Abstract
Evidence supports the efficacy of hypnotic treatments, but there remain many unresolved questions regarding how hypnosis produces its beneficial effects. Most theoretical models focus more or less on biological, psychological, and social factors. This scoping review summarizes the empirical findings regarding the associations between specific factors in each of these domains and response to hypnosis. The findings indicate that: (1) no single factor appears primary; (2) different factors may contribute more or less to outcomes in different subsets of individuals or for different conditions; and (3) comprehensive models of hypnosis that incorporate factors from all 3 domains may ultimately prove to be more useful than more restrictive models that focus on 1 or a very few factors.
A growing body of research supports the efficacy of hypnosis and hypnotic treatments for benefiting individuals with a variety of conditions including acute pain (Landolt & Milling, 2011; Madden, Middleton, Cyna, Matthewson, & Jones, 2012; G. H. Montgomery, DuHamel, & Redd, 2000; Patterson & Jensen, 2003; Tomé-Pires & Miró, 2012), chronic pain (Elkins, Jensen, & Patterson, 2007; Jensen & Patterson, 2006; Jensen, 2009; G. H. Montgomery et al., 2000; Patterson & Jensen, 2003; Tomé-Pires & Miró, 2012), irritable bowel syndrome (Rutten, Reitsma, Vlieger, & Benninga, 2013; Tan, Hammond, & Joseph, 2005), depression (Alladin, 2012), and anxiety (Golden, 2012; Hammond, 2010). Hypnosis has also been shown to enhance the efficacy and benefits of other therapeutic approaches, such as cognitive-behavioral therapy (Kirsch, Montgomery, & Sapirstein, 1995). Moreover, unlike many biological interventions such as analgesic medications for pain that can have significant financial costs (due to the need for ongoing use), negative side effects, and social costs (associated with problems with diversion), hypnotic interventions are relatively easy and inexpensive to provide, have a plethora of beneficial “side effects” (such as increased sense of control over pain and its impact, increased sense of well-being), and have very few negative side effects (Jensen et al., 2006). Given their efficacy, low cost, and positive side effect profile, the data suggest that training in self-hypnosis should be considered a “first-line” treatment for many chronic health conditions.
However, many questions remain regarding the mechanisms that explain the effects of hypnosis. Although a number of models have been proposed to explain these effects (Bányai, 1991; Barnier, Dienes, & Mitchell, 2008; Gruzelier, 1998; Kirsch, 1991; Lynn, Kirsch, & Hallquist, 2008; Nash, 2008; Rainville & Price, 2004; D. Spiegel, 2008; H. Spiegel, 2007; Wagstaff, David, Kirsch, & Lynn, 2010; Woody & Sadler, 2008), no single theory or model has been endorsed by the majority of hypnosis researchers and clinicians. Moreover, each existing model focuses on only some aspects of hypnosis at the exclusion of others (Hammond, 2005). For example, dissociation models, which date back to Pierre Janet (1901), focus on the degree of association and dissociation between different mental processes (cognitive monitoring, cognitive control, consciousness, sensory experience) (Woody & Sadler, 2008). Social cognitive theories focus on the hypnotic context and the subject’s abilities, attitudes, beliefs, expectancies, attributions, and motivations (Lynn et al., 2008). An ability-aptitude model hypothesizes a causal role for two key factors in influencing hypnotic responding (and each other): (1) a latent cognitive ability/talent for hypnotic responding and (2) the subject’s beliefs about their own future hypnotic responding (Benham, Woody, Wilson, & Nash, 2006). The social-psychobiological model stresses the importance of the relationship between the hypnotist and the subject, emphasizing how these interact with the physiological mechanisms as well as the personal characteristics of the hypnotist and the subject and physiological mechanisms (Bányai, 1991). However, even this relatively comprehensive model does not take into account a number of factors hypothesized to influence hypnotic responding by other theories.
Importantly, the available evidence can be interpreted to be consistent with each one of the existing theories and models of hypnosis; that is, each remains viable. This suggests that each model retains potential utility for understanding the components of hypnosis that they focus on, and supports continued work with and development of these models. On the other hand, the fact that none of the existing models takes into account all of the factors that have been shown to be associated with hypnotic responding suggests that each existing model also has important limitations. The field lacks an overarching model or framework for organizing the many factors that may contribute to hypnotic responding.
We propose in this review that a model hypothesizing roles for biological, psychological, and social factors, and their interactions – that is, a biopsychosocial model of hypnosis—might serve this organizing role. Biopsychosocial models have proven to be useful in understanding other complex issues, such as chronic pain (Novy, Nelson, Francis, & Turk, 1995), depression (Schotte, Van Den Bossche, De Doncker, Claes, & Cosyns, 2006), and substance abuse (Buckner, Heimberg, Ecker, & Vinci, 2013). Importantly, biopsychosocial models allow for the possibility that many factors can play a role and contribute to hypnotic responding. Such models therefore have the potential for having more explanatory power than more restrictive models (Novy et al., 1995).
The purpose of this scoping review is to explore the potential utility of a biopsychosocial framework for understanding hypnosis and hypnotic responding by performing a scoping review of what is now known regarding the associations between biological, psychological, and social factors that have been hypothesized to explain hypnosis and hypnotic analgesia by the primary more restrictive perspectives (Barnier et al., 2008; Gruzelier, 1998; Lynn et al., 2008; Nash, 2008; Rainville & Price, 2004; H. Spiegel, 2008; Woody & Sadler, 2008). Although the idea of using a biopsychosocial approach to understand hypnotic responding is not new (Bányai, 1991; Hammond, 2005), no review to date has sought to evaluate and summarize the empirical evidence with respect to the relative importance of factors within each of the biological, psychological, and social domains in the same paper.
A scoping review is a technique to “map” an area of relevant literature that provides comprehensive coverage but extracts a limited amount of information from published studies (Arksey & O’Malley, 2005). A scoping review can be contrasted to a systematic review, which seeks to answer a very specific question (e.g., “What is the average effects size of hypnotic analgesia?” (G. H. Montgomery et al., 2000) based on a small set of the highest quality studies available. A scoping review, on the other hand, seeks to answer more broad questions using selected studies from a large body of research. A scoping review is particularly useful when the body of research to be reviewed is so large that a systematic review is not practical; it can therefore identify the most important focused questions that can then be addressed with systematic reviews.
The primary goals of this review were to (1) identify the key biological, psychological, and social factors that have been hypothesized as having a potential role in explaining response to hypnotic suggestions and then (2) evaluate the relative importance of each factor for predicting response to hypnotic suggestions based on the available evidence. In particular, we were interested in determining if a single factor or a small subset of factors consistently emerged as strongly related to hypnotic responding, thereby supporting the development of more restrictive models that focus on just this factor or these factors, or if multiple factors emerged that tend to evidence weaker or more moderate associations to hypnotic responding, thereby supporting the development of more complex models (e.g., a “biopsychological” model if only biological and psychological factors were identified as important, or an even more comprehensive “biopsychosocial” model if factors in all three domains emerged as important).
METHOD
A number of articles and chapters have been published that have comprehensively reviewed the associations between biological (mostly, neurophysiological) factors and response to hypnosis and hypnotic suggestions (Barabasz & Barabasz, 2008; Crawford, 1990, 1994; Crawford & Gruzelier, 1992; De Pascalis, 1999; Gruzelier, 1998; Kihlstrom, 2013; Oakley, 2008; Oakley & Halligan, 2010; Ray, 1997). These reviews served as the primary sources for conclusions regarding current state of the science knowledge regarding the importance of biological factors, as well as sources to identify published articles supporting these conclusions. However, findings from a selected number of empirical studies as well as studies that have been published since these reviews were also included in our review if they illustrated key conclusions or provided additional important information not included in the existing reviews. To help identify these additional articles, we performed a search of the PubMed, CINAHL, and PsycINFO databases, using the search terms hypnosis combined with each of the following: EEG (for electroencephalogram), fMRI (for functional magnetic resonance imagery), and PET (for positron emission tomography).
As a starting point to identify articles reporting findings regarding the relative importance of psychological and social factors that could contribute to our understanding of the mechanisms of hypnosis, we read and extracted information from extant reviews and theoretical papers by prominent theoreticians in this area and that tended to focus on these factors (Bányai, 1991; Bányai, 1998; Barnier et al., 2008; Coe & Sarbin, 1977, 1993; Diamond, 1987; Evans, 2000; Hammond, 2005; Killeen & Nash, 2003; Kirsch, 1991; Kirsch & Lynn, 1995, 1997, 1999; Lynn et al., 2008; Lynn & Rhue, 1991; Spanos, 1991; Spanos & Chaves, 1989; Spanos & Coe, 1992; Wagstaff et al., 2010; Welch, 1947). Based on these reviews and the articles they cited, we identified five psychological domains and two social domains that were most commonly considered or studied.
The psychological factors identified included hypnotizability, expectations, motivation, absorption/imaginative involvement/fantasy proneness, and attitudes toward hypnosis, and the social factors identified were rapport (also referred to as “resonance” and “harmony”). Although historically some theorists may have associated the concept of “hypnotizability” with the concept of “hypnotic state” (i.e., with biological models of hypnosis), the concept of hypnotizability has also been explained from the perspective of psychosocial models (Wagstaff et al., 2010). Moreover, because hypnotizability is generally viewed as a stable trait and is assessed using behavioral scales (i.e., a person’s score is based on their behavioral response in a social context) and given that the biological section will focus on the correlates of hypnosis with objective physiological responses (e.g., brain activity as measured by fMRI, PET and EEG), the concept of hypnotizability seemed to us to fit better into the psychological domain than in the biological domain. However, we acknowledge that hypnotizability could reasonably be classified as either a psychological or biological factor. To ensure that we identified the key articles that might describe the associations between psychological and social factors and response to hypnosis, we performed searches of the PubMed, CINAHL, and PsycINFO databases that reported findings regarding these factors, using the search term hypnosis combined with each of the following: expectancies, attributions, motivation, absorption, imaginative involvement, fantasy proneness, attitudes, rapport, resonance, and social. We limited the search to studies published in English.
All of the relevant articles identified were read by pairs of authors for each of the three domains. MPJ and TA read all of the biological factors review articles and articles resulting from the search on the topic of biological factors, JM and CTP read all of the psychosocial theoretical and review articles and chapters, and articles from the search on the topic of psychological factors; JL and ZJO read all of the psychosocial theoretical and review articles and chapters, and articles that were identified from the search on the topic of social factors. These pairs of authors identified those articles and chapters that provided answers to the primary review question, and extracted the key findings from these articles for the current review.
RESULTS
Biological Factors Associated with Hypnotic Responding
In this section, we first introduce the reader to the primary brain imaging techniques used to study the neurophysiolgical correlates of hypnosis and hypnotic responding. This is followed by a discussion of the findings of the review, organized by the key conclusions that can be drawn from the existing body of research.
Measures of neurophysiolgical structure and responding
The primary strategies used to assess neurophysiological structure and responding in hypnosis research assess (1) activity in specific areas of the brain (initially using mostly positron emission tomography [PET] and then using mostly functional magnetic resonance imaging [fMRI], but occasionally using magnetoencephalography [MEG]), (2) brain structure (usually using structural magnetic resonance imaging), and (3) brain states (usually using electroencephalography).
PET, fMRI, and MEG can all produce three-dimensional images of brain function. Both PET and fMRI measure blood flow in specific areas, which is thought to reflect activity in the central nervous system. With PET, a radioactive tracer is injected into (or inhaled by) the subject, and by measuring the locations associated with more versus less radioactive decay, the PET scanner can identify areas of more or less blood flow. fMRI assesses local magnetic fields. Because hemoglobin (which carries oxygen in the blood) has different magnetic properties when it is oxygen rich than when it is oxygen poor, the fMRI scanner is able to identify specific brain areas that are using more or less oxygen at any one time. MEG records the magnetic fields produced by the electrical activity of neurons in the brain, and therefore is more closely associated with actual neuronal activity (as opposed to the proxy measure of brain activity – blood flow and blood oxygen use – assessed by PET and fMRI) than PET or fMRI. However, MEG has less spatial resolution than either PET or fMRI. Structural and diffusion magnetic resonance imaging (MRI) are used to assess the density of regional grey matter (neural cell bodies) and white matter (neural axons), as well as the anatomical connectivity between brain areas, respectively.
EEG uses electrodes placed directly on the scalp to record electrical activity in cortical neurons. Activity can be measured from just one scalp site using one electrode or up to 256 different scalp sites using a “net” of electrodes. The more sites used, the more able an investigator is to localize the source of electrical activity. However, because EEG assesses activity only from the very top layers of the cortex (i.e., not deeper structures), and assesses that activity through the skull, skin, and dura matter, the spatial resolution of the EEG is very low, especially when compared to PET or fMRI. On the other hand, temporal resolution with EEG is very high, which makes EEG a powerful tool for measuring brain activity changes over time. Importantly, the EEG signal can be filtered into specific frequency ranges, each of which has behavioral correlates reflecting brain states. A preponderance of very slow delta waves (up to 4 cycles per second or 4 Hz) is associated with sleep. Theta waves (between 4–8 Hz) are associated with drowsiness as well as both focused attention and memory functions. Alpha waves (between 8–12 Hz) tend to occur in posterior regions and are associated with feelings of relaxation, as well as a lack of sensory input (e.g., eye closure). Beta waves (13–35 Hz) tend to occur more frontally and are associated with feeling alert and active, or even anxious. Gamma waves (38 + Hz) occur during memory tracing and recall and are also associated with cross-modal sensory processing (binding different sensory experiences processed in different parts of the brain into a coherent whole; for example, the sights, smells, and touch senses of an object or experience).
However, a great deal has yet to be learned about the mechanisms of different brain oscillations. Moreover, the standard boundaries used to differentiate between the different oscillation bandwidths are based mostly on convention, and can therefore be considered as somewhat arbitrary. There is also between-person variability in peak levels of each bandwidth. Thus, it is possible that “low alpha” (9 Hz activity) in one individual might operate like and contribute to the same processes as theta oscillations (4–8 Hz) do in another individual. Similarly, “high beta” (33 Hz) might serve the same function as gamma usually does in a specific sample or individual. The distinctions between theta and alpha, or between beta and gamma, may not therefore be strong or clear, and can contribute to inconsistencies in the research findings. Finally, it is also important to note that the traditional bandwidths are quite broad, and evidence suggests that each one contains more than one narrower bandwidth which may have different functions (Michels et al., 2010; Vogel, Broverman, & Klaiber, 1968; Williams & Gruzelier, 2001); traditionally operationalized theta, alpha, beta, and gamma oscillations can all serve different purposes.
