (partial agonist), compared to methadone (full agonist), opioids already taken may be displaced from the mu receptors, thus precipitating withdrawal (Jones 2004). Better outcomes may be achieved if patient induction on to buprenorphine/naloxone occurs only after having clear evidence of the signs and symptoms of opioid withdrawal (e.g., minimum score of 5 on the Clinical Opiate Withdrawal Scale [COWS]). As expectations about how medications might or might not “work” may affect patient experience and outcomes (Schwartz et al. 2008), the importance of education about medications for both patients and staff cannot be overemphasized, especially during the initial phases of treatment (Demetrovics et al. 2009).
We found that participants left START early because of negative effects of the medication or because they continued to experience craving for opioids. Some of the negative effects may have been avoided through appropriate dosing adjustment to alleviate withdrawal symptoms, craving, or negative reactions attributed to the medication, which might improve retention (Fareed et al. 2012). Although some participants left START because the medication “did not work,” others dropped out because the buprenorphine/naloxone “worked too well.” These participants were ambivalent about treatment and decided that they wanted the option to continue to use opioids to get high. As opioid dependence in this population is a chronic problem, efforts at reengaging patients who drop out (Coviello et al. 2006) should be employed in the community. Further research could help determine factors that contribute to patients not feeling ready to quit opioid use.
Finally, our findings indicate that support and encouragement, particularly from caring and attentive staff, and the experience of feeling “normal” while on the medication can help retain some patients. Perhaps because many participants who were not familiar with buprenorphine/naloxone could not turn to peers who experienced success with the medication, they needed more attention and care from staff. The finding that buprenorphine/naloxone made some participants feel “normal” has been reported in other studies (Barry et al. 2009). Research is needed to understand why buprenorphine/naloxone “works” for some, but not for others (e.g., biology, motivation, psychiatric symptoms).
Several limitations should be considered. First, findings are drawn from a convenience sample of participants provided with buprenorphine/naloxone who terminated early from and completed START. Their perspectives may not represent those of participants in START with buprenorphine/naloxone who did not participate in the interviews. However, ours is a large sample for a qualitative study, with 105 participants interviewed in person across eight sites located in different parts of the country. Second, the interviews were conducted up to three-and-a-half years after participation ended in START; therefore, some participants may have had trouble recalling their thoughts and experiences during the study. Events following their participation in START may have altered participants’ perceptions of their experiences while they were in START in significant ways. Third, since the interviews were conducted at clinics where participants were enrolled in START and some were currently receiving treatment there, they may not have felt free to answer questions candidly. However, we assured each participant that the transcripts of the voice recordings would only be shared amongst the Retention Study research team, and that information that might identify individuals would be kept confidential. Finally, the community-based experiences of the buprenorphine/naloxone participants in this trial may not represent the experiences of patients receiving treatment in the more traditional office-based settings. Nevertheless, our sample of 67 participants who did not complete START and the 38 participants who did complete provides a wide range of perspectives of and experiences with buprenorphine/naloxone treatment.
CONCLUSIONS
Findings contribute to the sparse literature on patient perspectives of barriers to retention among individuals receiving treatment with buprenorphine/naloxone for opioid dependence. The themes identified underscore the complexity and nuances of providing a relatively new medication for treating opioid dependent patients within community-based settings. They also highlight the importance of considering each patient’s experience with and knowledge about buprenorphine/naloxone and methadone, medication preference, personal circumstances, and motivation to abstain from non-prescribed use of opioids, as they may influence retention in with buprenorphine/naloxone treatment. Our findings suggest a patient-centered approach to identify the treatment option that best meets the patients’ needs at the time. Ongoing education of patients and staff about buprenorphine/naloxone, especially in comparison to methadone, and support (from staff and peers) is essential. Finally, local, state and federal regulations and policies may need to be revisited to increase patient options to be treated with all FDA-approved medications for opioid dependence in as many OTPs and physician office-based practices as possible.
Acknowledgments
FUNDING
Funding for this study was provided by the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) (U10 DA13045). Dr. Hser is currently supported by K05 DA017648. We wish to acknowledge the START sites (Bay Area Addiction Research & Treatment [BAART], Bi-Valley Medical Clinic, Inc., Connecticut Counseling Centers, Inc., CODA, Inc., Evergreen Treatment Services, Hartford Dispensary, Matrix Institute on Addictions, NET Steps) for their support and participation in the Retention Study.