Given this background information regarding the most common biological variables studied in hypnosis research, we now discuss and summarize the key conclusions that can be drawn from the available research on biological correlates of hypnotic responding, citing the reviews and specific articles that support each one.
People who score high on hypnotizability scales demonstrate different patterns of neurophysiological responses to hypnotic inductions and suggestions than people who score low on hypnotizability scales
Perhaps the most common experimental design used to study the neurophysiological correlates of hypnosis and hypnotic responding involves selecting study participants who score either very high (highs) or very low (lows) on standardized tests of hypnotizability. Some investigators screen for hypnotizability two or even three times, to ensure that they have true (or extreme) highs and true lows. Less often, individuals who score in the mid-range (mediums) are included in research studies, and compared to both highs and lows. The procedures then often involve comparing the groups on measures of brain activity and assume that any between-group differences found reflect responses and processes important to hypnotic responding.
One of the most consistent findings from this body of research is that the brains of highs consistently react differently to hypnosis and hypnotic suggestions than the brains of lows on a number of key neurophysiology measures, including (1) specific areas of the brain that respond to hypnosis in general and specific hypnotic suggestions in particular and (2) shifts in brain states following hypnosis as measured by EEG activity (Barabasz & Barabasz, 2008; Crawford, 1994; De Pascalis, 1999; Gruzelier, 1998). Details regarding some of the most consistent findings with respect to brain areas and processes that evidence differences between highs and lows are summarized in the sections that follow. But the general conclusion from this body of research is clear: people who are more likely to respond to hypnosis and hypnotic suggestions consistently show different patterns of brain responses than those who do not respond to hypnotic analgesia suggestions.
Virtually all of the brain areas involved in the processing of pain have been shown to be impacted by hypnosis and hypnotic analgesia
Our understanding of how the body and brain work together to create the experience of pain has expanded significantly in the last 50 years, and especially in the last 2 decades as brain imaging technology has advanced to allow for real-time assessment of brain activity (e.g., PET and fMRI scans) and brain states (e.g., EEG assessments) while people experience more or less pain. Instead of a simple Cartesian notion of the brain as a passive recipient of information from sensory nerves in the periphery regarding physical damage, we now understand pain as the end product of a dynamic series of multiple neurophysiological mechanisms that modulate information about physical damage at many sites and levels, including the periphery, the spinal cord, and supraspinal sites such as the thalamus (where the majority of sensory information from the periphery first enters the brain), the sensory cortices (where information about the location, magnitude, and sensory qualities of pain is processed), the insula (where information regarding the need to “do something” about pain is processed), the anterior cingulate cortex (where information regarding the affective/emotional aspects of pain are processed), and the prefrontal cortex (where information about the meaning of pain to the individual is processed)(Apkarian, 2013; Apkarian, Bushnell, Treede, & Zubieta, 2005; Craig, 2003; Jensen, 2008). There is no such thing as a “pain center” in the brain.
With respect to the effects of hypnosis and hypnotic analgesia on the brain areas and processes that underlie the experience of pain, it has become increasingly clear that hypnosis and hypnotic analgesia can influence all of these sites and processes (De Benedittis, 2003; Jensen, 2008). Hypnotic analgesia suggestions have been shown to reduce the inflammatory processes associated with heat injury and increased sensitivity to pain in the periphery (Chapman, Goodell, & Wolff, 1959). Effective hypnotic analgesia also influences spinal reflexes—a reflex that cannot be influenced by conscious efforts (Danziger et al., 1998; Kiernan, Dane, Phillips, & Price, 1995). When effective, hypnotic analgesia suggestions have been shown to reduce activity in the thalamus, sensory cortices, insula, anterior cingulate cortex, and prefrontal cortex (Hofbauer, Rainville, Duncan, & Bushnell, 2001; Rainville, Duncan, Price, Carrier, & Bushnell, 1997; Wik, Fischer, Bragee, Finer, & Fredrikson, 1999). Thus, just as our initial models of pain as a simple sensory response to stimulation or damage in the periphery have evolved into a much more complex understanding of the many processes involved in the creation of pain, our neurophysiological models of hypnotic analgesia mechanisms have evolved to encompass the many ways that hypnosis can influence pain (and comfort)(Jensen, 2008). Just like there is no such thing as a single “pain center” in the brain, there is no such thing as a single “physiological mechanism” of hypnotic analgesia. Rather, because hypnosis can influence pain in a large variety of ways, it makes more sense to speak of physiological mechanisms of hypnotic analgesia.
Findings regarding hypnosis and EEG-assessed measures of brain states show (1) a fairly consistent pattern of more theta activity among highs, relative to lows; (2) both increases and decreases in most bandwidths with hypnosis except for theta, which tends to show increases with hypnosis in both highs and lows, (3) stronger effects of hypnosis and hypnotic suggestions on brain activity among highs than lows
Researchers have been examining the associations between hypnotizability and EEG bandwidth activity, as well as the effects of hypnotic inductions on EEG, for close to half a century. In the initial studies in the 1970s in this area, researchers hypothesized that hypnosis would be associated with more alpha activity, given the interest in this bandwidth as being associated with relaxation and a variety different meditative states (Kihlstrom, 2013; Oakley & Halligan, 2010). However, although hypnotic inductions sometimes result in increases in alpha activity (De Pascalis & Palumbo, 1986; Graffin, Ray, & Lundy, 1995; Macleod-Morgan, 1979; Morgan, Macdonald, & Hilgard, 1974), effects on alpha activity are not consistent (Crawford, 1990; Kihlstrom, 2013; Ray, 1997; Sabourin, Cutcomb, Crawford, & Pribram, 1990).
The most consistent finding with respect to differences between highs and lows in baseline EEG activity, noted by a number of reviewers (Barabasz & Barabasz, 2008; Crawford, 1994; Crawford & Gruzelier, 1992; Kihlstrom, 2013; Ray, 1997), is that individuals who score higher on hypnotizability tests evidence higher baseline levels of theta activity than individuals who score lower on hypnotizability tests (Freeman, Barabasz, Barabasz, & Warner, 2000; Galbraith, London, Leibovitz, Cooper, & Hart, 1970; Kirenskaya, Novototsky-Vlasov, & Zvonikov, 2011; D. D. Montgomery, Dwyer, & Kelly, 2000; Sabourin et al., 1990; Tebecis, Provins, Farnbach, & Pentony, 1975). In addition, there is a tendency for individuals—especially highs—to respond to hypnotic inductions with increases in theta activity (Sabourin et al., 1990; D. White, Ciorciari, Carbis, & Liley, 2009; Williams & Gruzelier, 2001). However, this effect has not been observed in either highs or lows in response to hypnotic analgesia suggestions (De Pascalis & Perrone, 1996). In fact, in one hypnotic analgesia study, reductions in low theta (4 – 5.75 Hz) were observed in the left hemisphere of highs (De Pascalis & Perrone, 1996).
De Pascalis (2007) recently reviewed the literature with respect to gamma activity and hypnosis. He described an overall inconsistency in the research findings on these effects. Some research has found higher baseline gamma activity in highs relative to lows (Akpinar, Ulett, & Itil, 1971; Schnyer & Allen, 1995), and also more gamma activity in response to hypnosis (De Pascalis, 1993). However, other studies have found lower levels of gamma power in highs, relative to lows (De Pascalis, Marucci, Penna, & Pessa, 1987) and decreases in gamma with hypnotic analgesia suggestions among highs (De Pascalis, Cacace, & Massicolle, 2004). In a follow-up study using a “obstructive imagery treatment” (instructions to relax and focus attention on the right hand and imaging a glove that attenuates all sensations; i.e., not formal hypnosis but something much like hypnosis) a decrease in gamma was specific to higher gamma frequencies (38–42 Hz and 42–46 Hz), and to central areas (Cz in the International 10/20 system (De Pascalis & Cacace, 2005); see also, Croft, Williams, Haenschel, & Gruzelier, 2002). Yet other studies report that highs evidence both (1) a greater increase in gamma density during recollection of positive emotional events than lows and (2) a greater reduction in left hemisphere gamma activity and increase right hemisphere activity in gamma, relative to lows during hypnosis (De Pascalis, Marucci, & Penna, 1989; De Pascalis et al., 1987). More recent research has found levels of gamma to be associated with hypnotic depth (Cardeña, Jonsson, Terhune, & Marcusson-Clavertz, 2013). Another study found an association between gamma activity and pain intensity (in response to painful stimulation) evident in both highs and lows which disappeared with hypnosis in highs only (Croft et al., 2002).
Two tentative conclusions can be made from this body of research regarding gamma activity. First, it appears that gamma activity often changes following hypnotic inductions and hypnotic suggestions. Second, there is a tendency for higher levels of gamma to be associated with more hypnotizability and response to hypnotic suggestions. But this tendency is not entirely consistent across samples or hypnotic suggestions. It appears that whether gamma increases or decreases with hypnosis may depend more on the content of the suggestions than whether more or less gamma is linked to hypnotic responding (De Pascalis, 2007).
The findings with respect to beta bandwidths also do not yet provide a clear picture regarding their involvement, one way or the other, in hypnotic responding. For example, a number of studies find no impact of hypnosis on beta activity (De Pascalis & Cacace, 2005). When an effect has been found, highs evidence decreases in low beta (13–15.75 Hz) with hypnotic analgesia suggestions (De Pascalis & Perrone, 1996), and increases in beta (13–16 Hz, 16 – 20 Hz, and 20–36 Hz) in the left hemisphere during age regression suggestions (De Pascalis, 1993).
In sum, when changes in EEG-measured brain activity following hypnosis (including hypnotic analgesia) are compared between highs and lows, differences are almost always found (Graffin et al., 1995; Ray, 1997; Williams & Gruzelier, 2001). These differences tend to be observed more often in the theta and gamma bandwidths, with higher levels of theta tending to be associated with more hypnotizability and hypnotic responding, and greater differences and changes (both directions) in gamma associated with more hypnotizability and hypnotic responding. But changes in activity in other bandwidths are also sometimes observed, with a tendency for there to be (1) differences in the direction of changes between highs and lows (i.e., sometimes when highs show increases, lows show decreases) and/or (2) greater changes in highs, relative to lows (i.e., if both show increases in a bandwidth, highs tend to show greater increases than lows). These general conclusions are also consistent with findings showing differences between highs and lows in both the patterns of associations between EEG-assessed bandwidth activity and subjects’ phenomenological experience of hypnosis (Cardeña et al., 2013), and in the brain areas (source locations) associated with theta and beta activity (Isotani et al., 2001).
Although a number of specific brain areas can be impacted by hypnosis, frontal (in particular dorsolateral prefrontal cortex) and cingulate cortical areas appear to be impacted most often
One of the clear conclusions from a number of reviews of research studies examining neurophysiological responses to hypnosis is that a number of different brain areas, many of which subsume the cognitive process of attention and consciousness, are impacted by hypnosis (Crawford, 1994; De Benedittis, 2003; Jensen, 2011; Rainville & Price, 2004). However, two brain areas appear to be most consistently linked to hypnotic responding in general and hypnotic analgesia in particular: the frontal and cingulate cortices.
Frontal cortices
The frontal cortices are an integral part of the executive function network, and are involved in planning, goal setting, selective attention, and modulation (mostly via inhibition) of other brain functions, including sensory functions and those that underlie motor behavior. Moreover, a number of neurophysiological models of hypnosis argue for a central role for frontal functions in hypnotic responding. For example, Gruzelier (1998, 2006) describes a three-phase model of hypnosis in which specific brain areas are hypothesized to become involved and become either more or less active, depending on the phase of the hypnotic procedure. In the first phase, when the subject is invited to focus their attention on an object during the induction, frontolimbic structures are hypothesized to become engaged and active. In the second phase, and in response to suggestions for tiredness (from fixation during the first phase) and relaxation, an inhibition in and/or dissociation within the frontolimbic structures is hypothesized, during which time the subject is more open to respond to suggestions. In the third phase, to the extent that the hypnotic procedures then involve suggestions for imagery, right-sided temporoposterior regions are hypothesized to be activated. Note that this model does not hypothesize that activity in these structures changes in this stepwise pattern in response to all hypnotic procedures, but only those that involve a fixation induction followed by suggestions for relaxation (“letting go”) and then for imagery. Research findings support this model, in particular findings indicating a reduction in frontal cortical activity and dissociation of frontal structures from other brain areas in response to hypnosis, as summarized by Gruzelier (Gruzelier, 1998, 2006). Additional evidence supporting a role for a reduction in frontal activity in hypnotic responding comes from two recent studies showing an increase in response to hypnotic suggestions following: (1) disruption of activity in the dorsolateral prefrontal cortex with repetitive transcranial magnetic stimulation (Dienes & Hutton, 2013) and (2) alcohol intake, which is known to impair frontal executive functioning (Semmens-Wheeler, Dienes, & Duka, 2013).
However, although inhibition of the executive functioning system (i.e., frontal areas) might be expected to be associated with response to many (if not most) hypnotic suggestions, an increase in frontal activity with hypnosis might be expected when or if such activity is necessary or useful when responding to a suggestion—for example, to inhibit sensory experiences. Consistent with this idea, evidence also indicates an increase in frontal activity (in highs, but not necessarily lows) with hypnotic suggestions for both motor imagery (Muller, Bacht, Schramm, & Seitz, 2012) and analgesia (Crawford, Gur, Skolnick, Gur, & Benson, 1993). As a group, these findings support the conclusion than frontal cortical areas are involved in hypnosis and the response to hypnotic suggestions. However, the extent to which a reduction or interruption of versus an increase in frontal activity (or, perhaps, in the specific neuronal assemblies within frontal areas) facilitates response to hypnotic suggestions appers to depend on the specific hypnotic procedures used or suggestions made.
Midcingulate and anterior cingulate cortices
The midcingulate and anterior cingulate cortices (MCC and ACC) are areas of the (frontal) limbic system involved in many different functions, including reward anticipation, error detection, attention, motivation, and emotion. In fact, the cingulate cortices are involved in so many aspects of human experience and behavior that it would be difficult to imagine these areas not being involved in many if not most responses to hypnotic suggestions, which by definition involve changes in experience, behavior, or both.
However, whether response to hypnotic suggestions is linked to increases or decreases in cingulate activity depends on the suggestions being made. Increases in ACC activity as measured by PET scales have been shown to be associated with ratings of more hypnotic absorption in highs (Rainville, Hofbauer, Bushnell, Duncan, & Price, 2002). Increases in ACC have also been shown to be associated with hypnotic suggestions to vividly imagine a pleasurable autobiographical memory; a suggestion that also results in decreases in pain intensity and unpleasantness (Faymonville et al., 2000). In a separate study, such suggestions were found to be associated with increases in activity in the MCC (Faymonville, Boly, & Laureys, 2006). On the other hand, hypnotic suggestions to decrease pain unpleasantness (Rainville, Carrier, Hofbauer, Bushnell, & Duncan, 1999) and pain intensity directly (Derbyshire, Whalley, & Oakley, 2009; Derbyshire, Whalley, Stenger, & Oakley, 2004) have both been shown to be associated with decreases in ACC activity. Thus, it appears that the MCC and ACC are involved in hypnotic responding, but they are engaged to the extent that changes in activity – either more or less activity – are required in the response.
Hypnosis is associated with hemispheric asymmetry
Some researchers have suggested that hypnotic responding may be associated more with right hemisphere than left hemisphere processing, because hypnotic responding appears to be associated more with right than left hemisphere cognitive activities and processes, such as creativity, intuition, and nonverbal/nonanalytic thinking (e.g., metaphors, stories, and imagery). Indeed, some preliminary support for this idea came from studies demonstrating greater dominance in the right hemisphere with hypnosis in general (Edmonston & Moscovitz, 1990; MacLeod-Morgan & Lack, 1982) and hypnotic analgesia in particular (Chen, Dworkin, & Bloomquist, 1981). However, research indicates no difference in hypnotizability between individuals with left versus right hemisphere lesions (Kihlstrom, Glisky, McGovern, Rapcsak, & Mennemeier, 2013). In addition, even though as cited above, greater right hemisphere activity has sometimes been shown to be associated with hypnotizability, greater left hemisphere activity has also been found to be associated with more hypnotizability in some studies (Sabourin et al., 1990), and findings from other studies show no differences in right versus left hemisphere activity (Graffin et al., 1995; Morgan et al., 1974).
Thus, hypnosis and hypnotic responding is not consistently associated with greater right hemisphere activity. On the other hand, highs have been shown to evidence greater differences in hemisphere activity than lows, regardless of the direction of those differences (i.e., sometimes right > left, sometimes left > right) (Crawford, 1990; De Pascalis & Perrone, 1996). Thus, the findings are more consistent with a model of hypnosis hypothesizing greater overall physiological flexibility among highs (i.e., shifting from left to right, anterior to posterior, as needed to respond to the suggestion), rather than a general tendency to simply shift from left to right hemisphere processing with hypnosis (Crawford, 1989).
Hypnosis and hypnotic responding is associated with both increases and decreases in functional connectivity between brain areas
Hypnosis also influences measures of connectivity between brain regions (Faymonville et al., 2006; Gruzelier, 1998; Oakley, 2008). However, the direction of effects can be variable and, like many of the other physiological activity measures discussed so far, differ as a function of both general hypnotizability and the contents of the hypnotic suggestions. One study, for example, reported a decrease in general connectivity between brain areas during a hypnotic induction (Cardeña et al., 2013), and another reported a decrease in connectivity between frontal midline areas and left lateral scalp sites in highs (but not lows) after hypnosis (Egner, Jamieson, & Gruzelier, 2005). Similarly, Gruzelier and colleagues found that a hypnotic induction resulted in decreases in connectivity between prefrontal regions and other areas in highs, but an increase in connectivity between these sites in lows (Gruzelier, 1998). One case study found mostly decreases in connectivity (and mostly between frontal and other areas) with hypnosis in a single highly hypnotizable subject with hypnosis, although there was also one consistent and strong increase in connectivity (between the left temporal and right occipital areas) with hypnosis (Fingelkurts, Kallio, & Revonsuo, 2007). Other research has shown increases in connectivity between precuneus and other regions with hypnotic paralysis (Cojan et al., 2009; Pyka et al., 2011). In sum, the findings in studies of hypnosis not involving analgesia suggest mostly decreases in connectivity (and mostly with highs) with hypnosis, but some increases in connectivity, depending on the sites examined as well as the level of hypnotizability.
With respect to hypnotic analgesia specifically, increases in connectivity have been found in response to hypnotic suggestions to re-experience a pleasurable autobiographical memory, which also resulted in analgesia (Faymonville et al., 2000; Faymonville et al., 2003; Vanhaudenhuyse et al., 2009). However, the extent to which these increases are or are not associated with other hypnotic suggestions that can result in analgesia (e.g., direct suggestions to experience a decrease in pain intensity, or suggestions to experience dissociation from parts of the body that are sometimes painful) is not known.
Hypnotizability is associated with higher levels of structural connectivity
In contrast to the findings that hypnotic inductions and suggestions can both decrease and increase functional connectivity between areas of the brain, depending on both hypnotizability and the specific suggestions made, two studies suggest the possibility of greater structural connectivity between brain areas as a functioning of hypnotizability. For example, using diffusion MRI, Hoeft and colleagues showed stronger connectivity between the prefrontal cortex and ACC in highs, relative to lows during resting state (Hoeft et al., 2012). Similarly, Horton and colleagues found that highs (who were also able to eliminate pain perception with hypnotic suggestions) had significant and substantially larger (32%) rostrum (the anterior portion of the corpus callosum, involved in transfer of information between the frontal cortices) than lows (Horton, Crawford, Harrington, & Downs, 2004). Thus, while there is relatively little research regarding structural differences between highs and lows, these two findings suggest the intriguing possibility that not only do the brains of highs and lows differ in how they respond to hypnosis, but may actually show differences in how they are hardwired, with highs evidencing greater overall structural connectivity than lows.
Summary of the findings regarding biological correlates of hypnosis
Although the research findings regarding the associations between hypnotizability, hypnotic responding, and brain activity measures are complicated, a picture is emerging and some general conclusions appear possible. First, there is not (yet) a clear brain “signature” of hypnosis. Brain activity measures change in response to hypnotic inductions and suggestions, but for the most part, the areas and type (more versus less) of activity seems to be more closely related to and consistent with the suggestions made, rather than hypnosis per se. Suggestions for “letting go” may be associated with decreased frontal activity, while suggestions for analgesia appear to be associated with increases in frontal activity (as executive systems are engaged to suppress the experience of pain). Moreover, the neurophysiological responses to hypnosis and hypnotic suggestions are consistently shown to differ between individuals with high versus low hypnotizability. The biological measures that seem to be most closely associated with hypnosis and hypnotic responding include (1) higher levels of theta activity in highs and in response to hypnotic inductions and (2) perhaps higher levels of structural connectivity between left and right hemisphere frontal areas and between frontal areas and the ACC. It is possible that differences in these biological domains in particular—that is, theta activity and structural connectivity—may facilitate hypnotic responding. This possibility should be examined further.
Psychological factors associated with hypnotic responding
As described previously, based on our reading of the articles and chapters presenting theories of hypnosis that focus on or include psychological factors, we identified five psychological factors as being the ones most consistently mentioned as important: hypnotizability, expectancies, motivation, absorptive capacity/fantasy proneness, and attitudes towards hypnosis. In the sections that follow, each of these factors is discussed under a heading that provides a summary of the key results from our review of the evidence regarding that factor.
The strength of the associations found between measures of general hypnotizability and response to specific hypnotic suggestions is variable
Most of the research studying the associations between general hypnotizability1 and response to specific suggestions has focused on pain. As described by a number of scientists who have reviewed the literature in this area, research shows that an individual’s ability to experience and respond to hypnosis in general, is usually—but not always—associated positively with response to specific hypnotic analgesia suggestions (Accardi & Milling, 2009; Jensen & Patterson, 2006; G. H. Montgomery et al., 2000; Patterson & Jensen, 2003; Tomé-Pires & Miró, 2012). However, there is a great deal of variability in the strength of the associations found. The strongest associations have been reported for the treatment of acute and procedure-related pain in children ranging from .50 to .81 (Liossi & Hatira, 1999, 2003; Liossi, White, & Hatira, 2006). Weaker, and in some studies nonsignificant, associations are found in chronic pain studies in adults (Jensen, Barber, Romano, Hanley et al., 2009; Jensen, Barber, Romano, Molton et al., 2009; Jensen et al., 2005). When reported, the strength of the associations in studies of laboratory pain in healthy subjects tend to range from moderately weak (e.g., r = .25) to large (e.g., r = .55 or Cohen’s d = > 1.00 between highs and lows) (Appel & Bleiberg, 2005; De Pascalis, 1999; Hilgard & Hilgard, 1994; Milling, Kirsch, Meunier, & Levine, 2002).
The high degree of variability in the associations found, as well as the differences in the strength of the associations as a function of study population (laboratory pain, acute procedural pain, chronic pain) strongly suggest the existence of moderating factors that influence the extent to which hypnotizability is associated with outcome. A number of moderating factors have been hypothesized by investigators in this area. For example, Hilgard (Hilgard & Hilgard, 1994) and Hammond (2005) have both noted that response to relatively “easy” suggestions (e.g., for ideomotor phenomenon and at least some analgesic response) does not require a great deal of hypnotic ability. For such suggestions and responses, general hypnotizability may play a relatively minor role in outcome. Other hypnotic suggestions and responses (e.g., for hallucinations, profound amnesia, or the complete elimination of pain during noxious stimulation) may require much more dissociative capacity, and therefore greater hypnotic ability may be required. Thus, the specific type and wording of suggestions may influence the role that hypnotizability plays on outcome. Other moderating factors in addition to type of suggestions surely exist. However, to date very few researchers have examined hypnotizability as a potential moderator of hypnotic responding (see Milling & Breen, 2003, and Milling, Reardon, & Carosella, 2006, for two rare exceptions). Additional research focusing on this question would have important theoretical and clinical implications.
The strength of the associations found between measures of expectancies and response to specific hypnotic suggestions is variable
Outcome expectancies represent the extent to which the subject believes that he or she will experience a specific response following a hypnotic intervention (e.g., that hypnosis will lead to analgesia, or that the dominant hand will raise seemingly of its own accord following an induction). Expectancies are thought to reflect automatic processes influenced by past experience, the current context, and the interaction. Expectancies are important, if not central to, a number of hypnosis theories (Kirsch, 1991; Kirsch & Lynn, 1995, 1997, 1999; Lynn et al., 2008; Lynn & Rhue, 1991; Spanos & Chaves, 1989; Wagstaff et al., 2010).
Evidence from a number of studies is consistent with the hypothesis that expectancies play a role in hypnotic responding, especially in laboratory settings (Benham et al., 2006; Braffman & Kirsch, 1999; Kirsch, Silva, Comey, & Reed, 1999). However, research also suggests the importance of expectancies may be less in response to hypnotic treatment for chronic pain (Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009; Jensen et al., 2005). One study examining the relative importance of outcome expectancies as predictors of the benefits of hypnosis for a variety of symptoms found that the strength of the associations varied as a function of the symptom being considered, with outcome expectancies playing a smaller role for predicting improvements in nausea (r = .20) and “discomfort” (r = .23) than for predicting improvements in pain intensity (r = .52) and fatigue (r = .62) (G. H. Montgomery & Bovbjerg, 2004). Another study found larger effects of expectancy on response to some more difficult hypnotic suggestions (e.g., audio hallucination, amnesia) than some easier suggestions (e.g., ideomotor suggestions) and to be larger among highs than lows (Kirsch et al., 1999).
One methodologically strong approach for evaluating the importance of any potential mechanism factor in hypnotic responding is to use mediational analyses (Baron & Kenny, 1986; Sobel, 2008). In such analyses, if a factor is primary (i.e., explains most if not all of an outcome), when the factor is controlled, then the significant benefits of a treatment become nonsignificant. If a factor plays a limited but not primary role (i.e., explains a portion of an outcome), the significant effects of a treatment are reduced when that variable is controlled, but some portion of the treatment effects remain. In this instance, the factor is said to be a “partial” mediating variable.
A number of studies have examined the extent to which outcome expectancies are full mediators versus partial mediators versus non mediators of the effects of hypnosis. One study found evidence for full mediation for expectancies in response to hypnotic analgesia for laboratory pain (Baker & Kirsch, 1993). Most other studies have found evidence for partial mediation in laboratory pain (Milling & Breen, 2003; Milling et al., 2002; Milling, Levine, & Meunier, 2003), medical procedure (breast biopsy) pain, (G. H. Montgomery, Weltz, Seltz, & Bovbjerg, 2002), and postsurgical pain and fatigue, (G. H. Montgomery et al., 2010). One study found no mediation effects of expectancy on the effects of hypnosis for reducing postsurgical nausea (G. H. Montgomery et al., 2010). In short, the findings regarding mediation are consistent with the research examining expectancies as a predictor of outcome; that is, the mediation effects of expectancies are supported more often than not, but the strength of those effects vary to some degree across populations and symptoms studied. As is the case with hypnotizability, this variability suggests the likely presence of moderating factors that influence the role that expectancies play in response to hypnotic treatments.
Trait absorptive and imaginative ability are consistently associated (moderately) with response to hypnosis
As noted by Kirsch and Council (1992), since the 1960s research on personality correlates of hypnotic responsiveness has been focused primarily on absorption, imaginative involvement, and fantasy proneness, which are constructs so closely related to one another that it is unclear that they represent different domains. A fair number of studies have examined the associations between measures of these domains and response to hypnosis and hypnotic suggestions, and the findings are fairly consistent. Specially, that individuals scoring higher on these measures tend to evidence more hypnotic response (Glisky, Tataryn, Tobias, Kihlstrom, & McConkey, 1991; Poulsen & Mathews, 2003; Silva & Kirsch, 1992). When coefficients between measures of imaginative ability or absorption and response to hypnosis or hypnotic suggestions are reported, they tend to be weak to moderate (e.g., rs range = .17 to .44; (Glisky et al., 1991; Poulsen & Mathews, 2003; Silva & Kirsch, 1992). The strength and range of these associations indicates both (1) a role for absorptive ability and (2) the possibility that other factors may moderate the strength of the associations found.