Dr. Schwartz is co-investigator on a NIDA-funded study that receives buprenorphine at no charge to participants from Reckitt Benckiser. Dr. Hser has received a small educational grant from Reckitt Benckiser to support the CALDAR Summer Institute.
REFERENCES
- Al-Tayyib AA, Koester S. Injection drug users' experience with and attitudes toward methadone clinics in Denver, CO. Journal of Substance Abuse Treatment. 2011;41(1):30–36. doi: 10.1016/j.jsat.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates' experiences with buprenorphine or methadone maintenance. Journal of Psychoactive Drugs. 2010;42(3):339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment: Patients, Programs, Services, and Outcome. New York: Springer-Verlag; 1991. [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O'Connor PG, Schottenfeld RS, Fiellin DA. Integrating buprenorphine treatment into office-based practice: A qualitative study. Journal of General Internal Medicine. 2009;24(2):218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazazi AR, Yokell M, Fu JJ, Rich JD, Zaller ND. Illicit use of buprenorphine/naloxone among injecting and noninjecting opioid users. Journal of Addiction Medicine. 2011;5(3):175–180. doi: 10.1097/ADM.0b013e3182034e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel C, Ness DL. If you build it, will they come? Designing truly patient-centered health care. Health Affairs. 2010;29(5):914–920. doi: 10.1377/hlthaff.2010.0305. [DOI] [PubMed] [Google Scholar]
- Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104(7):1193–1200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison - A high risk of death for former inmates. New England Journal of Medicine. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Corsi KF, Mikulich-Gilbertson SK. Factors associated with methadone maintenance treatment retention among street-recruited injection drug users. Drug and Alcohol Dependence. 2004;74(2):177–185. doi: 10.1016/j.drugalcdep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Boyatzis RE. Thematic Analysis and Code Development: Transforming Qualitative Information. Thousand Oaks: Sage; 1998. [Google Scholar]
- Caplehorn JR, Dalton MS, Haldar F, Petrenas AM, Nisbet JG. Methadone maintenance and addicts' risk of fatal heroin overdose. Substanc Use and Misuse. 1996;31(2):177–196. doi: 10.3109/10826089609045806. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. Health Technology Assessment. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Coviello DM, Zanis DA, Wesnoski SA, Alterman AI. The effectiveness of outreach case management in re-enrolling discharged methadone patients. Drug and Alcohol Dependence. 2006;85(1):56–65. doi: 10.1016/j.drugalcdep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches (2nd Ed.) Thousand Oaks: Sage; 2003. [Google Scholar]
- Deck D, Carlson MJ. Retention in publicly funded methadone maintenance treatment in two Western States. Journal of Behavioral Health Services and Research. 2005;32(1):43–60. doi: 10.1007/BF02287327. [DOI] [PubMed] [Google Scholar]
- Demetrovics Z, Farkas J, Csorba J, Nemeth A, Mervo B, Szemelyacz J, Fleischmann E, Kassai-Farkas A, Petke Z, Orojan T, Rozsa S, Rigo P, Funk S, Kapitany M, Kollar A, Racz J. Early experience with Suboxone maintenance therapy in Hungary. Neuropsychopharmacologia Hungarica. 2009;11(4):249–257. [PubMed] [Google Scholar]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment Prevention Policy. 2008;3:17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JE, Netherland J, Gass J, Finkelstein R, Weiss L, Collaborative B. Patient perspectives on buprenorphine/naloxone treatment in the context of HIV care. Journal of Acquired Immune Deficiency Syndromes. 2011;56(Suppl 1):S46–S53. doi: 10.1097/QAI.0b013e3182097561. [DOI] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Casarella J, Drexler K. Treatment outcome for flexible dosing buprenorphine maintenance treatment. American Journal of Drug and Alcohol Abuse. 2012;38(2):155–160. doi: 10.3109/00952990.2011.643988. [DOI] [PubMed] [Google Scholar]
- Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stuhlinger G, Pezawas L, Aschauer HN, Kasper S. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94(9):1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clinical Microbiology Reviews. 2003;16(2):209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D, Group BNCS. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Glaser BG, Strauss AL. The Discovery of Grounded Theory. New York: Aldine de Gruyter; 1967. [Google Scholar]