Motivation has rarely been tested as a predictor of response to hypnosis, but when examined motivation shows positive associations with hypnotic responding
A number of theorists have hypothesized that successful outcome and response to hypnotic treatments depend at least in part on heightened subject motivation (Barber, 1969; Hammond, 2005; Lynn et al., 2008; Patterson, Adcock, & Bombardier, 1997; Spanos, 1986; R. W. White, 1941). However, this factor has been rarely tested as a predictor of response to hypnosis. In fact, our search only yielded one study that examined this factor directly. In this study, Braffman and Kirsch assessed motivation using a 5-point Likert scale assessing “the degree to which [the participant] wanted to experience” hypnotic suggestions, and found weak to moderate positive correlation coefficients between these ratings and response to both behavioral and phenomenological responses to hypnotic suggestions ranging from .13 (NS) to .41 (p < .001) (Braffman & Kirsch, 1999).
Attitudes towards hypnosis have rarely been tested as predictors of response to hypnosis
Attitudes about hypnosis have been proposed as important predictors of hypnotic responding by a number of theorists for at least 40 years (Barber, 1969; Lynn & Kirsch, 2006; Spanos & Chaves, 1989). However, despite the fact that two adequately reliable measures of attitudes towards hypnosis exist (the Attitudes Towards Hypnosis scale or ATH, (Milling, 2012; Spanos, Brett, Menary, & Cross, 1987); the Valencia Scale of Attitudes and Beliefs Toward Hypnosis scale, or VSABTH (Capafons, Cabanas, Espejo, & Cardeñåa, 2004; Capafons, Espejo, & Mendoza, 2008), very little research has been performed that has examined its importance to hypnotic responding (Milling, 2012). We were only able to find two studies that reported associations between these scales and measures of hypnotic responding. In the first of these, the total ATH score, assessing (1) positive beliefs about hypnosis, (2) an absence of fear concerning hypnosis, and (3) beliefs about the mental stability of hypnotizable people evidenced weak to moderate (rs = .19 to .31) associations with subscales of the Carlton University Responsiveness to Suggestion Scale (Spanos et al., 1987). The investigators also noted that the associations were curvilinear; that is, individuals with negative attitudes tended to have low hypnotizability, while those with positive attitudes tended to show a greater range in hypnotizability. The associations between the subjects’ scores of the VSABTH scales and hypnotizability were even more variable, ranging from r = −.10 to .29 (Green, 2012). The strongest associations (both rs = .29) were between the VSABTH Help and Interest scales (assessing beliefs about hypnosis’s potential helpfulness and the respondent’s interest in being hypnotized, respectively) and hypnotizability. In short, while not all attitudes about hypnosis appear important, some may be as important as other factors (e.g., expectancies, motivation) found to be associated with hypnotic responding.
Summary of the findings regarding psychological correlates of hypnosis
The psychological factors most often hypothesized as playing a role in response to hypnosis and hypnotic suggestions include hypnotizability, expectancies, absorptive/imaginative ability, motivation, and attitudes towards hypnosis. While the associations between measures of these factors and response to hypnosis and various hypnotic suggestions have not been extensively studied, the findings that are available provide a fairly consistent picture. First, when tested, the findings show weak to moderate associations between each of these factors and hypnotic responding. Second, although very few studies have examined the mediating role of these factors using more sophisticated mediational analyses—and of those that have been performed, only expectancies have been tested as possible mediators—evidence supports a partial mediational role for expectancies. Third, the variability in associations between these factors and response to hypnosis suggests the existence of factors that likely influence (moderate) their role. Preliminary findings support the following as moderators for expectancy effects: (1) the specific symptom being examined (e.g., expectancies may be more important for hypnotic analgesia than hypnotic relief from nausea); (2) type of suggestion (e.g., expectancies may play a larger role for responding to more difficult suggestions than easier suggestions); and, (3) with respect to hypnotic analgesia, type of pain (e.g., expectancies may play a larger role for laboratory and acute pain than for chronic pain). The possible role of moderating factors that might influence the importance of other psychological factors and response to hypnosis has not yet been adequately examined.
Social Factors Associated with Hypnotic Responding
While sociocognitive models of hypnosis argue that hypnotic responding has both cognitive and social components, Radtke and Stam (2008) have argued that these models tend to focus more on cognitive factors (e.g., the factors discussed in the previous section) than the social aspects of hypnosis. This may be one of the reasons that only two social factors have been examined with respect to their association to responses to hypnosis: rapport (also referred to as “therapeutic alliance,” “resonance,” and “harmony,” among other labels) and social context.
Rapport and therapeutic alliance
The collaborative and affective bond between a clinician and client/patient is often considered to be an essential component in any therapeutic relationships including hypnosis. In support of this idea, a meta-analysis based on 68 (nonhypnosis) clinical studies that examined the contribution of therapeutic alliance suggested that positive alliance was weakly but consistently associated with better therapeutic outcomes (weighted estimate r = .22; Martin, Garske, & Davis, 2000). Research in hypnosis also supports a role for this variable in outcomes. For example, Sheehan (1980) manipulated the interaction approach of the hypnotist (which presumably would influence rapport) to determine if rapport might influence the subjects' receptiveness towards the hypnotist's suggestions, even when the suggestions contradicted the subject’s previous conception of how they should respond (also known as countering). The results indicate that subjects who are exposed to negative rapport conditions (where the therapist depersonalized himself or herself from the session or critically evaluated the subjects) exhibited less countering response, although the facilitation of positive rapport (through interaction that is characterized as spontaneous, positive, warm, and involved) did not result in significantly higher rate of countering response. In other words, poor rapport (or at least, clinician behaviors that could contribute to poor rapport) may inhibit subjects' hypnotic responsiveness. In another study, a group of low and medium hypnotizability subjects were given hypnotizability modification training with varying levels of hypnotist interpersonal involvement and asked to complete measures of rapport (Gfeller, Lynn, & Pribble, 1987). With respect to rapport, the results revealed that: (1) rapport was moderately correlated with posttraining hypnotizability (r = .49, p < .001) and (2) rapport was significantly associated with hypnotic responding (r = .42, p < .001).
Research also suggests that hypnotizability may moderate the effects of rapport on hypnotic responding. Lynn and colleagues found increases in hypnotizability in a high-interpersonal condition in lows, but not in highs (Lynn et al., 1991). Finally, Varga and colleagues (Varga, Józsa, Bányai, & Gösi-Greguss, 2001) administered the Dyadic Interactional Harmony questionnaire (DIH) to two samples of healthy volunteers, along with standardized measures of hypnotizability. The DIH assesses four domains of perceived hypnotist-subject interaction: intimacy, communion, playfulness, and tension. They found that the three scales representing positive interactions demonstrated consistently positive weak to moderate associations (rs range, .18 to .33) with hypnotizability, and the negative interaction scale assessing perceived tension was negatively associated (rs range, −.07 to −.22) with hypnotizability.
Social context and influence
Sociocognitive theorists have argued that expectations are often context dependent, and that putting subjects in a hypnotic context may facilitate expectations and other psychological processes that then facilitate increases in hypnotic responding (Coe & Sarbin, 1977, 1993; Spanos, 1991; Spanos & Coe, 1992). Consistent with this idea, Hylands-White and Derbyshire (Hylands-White & Derbyshire, 2007) found that a relaxation procedure that was labeled as "hypnosis" produced more pain reduction than the same procedure labeled as "relaxation."
Spanos and colleagues (Spanos, Kennedy, & Gwynn, 1984) demonstrated that the hypnotic context can moderate the association between hypnotizability and response to hypnotic analgesia suggestions. In their study, subjects with different levels of hypnotizability (high, medium, and low) were given either (1) brief instructions to try to reduce pain, (2) a hypnotic induction followed by the same instructions, and (3) neither a hypnotic induction nor instructions. Results indicated that subjects in both the instruction-alone and hypnosis conditions reported pain reductions more than the control subjects. In a subsequent study, Spanos and colleagues (Spanos, Gabora, Jarrett, & Gwynn, 1989) found that defining an imagination task as "imagination" instead of "hypnosis" reduced the strength of the association between hypnotizability and task performance (from r = .65 to r = .34).
Summary of the findings regarding social factor correlates of hypnosis
Although virtually all theorists recognize that hypnosis has a social context, and a number of theories specifically include “social” in the label they give to their theory, there has been a paucity of research examining the associations between measures of social factors (such as rapport and context) and response to hypnosis and hypnotic suggestions (Radtke & Stam, 2008). The little research that has been performed supports both direct and moderating roles for social variables – at least the ones that have been studied to date – and hypnotic responding. Although the range of the strength of the associations found tends to vary from weak to moderate, the direction of the associations is consistent, supporting a reliable finding. Higher levels of rapport are associated with more response to hypnotic suggestions, and defining an interaction as “hypnosis” also tends to increase hypnotic responding. The effects of social factors may also be moderated by trait hypnotizability (with “negative” rapport perhaps decreasing the likelihood of response in highs, and “positive” rapport increasing the likelihood of response in lows).
DISCUSSION
The key findings from this scoping review are summarized in Figure 1 and indicate that: (1) measures of a number of biological, psychological, and social factors are all associated with hypnotic responding; (2) there is variability between studies in the strengths of the associations found for many factors; and (3) there are differences between studies in the directions of the associations found for some but not all factors. The findings have important theoretical and clinical implications for understanding the mechanisms and enhancing the efficacy of hypnosis and hypnotic responding.
Figure 1.
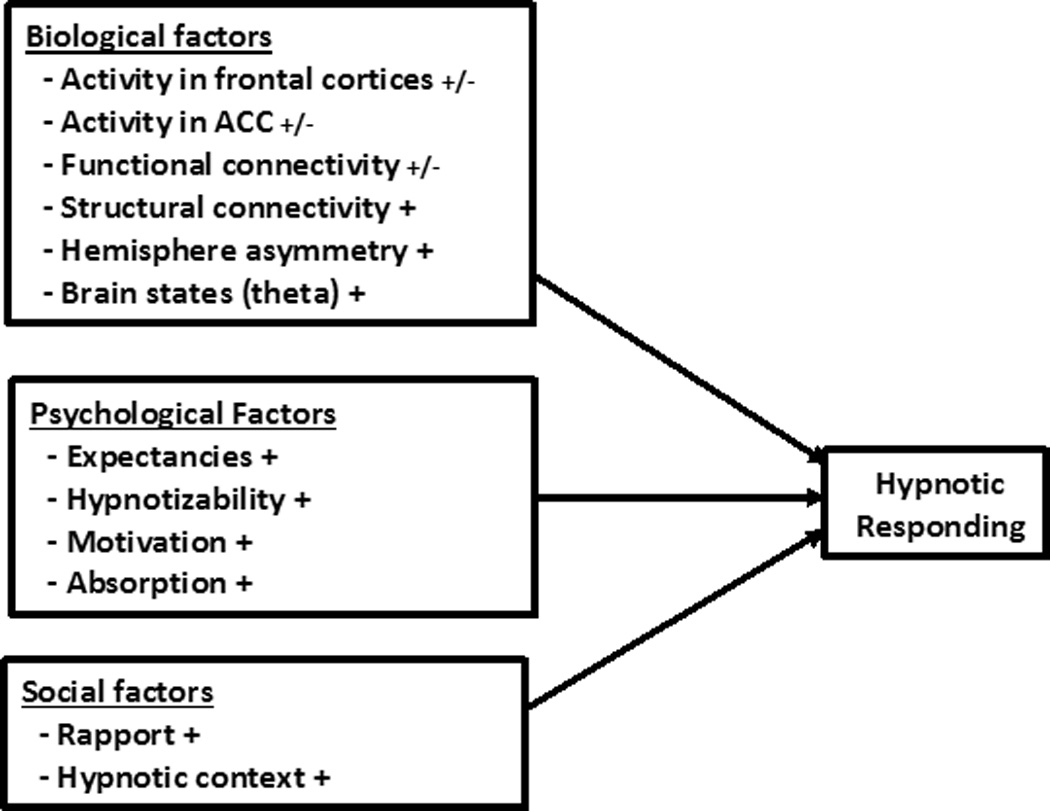
Summary of state of the science findings regarding biological, psychological, and social factors that contribute to response to hypnosis and hypnotic suggestions.
Note: “+/−“ indicates that the factor has demonstrated both positive and negative associations with hypnotic responding; “+” indicates that the factor has demonstrated mostly positive and consistent associations.
Theoretical Implications of the Findings
Support for biopsychosocial models of hypnosis
The significant associations found between a number of biological, psychological factors, and social factors, and measures of hypnotic responding are more consistent with comprehensive models that take into account factors from all three domains (i.e., biopsychosocial models of hypnosis; Bányai, 1991; Hammond, 2005) than with more restrictive models that focus on only one or a some small subset of domains and factors. This conclusion parallels the conclusions made by scientists studying other complex human responses and experiences, such as chronic pain (Novy et al., 1995). Although we believe that the development and testing of theoretical models that explain how specific factors may operate and interact with other factors to influence hypnotic responding have contributed a great deal to our understanding of hypnosis, the findings from this review indicate that restrictive models will not likely provide an adequate explanation of hypnotic phenomenon.
As mentioned previously, we are not the first authors to suggest the potential utility of a biopsychosocial model of hypnosis (Bányai, 1991; Hammond, 2005). However, to our knowledge, no one has yet performed a state of the science review that summarizes the empirical evidence regarding the biological, psychological, and social factors most often proposed as contributing to hypnotic responding by contemporary hypnosis theorists. To the extent that a biopsychosocial model is appropriate for understanding hypnosis, the findings from this review provide an empirical basis for determining the specific biological, psychological, and social factors that should be included in such a model (see Figure 1). In our view, there is no longer a need for research to determine if the 12 factors identified in this review are associated with hypnotic responding; the evidence indicates that they all are. Instead, we view the most important next steps as being to (1) determine which of these factors play a causal role (i.e., influence) and which ones merely reflect hypnotic responding; (2) identify factors that moderate the influence of these 12 factors on hypnotic responding; and (3) identify and explore additional possible biological, psychological, and social factors that should be included in the model. The rest of this Discussion section addresses each of these points.
Neurophysiological models and correlates of hypnosis
The findings provide strong support for a number of consistent associations between measures of central nervous system functioning and structure and response to hypnosis and hypnotic suggestions. The findings are consistent with theoretical models of hypnosis that focus on its neurophysiological substrates (Dienes & Hutton, 2013; Gruzelier, 1998, 2006; Jensen, 2008; Rainville et al., 2002; Rainville & Price, 2004). This conclusion is perhaps neither surprising nor controversial, given that all experience and behaviors – including those involved in response to hypnotic inductions and suggestions – are related to neurophysiological processes.
Questions remain, however, regarding the meaning of the specific associations found. Do the neurophysiological structures and functions linked to hypnosis simply reflect hypnotic responding (as a spinning pinwheel held outside of the window of a moving automobile “reflects” the speed of the automobile), or do (some of) these structures and processes themselves contribute to (influence) hypnotic responding, like the horsepower of an engine influences the 0 to 60 mph time or top speed at which an automobile can travel?
Athough the findings from this review cannot be used to answer causal questions definitively, because so much of the existing research is based on correlational data, the results can provide evidence that may be used to identify some of the biological factors that are more or less likely to reflect hypnosis versus those that may play a causal role in producing or facilitating hypnotic responses. Specifically, we propose that if a measure of a neurophysiological variable evidences both positive and negative associations with hypnotic responding (i.e., activity in frontal cortices, activity in the ACC, and functional connectivity, see Figure 1), the factor assessed by that measure is more likely to reflect a factor associated with hypnotic responding to a specific suggestion (i.e., a factor that reflects a hypnotic response) rather than one that influences hypnotic responding in general. On the other hand, if a measure tends to evidence consistent associations with hypnotic responding (e.g., always positive), then the factor assessed by the measure remains viable as one that might influence or otherwise be important to hypnotic responding. Continuing with the automobile analogy to illustrate this logic, the fact that a pinwheel would spin in one direction when an automobile moves forward and another direction when the automobile moves backward is consistent with the idea that pinwheel spinning may reflect automobile speed (and direction), but has no causal influence on automobile speed. On the other hand, engine horsepower would tend to show a consistently positive association with automobile speed; when tested and when significant associations are found, more horsepower would tend to be positively associated with shorter 0 to 60 mph times and faster top speeds; negative associations are very unlikely. This finding would be consistent with engine horsepower as being a factor that could facilitate automobile speed.
With this metaphor in mind, and based on the findings from our review, given the inconsistent (i.e., both positive and negative associations) results with respect to activity in the frontal cortices and ACC, as well as functional connectivity, the findings suggest that these may underlie processes that reflect but do not facilitate hypnotic responding. For example, when a hypnotic suggestion calls for the subject to do or experience something that requires more activity in the frontal cortices, such as the suppression of pain via frontal inhibitory systems, an increase in frontal cortical activity can be expected (Crawford et al., 1993). When a suggestion calls for a reduction in executive control or “letting go,” such as is the case for a number of hypnotic inductions and suggestions, a decrease in frontal activity can be anticipated (Gruzelier, 1998, 2006). If the suggestions call for changes in perceptions of color, then changes in activity in the brain areas that process color information can be expected (Kosslyn, Thompson, Costantini-Ferrando, Alpert, & Spiegel, 2000). Similarly, procedures that effectively reduce frontal activity could be expected to increase response to some hypnotic suggestions but not others, rather than increase general hypnotic responsivity (Dienes & Hutton, 2013).
On the other hand, the more consistent findings (with respect to the direction of associations) between hypnotic responding and measures of structural connectivity, hemisphere asymmetry, and theta bandwidth activity (see Figure 1) is consistent with the possibility that these may reflect factors that facilitate response to hypnotic suggestions in general. Thus, given that individuals with high hypnotizability have greater structural connectivity between left and right hemisphere frontal cortices and between left dorsolateral prefrontal cortex and the ACC (that is, those areas specifically involved in many hypnotic responses) than individuals with low hypnotizability (Hoeft et al., 2012; Horton et al., 2004), it is possible that more structural connections that facilitate communication between and control over different brain structures may make hypnotic responding easier. Similarly, higher levels of hemisphere asymmetry may measure processes that facilitate response to hypnosis, such as greater overall neurophysiological flexibility (Crawford, 1989). Slow wave brain oscillations, in particular theta bandwidth activity but also some alpha, has been linked to better memory tracing and recall functions (Basar & Guntekin, 2012; Buzsáki, 2006; Buzsaki & Moser, 2013; Klimesch, 2012). Given the central importance of memory in most if not all human responses (e.g., we must recall words to speak and recall images and sensations in order to re-experience them), it is possible that activity in these bandwidths, in particular, may help facilitate response to a variety of hypnotic suggestions. Note that we are not proposing here that more structural connectivity between frontal sites and other brain structures, greater hemisphere asymmetry, and more theta activity are necessarily biological “signatures” of a hypnotic state; it is too early to draw such a conclusions. Instead, the findings indicate that if a biological signature or biological signatures of hypnosis exist, these three factors remain as possible contenders for playing such a role.
Psychological and social models and correlates of hypnosis
One of the findings from this review was the relative lack of research examining the importance of psychological and in particular social factors in hypnotic responding, despite the fact that theorists have been hypothesizing roles for both as factors that contribute to hypnotic responding for decades (Bányai, 1991; Bányai, 1998; Barnier et al., 2008; Coe & Sarbin, 1977, 1993; Diamond, 1987; Evans, 2000; Hammond, 2005; Killeen & Nash, 2003; Kirsch, 1991; Kirsch & Lynn, 1995, 1997, 1999; Lynn et al., 2008; Lynn & Rhue, 1991; Spanos, 1991; Spanos & Chaves, 1989; Spanos & Coe, 1992; Wagstaff et al., 2010; Welch, 1947).
The role that social factors, in particular, may play has not yet been adequately examined (Radtke & Stam, 2008). A rare exception to a lack of attention to social factors in the field is the work of Éva Bányai, Katlin Varga, and their colleagues at the Eötvös Loránd University in Budapest, who have developed an impressive program studying hypnosis in the context of its social and interactional elements (Bányai, 1985, 1998; Varga, 2013; Varga, Banyai, & Gosi-Greguss, 1994; Varga, Banyai, Gosi-Greguss, & Tauszik, 2013; Varga, Józsa, Bányai, & Gösi-Greguss, 2009; Varga, Jozsa, Banyai, Gosi-Greguss, & Kumar, 2001). Much of the work completed by this group to date focuses on the assessment of the phenomenological experiences of the hypnotist and subject and their interaction. It will be interesting to see in future studies how these phenomenological experiences are associated with response to different hypnotic suggestions.
There is also a need to move beyond correlational designs in the field. Although the findings from correlational research can be used to reject a factor as unimportant when it shows weak and nonsignificant associations with hypnotic responding, when significant associations are found (as they often are), causal conclusions cannot be drawn. Mediational analyses, while also a type of correlational study, provides a more sophisticated strategy for testing hypothesized mechanisms of hypnosis (Baron & Kenny, 1986; Sobel, 2008).
We were only able to identify five studies that used this approach to test for the potential mediation effects of a biopsychosocial variable (Baker & Kirsch, 1993; Milling & Breen, 2003; Milling et al., 2002; Milling et al., 2003; G. H. Montgomery et al., 2010). In each of these studies, the factor examined was expectancies. Four of these examined the mediation effects of expectancy on analogue (laboratory) pain, and only one (G. H. Montgomery et al., 2010) examined mediational effects in a clinical population. More mediational studies such as these would be extremely useful to advance our knowledge regarding the role that other psychological and social factors play in influencing hypnotic responding.
Whereas mediational analyses can provide information regarding why hypnosis may be effective, moderation analyses can provide information regarding for whom hypnosis is most effective, or for whom a specific factor may play a greater or lesser role (Baron & Kenny, 1986). In a moderation analyses, two factors are examined together with respect to their interaction effects in predicting outcome. When a significant interaction effect emerges, it indicates that the strength or importance of one of the factors depends on the level of the other one.
The results of this review indicate that moderation analyses may be particularly relevant for better understanding the roles of expectancies and hypnotizability in understanding hypnotic responding. For both of these psychological variables, although they tended to show positive associations with hypnotic responding, the strength of the associations found demonstrated a great deal of variability from study to study, ranging from very weak and nonsignificant to (mostly) moderate. This level of variability provides support for the presence of moderator variables that could influence how important each factor would be in any particular situation. Unfortunately, interaction effects are rarely examined in hypnosis research. In support of the potential utility of such analyses, in the rare instance where moderation effects have been considered, and as cited in the Results section of this review, moderation effects have been found. For example, Milling and colleagues (Milling et al., 2006) found that hypnotizability moderated the outcome for an analogue hypnotic analgesia treatment, but not for a distraction or cognitivebehavioral treatment. G. H. Montgomery and Bovbjerg (2004) found that outcome expectancies were more important for predicting response to hypnotic analgesia suggestions than for suggestions for reductions in nausea or “discomfort,” and Kirsch and colleagues found that expectancies played a larger role in response to more difficult hypnotic suggestions than easier hypnotic suggestions (Kirsch et al., 1999).
Research is needed to determine the extent to which these moderation findings replicate across different populations, as well as to identify additional moderating variables. For example, we can envision the possibility that individuals with high trait hypnotizability may be more responsive (vulnerable) to their own self-suggestions (i.e., both positive and negative). Such a model would predict an Expectancy X Hypnotizability interaction predicting response to hypnotic cognitive therapy. Findings supporting such an interaction effect across populations would support the importance of teaching individuals with more hypnotizability how to protect themselves from the impact of negative self-suggestions (Jensen et al., 2011) as well as how to take advantage of their “talent” by benefiting from systematic positive self-suggestions. Research testing for other possible interaction effects between the factors within domains (e.g., Motivation X Expectancy) or even across domains (e.g., Theta Bandwidth Power X Expectancy, Rapport X Absorption) could help us identify additional variables that influence the role of psychological and social factors in hypnotic responding.
Clinical Implications
Perhaps the most important conclusion for clinicians from this review is the confirmation that an individual’s response to hypnosis is highly complex, and not easily or strongly predicted by any one biological, psychological, or social factor. Many if not all clinicians who use hypnosis regularly see evidence for this conclusion in their daily practice, when a client or patient is seen to respond favorably to hypnotic treatment despite (1) a noisy environment not thought to be conducive to hypnotic responding, (2) low scores on measures of trait hypnotizability, (3) adamant proclamations of the belief that hypnosis will not be helpful or effective, and (4) the presence of evidence for significant secondary gain for having a symptom (i.e., low apparent motivation to respond). None of these factors impact response in everyone and in every situation.
Does evidence for “only” weak to moderate associations between biological, psychological, and social factors and response to hypnosis mean that these factors should therefore be ignored? We do not believe so. Each of these factors has evidence supporting its importance in some individuals. The experienced clinician, who wishes his or her clients and patients to obtain the most benefit possible, will do everything reasonable to enhance outcomes. This can, and should we believe, include: (1) facilitating a reasonable level of hope and outcome expectancies; (2) enhancing motivation for treatment outcome; (3) enhancing positive rapport; and (4) creating a “hypnotic” environment for treatment. As more is learned about the role that biological factors play in hypnotic responding, it might even be possible to prepare clients and patients to respond more strongly to hypnosis treatment using strategies that enhance certain brain states (Batty, Bonnington, Tang, Hawken, & Gruzelier, 2006).
Limitations
Perhaps the most important limitation of this review is that it is scoping and not a systematic review. As such, we based our search for articles to review mostly on the reference lists of primary review articles as well as some key articles identified via a literature search using very specific—but limited—search terms. Thus, it is likely that some articles presenting findings relevant to this review were missed. However, we did not believe that a systematic review of the topics covered in this paper was feasible, both in terms of the resources that such a review would require, as well as the number of pages that would be available in an article to present the findings (of all published studies regarding the associations between all biological, psychological, and social factors ever examined). Moreover, the results that did emerge from the review still provide clear findings that have important theoretical and clinical implications. We would encourage future researchers to perform systematic reviews on more focused domains or factors described here (e.g., a systematic review of the importance of outcome expectancies in hypnotic responding) to help confirm the general conclusions we made from the articles we identified and cited.
Relatedly, because we only reviewed studies involving those factors that have been studied most often in the research literature, we did not report on or discuss findings regarding a number of factors for which there are positive preliminary findings, and which may ultimately prove to be as or even more important than the factors identified here. For example, researchers are beginning to examine additional biological factors associated with hypnosis such as changes in peripheral hormone levels (Bryant, Hung, Guastella, & Mitchell, 2012; Varga & Kekecs, 2014), heart rate and heart rate variability (Santarcangelo et al., 2012; Santarcangelo, Varanini et al., 2013; Sebastiani, Simoni, Gemignani, Ghelarducci, & Santarcangelo, 2003; Yuksel, Ozcan, & Dane, 2013), respiratory rate (Sebastiani et al., 2003), and blood pressure (Santarcangelo, Paoletti et al., 2013). Doubtless some of these factors as well as other biological, psychological, and social factors not examined here will be identified as being associated with, and may ultimately be shown to influence, hypnotic responding.
SUMMARY
Despite the limitations associated with this review, the findings support a number of important conclusions that have both theoretical and clinical relevance. First, the findings indicate that hypnosis and hypnotic responding are probably best explained by more comprehensive models that take into account factors from biological, psychological, and social domains. More restrictive models that focus only or primarily on a single factor or subset of factors, while useful for increasing our understanding the role of these factors may play, are unlikely to be adequate for providing a thorough understanding of the domain of hypnosis. Second, the findings provide preliminary evidence regarding the variables that remain viable as factors that might facilitate hypnotic responding; that is, structural connectivity, hemisphere asymmetry, higher levels of theta bandwidth activity, expectancies, trait hypnotizability, motivation, absorptive capacity, rapport, and context. A greater focus on examining the causal (versus only correlational) role that these factors may play in hypnotic responding is warranted. Third, the findings point to the need for additional research to better understand how the factors discussed both mediate (explain) and work together (interact) to predict hypnotic responding. Finally, clinicians can use the findings from this review to help identify targets for potentially enhancing response to hypnotic treatment. Our hope is that the findings reported in this review encourage theoreticians and clinicians to “think outside of” our preferred theoretical boxes, and consider how we might expand our views and models, so as to better understand hypnosis and ultimately improve its beneficial effects.