- Gossop M, Marsden J, Stewart D, Kidd T. Reduction or cessation of injecting risk behaviours? Treatment outcomes at 1-year follow-up. Addictive Behaviors. 2003;28(4):785–793. doi: 10.1016/s0306-4603(01)00279-9. [DOI] [PubMed] [Google Scholar]
- Hser Y, Joshi V, Maglione M, Chou C, Anglin D. Effects of program and patient characteristics on retention of drug treatment patients. Evaluation and Program Planning. 2001;24:331–341. [Google Scholar]
- Hser YI. Predicting long-term stable recovery from heroin addiction: Findings from a 33-year follow-up study. Journal of Addictive Diseases. 2007;26(1):51–60. doi: 10.1300/J069v26n01_07. [DOI] [PubMed] [Google Scholar]
- Hubbard RL, Craddock SG, Flynn PM, Anderson J, Etheridge RM. Overview of 1-year follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychology of Addictive Behaviors. 1997;11(4):261–278. [Google Scholar]
- Hubbard RL, Craddock SG, Anderson J. Overview of 5-year follow-up outcomes in the Drug Abuse Treatment Outcome Studies (DATOS) Journal of Substance Abuse Treatment. 2003;25(3):125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Huberman AM, Miles MB. Data management and analysis methods. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks: Sage; 1994. [Google Scholar]
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Broome KM. Effects of readiness for drug abuse tratment on client retention and assessment of process. Addiction. 1998;93(8):1177–1190. doi: 10.1080/09652149835008. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New England Journal of Medicine. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson RE, McCagh JC. Buprenorphine and naloxone for heroin dependence. Current Psychiatry Reports. 2000;2(6):519–526. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Jones HE. Practical considerations for the clinical use of buprenorphine. Science & Practice Perspectives. 2004;2(2):4–20. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamien JB, Branstetter SA, Amass L. Buprenorphine-naloxone versus methadone maintenance therapy: A randomised double-blind trial with opioid-dependent patients. Heroin Addiction and Related Clinical Problems. 2008;10(4):5–8. [Google Scholar]
- Kelly SM, O'Grady KE, Mitchell SG, Brown BS, Schwartz RP. Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug and Alcohol Dependence. 2011;117(2–3):170–175. doi: 10.1016/j.drugalcdep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber J, Copeland L, Hickman M, Macleod J, McKenzie J, De Angelis D, Robertson JR. Survival and cessation in injecting drug users: Prospective observational study of outcomes and effect of opiate substitution treatment. British Medical Journal. 2010;341:c3172. doi: 10.1136/bmj.c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester S, Anderson K, Hoffer L. Active heroin injectors' perceptions and use of methadone maintenance treatment: Cynical performance or self-prescribed risk reduction? Substance Use and Misuse. 1999;34(14):2135–2153. doi: 10.3109/10826089909039442. [DOI] [PubMed] [Google Scholar]
- Kosten TR. Buprenorphine for benzodiazepine-abusing heroin addicts. American Journal of Psychiatry. 1994;151(1):151. doi: 10.1176/ajp.151.1.151a. [DOI] [PubMed] [Google Scholar]
- Kristensen O, Espegren O, Asland R, Jakobsen E, Lie O, Seiler S. Buprenorphine and methadone to opiate addicts - A randomized trial. Tidsskrift for den Norske Laegeforening. 2005;125(2):148–151. [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA, Jr, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction. 1998;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Archives of General Psychiatry. 1996;53(5):401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Ali R, White JM, O'Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: A randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98(4):441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: An 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes. 1993;6(9):1049–1056. [PubMed] [Google Scholar]
- Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, Jaffe JH. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.08.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Kelly SM, Brown BS, Reisinger HS, Peterson JA, Ruhf A, Agar MH, Schwartz RP. Incarceration and opioid withdrawal: The experiences of methadone patients and out-of-treatment heroin users. Journal of Psychoactive Drugs. 2009;41(2):145–152. doi: 10.1080/02791072.2009.10399907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MQ. Qualitative Research and Evaluation Methods. Newbury Park: Sage; 1990. [Google Scholar]
- Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. American Journal of Drug and Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O'Grady KE, Marrari EA, Brown BS, Agar MH. Premature discharge from methadone treatment: Patient perspectives. Journal of Psychoactive Drugs. 2009;41(3):285–296. doi: 10.1080/02791072.2009.10400539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades HM, Creson D, Elk R, Schmitz J, Grabowski J. Retention, HIV risk, and illicit drug use during treatment: Methadone dose and visit frequency. American Journal of Public Health. 1998;88(1):34–39. doi: 10.2105/ajph.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald PJ, Robertson JR, Elton RA. Continued drug use and other cofactors for progression to AIDS among injecting drug users. AIDS. 1994;8(3):339–343. doi: 10.1097/00002030-199403000-00007. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, Doraimani G, Tasissa G, Lokhnygina Y, Leimberger J, Bruce RD, McCarthy J, Wiest K, McLaughlin P, Bilangi R, Cohen A, Woody G, Jacobs P. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug and Alcohol Dependence. 2013;128(1–2):71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91(8):1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Archives of General Psychiatry. 1997;54(8):713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O'Grady KE, Mitchell SG, Peterson JA, Reisinger HS, Agar MH, Brown BS. Attitudes toward buprenorphine and methadone among opioid-dependent individuals. American Journal on Addictions. 2008;17(5):396–401. doi: 10.1080/10550490802268835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD. Treatment for drug abuse. Follow-up outcomes and length of time spent. Archives of General Psychiatry. 1981;38(8):875–880. doi: 10.1001/archpsyc.1981.01780330033003. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA, Greener JM. Drug abuse treatment process components that improve retention. Journal of Substance Abuse Treatment. 1997;14(6):565–572. doi: 10.1016/s0740-5472(97)00181-5. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Haggerty KP, Fleming CB, Catalano RF, Gainey RR. Opiate-addicted parents in methadone treatment: Long-term recovery, health, and family relationships. Journal of Addictive Diseases. 2011;30(1):17–26. doi: 10.1080/10550887.2010.531670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. International Journal of Neuropsychopharmacology. 2008;11(5):641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- Specter S. Drugs of abuse and infectious diseases. Journal of the Florida Medical Association. 1994;81(7):485–487. [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: Self-reports, urinalysis, and addiction severity index. Journal of Clinical Psychopharmacology. 1996;16(1):58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Comparison of buprenorphine and methadone in the treatment of opioid dependence. American Journal of Psychiatry. 1994;151(7):1025–1030. doi: 10.1176/ajp.151.7.1025. [DOI] [PubMed] [Google Scholar]
- Teesson M, Mills K, Ross J, Darke S, Williamson A, Havard A. The impact of treatment on 3 years' outcome for heroin dependence: Findings from the Australian Treatment Outcome Study (ATOS) Addiction. 2008;103(1):80–88. doi: 10.1111/j.1360-0443.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Opioid Drugs in Maintenance and Detoxification Treatment of Opiate Addiction; Proposed Modification of Dispensing Restrictions for Buprenorphine and Buprenorphine Combination as Used in Approved Opioid Treatment Medications. Substance Abuse and Mental Health Services Administration. Washington: Government Printing Office; 2012. [Google Scholar]
- Wallen MC, Lorman WJ, Gosciniak JL. Combined buprenorphine and chlonidine for short-term opiate detoxification: Patient perspectives. Journal of Addictive Diseases. 2006;25(1):23–31. doi: 10.1300/J069v25n01_05. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lintzeris N, Lea T. "Should I stay or should I go?" Coming off methadone and buprenorphine treatment. International Journal of Drug Policy. 2011;22(1):77–81. doi: 10.1016/j.drugpo.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Xiao L, Wu Z, Luo W, Wei X. Quality of life of outpatients in methadone maintenance treatment clinics. Journal of Acquired Immune Deficiency Syndromes. 2010;53(Suppl 1):S116–S120. doi: 10.1097/QAI.0b013e3181c7dfb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Friedmann PD, Gerstein DR. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98:673–684. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]