Acknowledgments
Mark P. Jensen is the author of two books related to the topic of this paper (Hypnosis for chronic pain management: Therapist guide and Hypnosis for chronic pain management: Workbook). He receive royalties for the sale of these books.
Funding
This research was supported by National Institutes of Health, National Institute of Child Health and Human Development, National Center for Medical Rehabilitation Research grant R01 HDD070973. The views presented here are not necessarily those of the National Institutes of Health.
Footnotes
Throughout the literature terms such as hypnotizability, suggestibility, hypnotic responsivity, hypnotic susceptibility, hypnotic preparedness, hypnotic responding, and hypnotic depth have been used interchangeably at various times and by various investigators. The terms “hypnotic susceptibility” and “hypnotic suggestibility” imply stable traits of individuals, whereas “hypnotic responsivity” and “hypnotic responding” refer more to an individual’s behavior in response to a specific suggestion or set of suggestions. Here we use the term “hypnotizability” to refer to an individual’s response to test suggestions after a hypnotic induction procedure” (Heap, Brown, & Oakley, 2004; I. R. Kirsch & Council, 1992).
Contributor Information
Mark P. Jensen, University of Washington, Seattle, USA
Tomonori Adachi, Osaka University, Osaka, Japan.
Catarina Tomé-Pires, Rovira and Virgili University, Tarragona, Catalonia, Spain.
Jikwan Lee, University Putra Malaysia, Selangor, Malaysia.
Zubaidah Jamil Osman, University Putra Malaysia, Selangor, Malaysia.
Jordi Miró, Rovira and Virgili University, Tarragona, Catalonia, Spain.
References
- Accardi MC, Milling LS. The effectiveness of hypnosis for reducing procedure-related pain in children and adolescents: a comprehensive methodological review. Journal of Behavioral Medicine. 2009;32:328–339. doi: 10.1007/s10865-009-9207-6. [DOI] [PubMed] [Google Scholar]
- Akpinar S, Ulett GA, Itil TM. Hypnotizability predicted by digital computer-analyzed EEG pattern. Biological Psychiatry. 1971;3(4):387–392. [PubMed] [Google Scholar]
- Alladin A. Cognitive hypnotherapy for major depressive disorder. American Journal of Clinical Hypnosis. 2012;54:275–293. doi: 10.1080/00029157.2012.654527. [DOI] [PubMed] [Google Scholar]
- Apkarian AV. A brain signature for acute pain. Trends in Cognitive Science. 2013;17(7):309–310. doi: 10.1016/j.tics.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Appel PR, Bleiberg J. Pain reduction is related to hypnotizability but not to relaxation or to reduction in suffering: a preliminary investigation. American Journal of Clinical Hypnosis. 2005;48(2–3):153–161. doi: 10.1080/00029157.2005.10401512. [DOI] [PubMed] [Google Scholar]
- Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- Baker SL, Kirsch I. Hypnotic and placebo analgesia: Order effects and the placebo label. Contemporary Hypnosis. 1993;10:117–126. [Google Scholar]
- Bányai ÉI. A social psychophysiological approach to the understanding of hypnosis: The interaction between hypnotist and subject. Hypnos. 1985;12:186–210. [Google Scholar]
- Bányai É. Toward a social-psychobiological model of hypnosis. In: JLynn S, WRhue J, editors. Theories of hyposis: Current models and perspectives. New York: Guilford Press; 1991. pp. 564–598. [Google Scholar]
- Bányai ÉI. The interactive nature of hypnosis: Research evidence for a social-psychobiological model. Contemporary Hypnosis. 1998;15:52–63. [Google Scholar]
- Barabasz AF, Barabasz M. Hypnosis and the brain. In: Nash MR, Barnier AJ, editors. The oxford handbook of hypnosis: Theory, research, and practice. Oxford, UK: Oxford University Press; 2008. pp. 337–364. [Google Scholar]
- Barber TX. Hypnosis: A scientific approach. New York: Van Nostrand Reinhold; 1969. [Google Scholar]
- Barnier AJ, Dienes Z, Mitchell CJ. How hypnosis happens: New cognitive theories of hypnotic responding. In: Nash MR, Barnier AJ, editors. The Oxford handbook of hypnosis: Theory, research, and practice. New York: Oxford University Press; 2008. pp. 141–177. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basar E, Guntekin B. A short review of alpha activity in cognitive processes and in cognitive impairment. International Journal of Psychophysiolgy. 2012;86(1):25–38. doi: 10.1016/j.ijpsycho.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Batty MJ, Bonnington S, Tang BK, Hawken MB, Gruzelier JH. Relaxation strategies and enhancement of hypnotic susceptibility: EEG neurofeedback, progressive muscle relaxation and self-hypnosis. Brain Research Bulletin. 2006;71(1–3):83–90. doi: 10.1016/j.brainresbull.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Benham G, Woody EZ, Wilson KS, Nash MR. Expect the unexpected: Ability, attitude, and responsiveness to hypnosis. Journal of Personality and Social Psychology. 2006;91:342–350. doi: 10.1037/0022-3514.91.2.342. [DOI] [PubMed] [Google Scholar]
- Braffman W, Kirsch I. Imaginative suggestibility and hypnotizability: an empirical analysis. Journal of Personality and Social Psychology. 1999;77(3):578–587. doi: 10.1037//0022-3514.77.3.578. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Hung L, Guastella AJ, Mitchell PB. Oxytocin as a moderator of hypnotizability. Psychoneuroendocrinology. 2012;37(1):162–166. doi: 10.1016/j.psyneuen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Ecker AH, Vinci C. A biopsychosocial model of social anxiety and substance use. Depression and Anxiety. 2013;30(3):276–284. doi: 10.1002/da.22032. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford ; New York: Oxford University Press; 2006. [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capafons A, Cabanas S, Espejo B, Cardeñåa E. Confirmatory factor analysis of the Valencia Scale on Attitudes and Beliefs Toward Hypnosis: an international study. International Journal of Clinical and Experimental Hypnosis. 2004;52(4):413–433. doi: 10.1080/00207140490888432. [DOI] [PubMed] [Google Scholar]
- Capafons A, Espejo B, Mendoza ME. Confirmatory factor analysis of the Valencia Scale on Attitudes and Beliefs toward Hypnosis, Therapist version. International Journal of Clinical and Experimental Hypnosis. 2008;56(3):281–294. doi: 10.1080/00207140802039748. [DOI] [PubMed] [Google Scholar]
- Cardeña E, Jonsson P, Terhune DB, Marcusson-Clavertz D. The neurophenomenology of neutral hypnosis. Cortex. 2013;49(2):375–385. doi: 10.1016/j.cortex.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Chapman LF, Goodell H, Wolff HG. Changes in tissue vulnerability induced during hypnotic suggestion. Journal of Psychosomatic Research. 1959;4:99–105. doi: 10.1016/0022-3999(59)90023-6. [DOI] [PubMed] [Google Scholar]
- Chen AC, Dworkin SF, Bloomquist DS. Cortical power spectrum analysis of hypnotic pain control in surgery. International Journal of Neuroscience. 1981;13(2–3):127–136. doi: 10.3109/00207458109043312. [DOI] [PubMed] [Google Scholar]
- Coe WC, Sarbin TR. Hypnosis from the standpoint of a contextualist. Annals of the New York Acadamy of Science. 1977;296:2–13. doi: 10.1111/j.1749-6632.1977.tb38157.x. [DOI] [PubMed] [Google Scholar]
- Coe WC, Sarbin TR. Role theory: Hypnosis from a dramaturgical and narrational perspective. In: JLynn SJ, Rhue JW, editors. Theories of hypnosis: Current models and perspectives. New York: Guildford Press; 1993. pp. 303–323. [Google Scholar]
- Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The Brain under self-control: Modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62:862–875. doi: 10.1016/j.neuron.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annual Review of Neuroscience. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Crawford HJ. Cognitive and psychological flexiability: Multiple pathways to hypnotic responsiveness. In: Ghorghui V, Netter H, Eysenck H, Rosenthal R, editors. Suggestion and suggestibility: Theory and research. Heidelberg: Springer; 1989. [Google Scholar]
- Crawford HJ. Cognitive and psychophysiological correlates of hypnotic responsiveness and hypnosis. In: Brown DP, Fass ML, editors. Creative master in hypnosis and hypnoanalysis: A festschrift for Erika Fromm. Hillsdate, NJ: Erlbaum; 1990. pp. 155–168. [Google Scholar]
- Crawford HJ. Brain dynamics and hypnosis: attentional and disattentional processes. International Journal of Clinical and Experimental Hypnosis. 1994;42(3):204–232. doi: 10.1080/00207149408409352. [DOI] [PubMed] [Google Scholar]
- Crawford HJ, Gruzelier JH. A midstream view of the neuropsychophysiology of hypnosis: Recent recearch and future directions. In: Fromm E, Nash MR, editors. Contemporary Hypnosis Research. New York: Guilford Press; 1992. pp. 227–266. [Google Scholar]
- Crawford HJ, Gur RC, Skolnick B, Gur RE, Benson DM. Effects of hypnosis on regional cerebral blood flow during ischemic pain with and without suggested hypnotic analgesia. Inernational Journal of Psychophysiology. 1993;15(3):181–195. doi: 10.1016/0167-8760(93)90002-7. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Williams JD, Haenschel C, Gruzelier JH. Pain perception, hypnosis and 40 Hz oscillations. Inernational Journal of Psychophysiology. 2002;46(2):101–108. doi: 10.1016/s0167-8760(02)00118-6. [DOI] [PubMed] [Google Scholar]
- Danziger N, Fournier E, Bouhassira D, Michaud D, De Broucker T, Santarcangelo E, Willer JC. Different strategies of modulation can be operative during hypnotic analgesia: a neurophysiological study. Pain. 1998;75(1):85–92. doi: 10.1016/S0304-3959(97)00208-X. [DOI] [PubMed] [Google Scholar]
- De Benedittis G. Understanding the multidimensional mechanisms of hypnotic analgesia. Contemporary Hypnosis. 2003;20:59–80. [Google Scholar]
- De Pascalis V. EEG spectral analysis during hypnotic induction, hypnotic dream and age regression. Inernational Journal of Psychophysiology. 1993;15(2):153–166. doi: 10.1016/0167-8760(93)90073-x. [DOI] [PubMed] [Google Scholar]
- De Pascalis V. Psychophysiological correlates of hypnosis and hypnotic susceptibility. International Journal of Clinical and Experimental Hypnosis. 1999;47(2):117–143. doi: 10.1080/00207149908410026. [DOI] [PubMed] [Google Scholar]
- De Pascalis V. Phase-ordered gamma oscillations and the modulations of hypnotic experience. In: Jamiseon GA, editor. Hypnosis and conscious states: The cognitive neuroscience perspective. New York: Oxford University Press; 2007. p. 67. [Google Scholar]
- De Pascalis V, Cacace I. Pain perception, obstructive imagery and phase-ordered gamma oscillations. Inernational Journal of Psychophysiology. 2005;56(2):157–169. doi: 10.1016/j.ijpsycho.2004.11.004. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Cacace I, Massicolle F. Perception and modulation of pain in waking and hypnosis: functional significance of phase-ordered gamma oscillations. Pain. 2004;112(1–2):27–36. doi: 10.1016/j.pain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Marucci FS, Penna PM. 40-Hz EEG asymmetry during recall of emotional events in waking and hypnosis: differences between low and high hypnotizables. Inernational Journal of Psychophysiology. 1989;7(1):85–96. doi: 10.1016/0167-8760(89)90034-2. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Marucci FS, Penna PM, Pessa E. Hemispheric activity of 40 Hz EEG during recall of emotional events: differences between low and high hypnotizables. Inernational Journal of Psychophysiology. 1987;5(3):167–180. doi: 10.1016/0167-8760(87)90003-1. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Palumbo G. EEG alpha asymmmetry: Task difficulty and hypnotizbaility. Perceptual and Motor Skills. 1986;62:139–150. doi: 10.2466/pms.1986.62.1.139. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Perrone M. EEG asymmetry and heart rate during experience of hypnotic analgesia in high and low hypnotizables. Inernational Journal of Psychophysiology. 1996;21(2–3):163–175. doi: 10.1016/0167-8760(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Whalley MG, Oakley DA. Fibromyalgia pain and its modulation by hypnotic and non-hypnotic suggestion: an fMRI analysis. European Journal of Pain. 2009;13(5):542–550. doi: 10.1016/j.ejpain.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23(1):392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Diamond MJ. The interactional basis of hypnotic experience: on the relational dimensions of hypnosis. International Journal of Clinical and Experimental Hypnosis. 1987;35(2):95–115. doi: 10.1080/00207148708416046. [DOI] [PubMed] [Google Scholar]
- Dienes Z, Hutton S. Understanding hypnosis metacognitively: rTMS applied to left DLPFC increases hypnotic suggestibility. Cortex. 2013;49(2):386–392. doi: 10.1016/j.cortex.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Edmonston WE, Jr, Moscovitz HC. Hypnosis and lateralized brain functions. International Journal of Clinical and Experimental Hypnosis. 1990;38(1):70–84. doi: 10.1080/00207149008414499. [DOI] [PubMed] [Google Scholar]
- Egner T, Jamieson G, Gruzelier J. Hypnosis decouples cognitive control from conflict monitoring processes of the frontal lobe. Neuroimage. 2005;27(4):969–978. doi: 10.1016/j.neuroimage.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Elkins G, Jensen MP, Patterson DR. Hypnotherapy for the management of chronic pain. International Journal of Clinical and Experimental Hypnosis. 2007;55:275–287. doi: 10.1080/00207140701338621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans FJ. The domain of hypnosis: a multifactorial model. American Journal of Clinical Hypnosis. 2000;43(1):1–16. doi: 10.1080/00029157.2000.10404252. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Boly M, Laureys S. Functional neuroanatomy of the hypnotic state. Journal of Physiology - Paris. 2006;99(4–6):463–469. doi: 10.1016/j.jphysparis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92(5):1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Roediger L, Del Fiore G, Delgueldre C, Phillips C, Lamy M, Laureys S. Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Brain Research: Cognitive Brain Research. 2003;17(2):255–262. doi: 10.1016/s0926-6410(03)00113-7. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Kallio S, Revonsuo A. Cortex functional connectivity as a neurophysiological correlate of hypnosis: an EEG case study. Neuropsychologia. 2007;45(7):1452–1462. doi: 10.1016/j.neuropsychologia.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Freeman R, Barabasz A, Barabasz M, Warner D. Hypnosis and distraction differ in their effects on cold pressor pain. American Journal of Clinical Hypnosis. 2000;43(2):137–148. doi: 10.1080/00029157.2000.10404266. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, London P, Leibovitz MP, Cooper LM, Hart JT. EEG and hypnotic susceptibility. Journal of Comparative and Physiological Psychology. 1970;72(1):125–131. doi: 10.1037/h0029278. [DOI] [PubMed] [Google Scholar]
- Gfeller JD, Lynn SJ, Pribble WE. Enhancing hypnotic susceptibility: interpersonal and rapport factors. Journal of Personality and Social Psychology. 1987;52(3):586–595. doi: 10.1037//0022-3514.52.3.586. [DOI] [PubMed] [Google Scholar]
- Glisky ML, Tataryn DJ, Tobias BA, Kihlstrom JF, McConkey KM. Absorption, openness to experience, and hypnotizability. Journal of Personality and Social Psychology. 1991;60(2):263–272. doi: 10.1037//0022-3514.60.2.263. [DOI] [PubMed] [Google Scholar]
- Golden WL. Cognitive hypnotherapy for anxiety disorders. American Journal of Clinical Hypnosis. 2012;54(4):263–274. doi: 10.1080/00029157.2011.650333. [DOI] [PubMed] [Google Scholar]
- Graffin NF, Ray WJ, Lundy R. EEG concomitants of hypnosis and hypnotic susceptibility. Journal of Abnormal Psychology. 1995;104(1):123–131. doi: 10.1037//0021-843x.104.1.123. [DOI] [PubMed] [Google Scholar]
- Green JP. The Valencia Scale of Attitudes and Beliefs Toward Hypnosis-Client version and hypnotizability. International Journal of Clinical and Experimental Hypnosis. 2012;60(2):229–240. doi: 10.1080/00207144.2012.648073. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. A working model of the neurophysiology of hypnosis: A review of the evidence. Contemporary Hypnosis. 1998;15:3–21. [Google Scholar]
- Gruzelier JH. Frontal functions, connectivity and neural efficiency underpinning hypnosis and hypnotic susceptibility. Contemporary Hypnosis. 2006;23:15–32. [Google Scholar]
- Hammond DC. An integrative, multi-factor conceptualization of hypnosis. American Journal of Clinical Hypnosis. 2005;48(2–3):131–135. doi: 10.1080/00029157.2005.10401508. [DOI] [PubMed] [Google Scholar]
- Hammond DC. Hypnosis in the treatment of anxiety- and stress-related disorders. Expert Reviews in Neurotherapeutics. 2010;10(2):263–273. doi: 10.1586/ern.09.140. [DOI] [PubMed] [Google Scholar]
- Heap M, Brown RJ, Oakley DA. The highly hypnotizable person : theoretical, experimental, and clinical issues. Hove, East Sussex ; New York, NY: Brunner-Routledge; 2004. [Google Scholar]
- Hilgard ER, Hilgard JR. Hypnosis in the relief of pain. Rev. ed. New York: Brunner/Mazel; 1994. [Google Scholar]
- Hoeft F, Gabrieli JD, Whitfield-Gabrieli S, Haas BW, Bammer R, Menon V, Spiegel D. Functional brain basis of hypnotizability. Archives of General Psychiatry. 2012;69(10):1064–1072. doi: 10.1001/archgenpsychiatry.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. Journal of Neurophysiology. 2001;86(1):402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Horton JE, Crawford HJ, Harrington G, Downs JH., 3rd Increased anterior corpus callosum size associated positively with hypnotizability and the ability to control pain. Brain. 2004;127(Pt 8):1741–1747. doi: 10.1093/brain/awh196. [DOI] [PubMed] [Google Scholar]
- Hylands-White N, Derbyshire SWG. Modifying pain perception: Is it better to be hypnotizable or feel that you are hypnotized? Contemporary Hypnosis. 2007;24:143–153. [Google Scholar]
- Isotani T, Lehmann D, Pascual-Marqui RD, Kochi K, Wackermann J, Saito N, Sasada K. EEG source localization and global dimensional complexity in high- and low- hypnotizable subjects: a pilot study. Neuropsychobiology. 2001;44(4):192–198. doi: 10.1159/000054942. doi: [DOI] [PubMed] [Google Scholar]
- Janet P. The mental state of hysteria. New York: Putnam; 1901. [Google Scholar]
- Jensen MP. The neurophysiology of pain perception and hypnotic analgesia: Implications for clinical practice. American Journal of Clinical Hypnosis. 2008;51:123–148. doi: 10.1080/00029157.2008.10401654. [DOI] [PubMed] [Google Scholar]
- Jensen MP. Hypnosis for chronic pain management: a new hope. Pain. 2009;146(3):235–237. doi: 10.1016/j.pain.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Jensen MP. Hypnosis for chronic pain management: Therapist guide. Oxford: Oxford University Press; 2011. [Google Scholar]
- Jensen MP, McArthur KD, Barber J, Hanley MA, Engel JM, Romano JM, Patterson DR. Satisfaction with, and the beneficial side effects of, hypnotic analgesia. International Journal of Clinical and Experimental Hypnosis. 2006;54:432–447. doi: 10.1080/00207140600856798. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, Patterson DR. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. International Journal of Clinical and Experimental Hypnosis. 2009;57(3):239–268. doi: 10.1080/00207140902881007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Barber J, Romano JM, Molton IR, Raichle K, Osborne TL, Patterson DR. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. International Journal of Clinical and Experimental Hypnosis. 2009;57:198–221. doi: 10.1080/00207140802665476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Ehde DM, Gertz KJ, Stoelb BL, Dillworth TM, Hirsh AT, Kraft GH. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. International Journal of Clinical and Experimental Hypnosis. 2011;59(1):45–63. doi: 10.1080/00207144.2011.522892. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, Patterson DR. Hypnotic analgesia for chronic pain in persons with disabilities: A case series. International Journal of Clinical and Experimental Hypnosis. 2005;53:198–228. doi: 10.1080/00207140590927545. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Patterson DR. Hypnotic treatment of chronic pain. Journal of Behavioral Medicine. 2006;29:95–124. doi: 10.1007/s10865-005-9031-6. [DOI] [PubMed] [Google Scholar]
- Kiernan BD, Dane JR, Phillips LH, Price DD. Hypnotic analgesia reduces R-III nociceptive reflex: further evidence concerning the multifactorial nature of hypnotic analgesia. Pain. 1995;60(1):39–47. doi: 10.1016/0304-3959(94)00134-Z. [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF. Neuro-hypnotism: prospects for hypnosis and neuroscience. Cortex. 2013;49(2):365–374. doi: 10.1016/j.cortex.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlstrom JF, Glisky ML, McGovern S, Rapcsak SZ, Mennemeier MS. Hypnosis in the right hemisphere. Cortex. 2013;49(2):393–399. doi: 10.1016/j.cortex.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Nash MR. The four causes of hypnosis. International Journal of Clinical and Experimental Hypnosis. 2003;51(3):195–231. doi: 10.1076/iceh.51.3.195.15522. [DOI] [PubMed] [Google Scholar]
- Kirenskaya AV, Novototsky-Vlasov VY, Zvonikov VM. Waking EEG spectral power and coherence differences between high and low hypnotizable subjects. International Journal of Clinical and Experimental Hypnosis. 2011;59(4):441–453. doi: 10.1080/00207144.2011.594744. [DOI] [PubMed] [Google Scholar]
- Kirsch I. The social learning theory of hypnosis. In: Lynn SJ, Rhue JW, editors. Theories of hypnosis: Current models and perspectives. New York: Guildford Press; 1991. pp. 439–466. [Google Scholar]
- Kirsch I, Lynn SJ. The altered state of hypnosis: Changes in the theoretical landscape. American Psychologist. 1995;50:846–858. [Google Scholar]
- Kirsch I, Lynn SJ. Hypnotic involuntariness and the automaticity of everyday life. American Journal of Clinical Hypnosis. 1997;40(1):329–348. doi: 10.1080/00029157.1997.10403402. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Lynn SJ. Automaticity in clinical psychology. American Psychologist. 1999;54:504–515. doi: 10.1037//0003-066x.54.7.504. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Montgomery G, Sapirstein G. Hypnosis as an adjunct to cognitive-behavioral psychotherapy: a meta-analysis. Journal of Consulting and Clinical Psychology. 1995;63(2):214–220. doi: 10.1037//0022-006x.63.2.214. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Silva CE, Comey G, Reed S. A spectral analysis of cognitive and personality variables in hypnosis: Empirical disconfirmation of the two-factor model of hypnotic responding. Journal of Personality and Social Psychology. 1999;69:167–175. [Google Scholar]
- Kirsch IR, Council J. Situational and personality correlated of hypnotic responsivness. In: Fromm E, Nash MR, editors. Contemporary Hypnosis Research. New York: Guilford; 1992. pp. 267–291. [Google Scholar]
- Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Science. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Costantini-Ferrando MF, Alpert NM, Spiegel D. Hypnotic visual illusion alters color porcessing in the brain. American Journal of Psychiatry. 2000;157:1279–1284. doi: 10.1176/appi.ajp.157.8.1279. [DOI] [PubMed] [Google Scholar]
- Landolt AS, Milling LS. The efficacy of hypnosis as an intervention for labor and delivery pain: a comprehensive methodological review. Clinical Psychology Review. 2011;31(6):1022–1031. doi: 10.1016/j.cpr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis versus cognitive behavioral training for pain management with pediatric cancer patients undergoing bone marrow aspirations. International Journal of Clinical and Experimental Hypnosis. 1999;47(2):104–116. doi: 10.1080/00207149908410025. [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure-related pain in pediatric oncology patients. International Journal of Clinical and Experimental Hypnosis. 2003;51(1):4–28. doi: 10.1076/iceh.51.1.4.14064. [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Hatira P. Randomized clinical trial of local anesthetic versus a combination of local anesthetic with self-hypnosis in the management of pediatric procedure-related pain. Health Psychology. 2006;25(3):307–315. doi: 10.1037/0278-6133.25.3.307. [DOI] [PubMed] [Google Scholar]
- Lynn S, Kirsch IR, Hallquist MN. Social cognitive theories of hypnosis. In: Nash JA, Barnier A, editors. The oxford handbook of hypnosis: Theory, research, and practice. Oxford, UK: Oxford University Press; 2008. pp. 111–139. [Google Scholar]
- Lynn SJ, Kirsch I. Essentials of clinical hypnosis: An evidenced based approach. Washington, DC: American Psychological Associations; 2006. [Google Scholar]
- Lynn SJ, Rhue JW. An integrative model of hypnosis. In: Lynn SJ, Rhue JW, editors. Theories of hypnosis: Current models and perspectives. New York: Guilford Press; 1991. pp. 397–438. [Google Scholar]
- Lynn SJ, Weekes JR, Neufeld V, Zivney O, Brentar J, Weiss F. Interpersonal climate and hypnotizability level: Effects on hypnotic performance, rapport, and archaic involvement. Journal of Personality and Social Psychology. 1991;60:739–743. [Google Scholar]
- Macleod-Morgan C. Hypnotic susceptibility, EEG theta and alpha waves, and hemispheric specificity. In: Burrows GD, Collinson DR, Dennerstein L, editors. Hypnosis 1979. Amsterdam: Elsevier; 1979. pp. 181–188. [Google Scholar]
- MacLeod-Morgan C, Lack L. Hemispheric specificity: a physiological concomitant of hypnotizability. Psychophysiology. 1982;19(6):687–690. doi: 10.1111/j.1469-8986.1982.tb02525.x. [DOI] [PubMed] [Google Scholar]
- Madden K, Middleton P, Cyna AM, Matthewson M, Jones L. Hypnosis for pain management during labour and childbirth. Cochrane Database of Systematic Reviews. 2012;11:CD009356. doi: 10.1002/14651858.CD009356.pub2. [DOI] [PubMed] [Google Scholar]
- Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2000;68(3):438–450. [PubMed] [Google Scholar]
- Michels L, Bucher K, Luchinger R, Klaver P, Martin E, Jeanmonod D, Brandeis D. Simultaneous EEG-fMRI during a working memory task: modulations in low and high frequency bands. PLoS One. 2010;5(4):e10298. doi: 10.1371/journal.pone.0010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milling LS. The Spanos Attitudes Toward Hypnosis Questionnaire: psychometric characteristics and normative data. American Journal of Clinical Hypnosis. 2012;54(3):202–212. doi: 10.1080/00029157.2011.631229. [DOI] [PubMed] [Google Scholar]
- Milling LS, Breen AC. Mediation and moderation of hypnotic and cognitive behavioural pain reduction. Contemporary Hypnosis. 2003;20:81–97. [Google Scholar]
- Milling LS, Kirsch I, Meunier SA, Levine MR. Hypnotic analgesia and stress inocluation training: Individual and combined effects in analog treatment of experimental pain. Cognitive Therapy and Research. 2002;26:355–371. [Google Scholar]
- Milling LS, Levine MR, Meunier SA. Hypnotic enhancement of cognitive-behavioral interventions for pain: an analogue treatment study. Health Psychology. 2003;22(4):406–413. doi: 10.1037/0278-6133.22.4.406. [DOI] [PubMed] [Google Scholar]
- Milling LS, Reardon JM, Carosella GM. Mediation and moderation of psychological pain treatments: response expectancies and hypnotic suggestibility. Journal of Consulting and Clinical Psychology. 2006;74(2):253–262. doi: 10.1037/0022-006X.74.2.253. [DOI] [PubMed] [Google Scholar]
- Montgomery DD, Dwyer KV, Kelly SM. Relationship between QEEG relative power and hypnotic susceptibility. American Journal of Clinical Hypnosis. 2000;43(1):71–75. doi: 10.1080/00029157.2000.10404256. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Bovbjerg DH. Presurgery distress and specific response expectancies predict postsurgery outcomes in surgery patients confronting breast cancer. Health Psychology. 2004;23(4):381–387. doi: 10.1037/0278-6133.23.4.381. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, DuHamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis? International Journal of Clinical and Experimental Hypnosis. 2000;48(2):138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Hallquist MN, Schnur JB, David D, Silverstein JH, Bovbjerg DH. Mediators of a brief hypnosis intervention to control side effects in breast surgery patients: response expectancies and emotional distress. Journal of Consulting and Clinical Psychology. 2010;78(1):80–88. doi: 10.1037/a0017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery GH, Weltz CR, Seltz M, Bovbjerg DH. Brief presurgery hypnosis reduces distress and pain in excisional breast biopsy patients. International Journal of Clinical and Experimental Hypnosis. 2002;50(1):17–32. doi: 10.1080/00207140208410088. [DOI] [PubMed] [Google Scholar]
- Morgan AH, Macdonald H, Hilgard ER. EEG alpha: Lateral asymmetry related to task, and hypnotizability. Psychophysiology. 1974;11(3):275–282. doi: 10.1111/j.1469-8986.1974.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Muller K, Bacht K, Schramm S, Seitz RJ. The facilitating effect of clinical hypnosis on motor imagery: an fMRI study. Behav Brain Res. 2012;231(1):164–169. doi: 10.1016/j.bbr.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Nash MR. A psychoanalytic theory of hypnosis: A clinically informed approach. In: Nash MR, Barnier AJ, editors. The oxford handbook of hypnosis: Theory, research, and practice. Oxford, UK: Oxford University Press; 2008. pp. 201–222. [Google Scholar]
- Novy DM, Nelson DV, Francis DJ, Turk DC. Perspectives of chronic pain: an evaluative comparison of restrictive and comprehensive models. Psychological Bulletin. 1995;118:238–247. doi: 10.1037/0033-2909.118.2.238. [DOI] [PubMed] [Google Scholar]
- Oakley DA. Hypnosis, trance and suggestion: evidence from neuroimaging. In: Nash MR, Barnier A, editors. The oxford handbook of hypnosis: Theory, research and practice. Oxford, UK: Oxford University Press; 2008. pp. 365–392. [Google Scholar]
- Oakley DA, Halligan PW. Psychophysiological foundations of hypnosis and suggestion. In: Lynn SJ, Rhue JW, Kirsch I, editors. Handbook of clinical hypnosis. 2nd ed. Washington, DC: American Psychological Association; 2010. pp. 79–117. [Google Scholar]
- Patterson DR, Adcock RJ, Bombardier CH. Factors predicting hypnotic analgesia in clinical burn pain. International Journal of Clinical and Experimental Hypnosis. 1997;45(4):377–395. doi: 10.1080/00207149708416139. [DOI] [PubMed] [Google Scholar]
- Patterson DR, Jensen M. Hypnosis and clinical pain. Psychological Bulletin. 2003;29:495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- Poulsen BC, Mathews WJ., Jr Correlates of imaginative and hypnotic suggestibility in children. Contemporary Hypnosis. 2003;20:198–208. [Google Scholar]
- Pyka M, Burgmer M, Lenzen T, Pioch R, Dannlowski U, Pfleiderer B, Ewert AW, Konrad C. Brain correlates of hypnotic paralysis—a resting-state fMRI study. Neuroimage. 2011;56:2173–2182. doi: 10.1016/j.neuroimage.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Radtke HL, Stam HJ. What happened to the social in contemporary accounts of hypnosis? Contemporary Hypnosis. 2008;25:192–201. [Google Scholar]
- Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82(2):159–171. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. Journal of Cognitive Neuroscience. 2002;14:887–901. doi: 10.1162/089892902760191117. [DOI] [PubMed] [Google Scholar]
- Rainville P, Price DD. The neurophemomenology of hypnosis and hypnotic analgesia. In: Price DD, Bushnell MC, editors. Psychological methods of pain control: Pain science and clinical perspectives, Progress in pain research and managment. Vol. 29. Seattle: IASP Press; 2004. [Google Scholar]
- Ray WJ. EEG concomitants of hypnotic susceptibility. International Journal of Clinical and Experimental Hypnosis. 1997;45(3):301–313. doi: 10.1080/00207149708416131. [DOI] [PubMed] [Google Scholar]
- Rutten JM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child. 2013;98(4):252–257. doi: 10.1136/archdischild-2012-302906. [DOI] [PubMed] [Google Scholar]
- Sabourin ME, Cutcomb SD, Crawford HJ, Pribram K. EEG correlates of hypnotic susceptibility and hypnotic trance: Spectral analysis and coherence. International Journal of Psychophysiology. 1990;10:125–142. doi: 10.1016/0167-8760(90)90027-b. [DOI] [PubMed] [Google Scholar]
- Santarcangelo EL, Paoletti G, Balocchi R, Carli G, Morizzo C, Palombo C, Varanini M. Hypnotizability modulates the cardiovascular correlates of subjective relaxation. International Journal of Clinical and Experimental Hypnosis. 2012;60(4):383–396. doi: 10.1080/00207144.2012.700609. [DOI] [PubMed] [Google Scholar]
- Santarcangelo EL, Paoletti G, Chiavacci I, Palombo C, Carli G, Varanini M. Cognitive modulation of psychophysical, respiratory and autonomic responses to cold pressor test. PLoS One. 2013;8(10):e75023. doi: 10.1371/journal.pone.0075023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarcangelo EL, Varanini M, Paoletti G, Castellani E, Palombo C, Carli G. Pain-inducing imagery as a function of hypnotisability and of the activity of Gray's Behavioral Inhibition/Activation Systems. Neuroscience Letters. 2013 doi: 10.1016/j.neulet.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Allen JJ. Attention-related electroencephalographic and event-related potential predictors of responsiveness to suggested posthypnotic amnesia. International Journal of Clinical and Experimental Hypnosis. 1995;43(3):295–315. doi: 10.1080/00207149508409972. [DOI] [PubMed] [Google Scholar]
- Schotte CK, Van Den Bossche B, De Doncker D, Claes S, Cosyns P. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23(5):312–324. doi: 10.1002/da.20177. [DOI] [PubMed] [Google Scholar]
- Sebastiani L, Simoni A, Gemignani A, Ghelarducci B, Santarcangelo EL. Autonomic and EEG correlates of emotional imagery in subjects with different hypnotic susceptibility. Brain Research Bulletin. 2003;60(1–2):151–160. doi: 10.1016/s0361-9230(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Semmens-Wheeler R, Dienes Z, Duka T. Alcohol increases hypnotic susceptibility. Conscious Cognition. 2013;22(3):1082–1091. doi: 10.1016/j.concog.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Sheehan PW. Factors influencing rapport in hypnosis. Journal of Abnormal Psychology. 1980;89(2):563–581. [PubMed] [Google Scholar]
- Silva CE, Kirsch I. Interpretive sets, expectancy, fantasy proneness, and dissociation as predictors of hypnotic response. Journal of Personality and Social Psychology. 1992;63(5):847–856. doi: 10.1037//0022-3514.63.5.847. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Identification of causal parameters in randomized studies with medating variables. Journal of Education and Behavioral Statistics. 2008;33:230–251. [Google Scholar]
- Spanos NP. Hypnotic behavior: A social-psychological interpretation of amnesia, analgesia, and “trance logic". Behavioral and Brain Sciences. 1986;9:449–502. [Google Scholar]
- Spanos NP. A sociocognitive approach to hypnosis. In: Lynn SJ, Rhue JW, editors. Theories of Hypnosis: Current Models and Perspectives. New York: Guilford Press; 1991. pp. 324–361. [Google Scholar]
- Spanos NP, Brett PJ, Menary EP, Cross WP. A measure of attitudes toward hypnosis: relationships with absorption and hypnotic susceptibility. American Journal of Clinical Hypnosis. 1987;30(2):139–150. doi: 10.1080/00029157.1987.10404174. [DOI] [PubMed] [Google Scholar]
- Spanos NP, Chaves JF, editors. Hypnosis: The cognitive-behavioral perspective. Buffalo, NY: Promethus Books; 1989. [Google Scholar]
- Spanos NP, Coe WC. A social-psychological approach to hypnosis. In: Fromm E, Nash MR, editors. Contemporary hypnosis research. New York: Guilford Press; 1992. pp. 102–130. [Google Scholar]
- Spanos NP, Gabora NJ, Jarrett LE, Gwynn MI. Contextual determinants of hypnotizability and of relationships between hypnotizability scales. Journal of Personality and Social Psychology. 1989;57:271–278. [Google Scholar]
- Spanos NP, Kennedy SK, Gwynn MI. Moderating effects of contextual variables on the relationship between hypnotic susceptibility and suggested analgesia. Journal of Abnormal Psychology. 1984;93:285–294. doi: 10.1037//0021-843x.93.3.285. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Intelligent design or designed intelligence? Hypnotizability as neurobiological adaptation. In: Nash MR, Barnier AJ, editors. The Oxford handbook of hypnosis: Theory, research, and practice. Oxford: Oxford University Press; 2008. [Google Scholar]
- Spiegel H. The neural trance: A new look at hypnosis. International Journal of Clinical and Experimental Hypnosis. 2007;55:387–410. doi: 10.1080/00207140701506367. [DOI] [PubMed] [Google Scholar]
- Tan G, Hammond DC, Joseph G. Hypnosis and irritable bowel syndrome: a review of efficacy and mechanism of action. American Journal of Clinical Hypnosis. 2005;47(3):161–178. doi: 10.1080/00029157.2005.10401481. [DOI] [PubMed] [Google Scholar]
- Tebecis AK, Provins KA, Farnbach RW, Pentony P. Hypnosis and the EEG. A quantitative investigation. Journal of Nervous and Mental Disease. 1975;161(1):1–17. doi: 10.1097/00005053-197507000-00001. [DOI] [PubMed] [Google Scholar]
- Tomé-Pires C, Miró J. Hypnosis for the management of chronic and cancer procedure-related pain in children. International Journal of Clinical and Experimental Hypnosis. 2012;60:432–457. doi: 10.1080/00207144.2012.701092. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Boly M, Balteau E, Schnakers C, Moonen G, Luxen A, Faymonville ME. Pain and non-pain processing during hypnosis: a thulium-YAG event-related fMRI study. NeuroImage. 2009;47(3):1047–1054. doi: 10.1016/j.neuroimage.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Varga K. The phenomenology of hypnotic interactions. New York: Nova; 2013. [Google Scholar]
- Varga K, Banyai EI, Gosi-Greguss AC. Parallel application of the experiential analysis technique with subject and hypnotist: a new possibility for measuring interactional synchrony. International Journal of Clinical and Experimental Hypnosis. 1994;42(2):130–139. doi: 10.1080/00207149408409346. [DOI] [PubMed] [Google Scholar]
- Varga K, Banyai EI, Gosi-Greguss AC, Tauszik K. Phenomenological aspects of hypnotic interactions: the effect of kinship. International Journal of Clinical and Experimental Hypnosis. 2013;61(4):401–415. doi: 10.1080/00207144.2013.810476. [DOI] [PubMed] [Google Scholar]
- Varga K, Józsa E, Bányai ÉI, Gösi-Greguss AC. A new way of characterizing hypnotic interactions: Dyadic Interactional Harmony (DIH) Questionnaire. Contemporary Hypnosis. 2001;24:151–166. [Google Scholar]
- Varga K, Józsa E, Bányai ÉI, Gösi-Greguss AC. Patterns of interactional harmony: The Phenomenology of hypnosis interaction. In: Koester GD, Delisle PR, editors. Hypnosis: Theories, research, and applications. New York: Nova Science Publishers; 2009. [Google Scholar]
- Varga K, Jozsa E, Banyai EI, Gosi-Greguss AC, Kumar VK. Phenomenological experiences associated with hypnotic susceptibility. International Journal of Clinical and Experimental Hypnosis. 2001;49(1):19–29. doi: 10.1080/00207140108410376. [DOI] [PubMed] [Google Scholar]
- Varga K, Kekecs Z. Oxytocin and cortisol in the hypnotic interaciton. International Journal of Clinical and Experimental Hypnosis. 2014;62:84–110. doi: 10.1080/00207144.2013.841494. [DOI] [PubMed] [Google Scholar]
- Vogel W, Broverman DM, Klaiber EL. EEG and mental abilities. Electroencephalogr Clin Neurophysiol. 1968;24(2):166–175. doi: 10.1016/0013-4694(68)90122-3. [DOI] [PubMed] [Google Scholar]
- Wagstaff GF, David D, Kirsch I, Lynn SJ. The cognitive-behavioral model of hypnotherapy. In: Lynn SJ, Rhue JW, Kirsch I, editors. Handbook of clinical hypnosis. 2nd edition ed. Washington, DC: American Psychological Association; 2010. pp. 179–208. [Google Scholar]
- Welch L. A behavioristic explanation of the mechanism of suggestion and hypnosis. Journal of Abnormal Psychology. 1947;42(3):359–364. doi: 10.1037/h0058998. [DOI] [PubMed] [Google Scholar]
- White D, Ciorciari J, Carbis C, Liley D. EEG correlates of virtual reality hypnosis. International Journal of Clinical and Experimental Hypnosis. 2009;57(1):94–116. doi: 10.1080/00207140802463690. [DOI] [PubMed] [Google Scholar]
- White RW. An analysis of motivation in hypnosis. Journal of General Psychology. 1941;24:145–162. [Google Scholar]
- Wik G, Fischer H, Bragee B, Finer B, Fredrikson M. Functional anatomy of hypnotic analgesia: a PET study of patients with fibromyalgia. European Journal of Pain. 1999;3(1):7–12. doi: 10.1053/eujp.1998.0093. [DOI] [PubMed] [Google Scholar]
- Williams JD, Gruzelier JH. Differentiation of hypnosis and relaxation by analysis of narrow band theta and alpha frequencies. International Journal of Experimental Hypnosis. 2001;49:185–206. doi: 10.1080/00207140108410070. [DOI] [PubMed] [Google Scholar]
- Woody EZ, Sadler P. Dissociation theories of hypnosis. In: Nash MR, Barnier A, editors. The oxford handbook of hypnosis: Theory, research, and practice. Oxford, UK: Oxford University Press; 2008. pp. 81–110. [Google Scholar]
- Yuksel R, Ozcan O, Dane S. The effects of hypnosis on heart rate variability. International Journal of Clinical and Experimental Hypnosis. 2013;61(2):162–171. doi: 10.1080/00207144.2013.753826. [DOI] [PubMed] [Google Scholar]


