Abstract
Background
Effects were compared in patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial of 2 mechanistically different strategies for treatment of hyperglycemia, insulin-sensitizing and insulin-providing strategies, on biomarker profiles reflecting the balance between fibrinolysis and thrombosis and the intensity of inflammation implicated in diabetic vasculopathy.
Methods and Results
A total of 2368 patients with type 2 diabetes mellitus and clinically stable, angiographically documented coronary artery disease were randomized to treatment with 1 of the 2 strategies and followed for an average of 5 years. Plasminogen activator inhibitor type 1 antigen and activity, tissue plasminogen activator antigen, fibrinogen, D-dimer, C-reactive protein, insulin, and hemoglobin A1c were assayed in blood samples acquired at baseline and at 12 regular intervals throughout the follow-up interval. Higher baseline D-dimer, fibrinogen, and C-reactive protein portended a poor prognosis in patients in both groups. In contrast to the insulin-providing strategy, the insulin-sensitizing strategy led to (1) lower plasma insulin; (2) lower plasminogen activator inhibitor type 1 antigen and activity and lower tissue plasminogen activator antigen (known to track with plasminogen activator inhibitor type 1); and (3) lower C-reactive protein and fibrinogen at all intervals after baseline (P<0.001 for each).
Conclusions
The insulin-sensitizing treatment strategy led to changes in biomarker profiles indicative of decreased insulin resistance, an altered balance between thrombosis and fibrinolysis favoring fibrinolysis, and diminished intensity of the systemic inflammatory state, factors that have been associated with cardiovascular risk.
Keywords: diabetes mellitus, fibrinolysis, mortality, myocardial infarction, revascularization
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial was designed to determine whether early revascularization and the use of insulin-sensitizing drugs reduce the 2- to 4-fold greater incidence of coronary artery disease and cardiovascular events including death and myocardial infarction (MI) occurring in patients with type 2 diabetes mellitus (DM) and angiographically proven coronary artery disease. A complex diabetic vasculopathy underlies the poorer outcomes in patients with DM. It is thought to result in part from augmented inflammation and impaired fibrinolysis. Although the incidences of cardiovascular events in BARI 2D were comparable overall in both glycemic control arms of the trial,1,2 we sought to explore baseline and subsequent biomarker profiles associated with a random assignment to an insulin-sensitizing (IS) or insulin-providing (IP) strategy for glycemic control. Our objective was to gain insights pertinent to the impact of the 2 strategies on potentially pathophysiological determinants of long-term prognosis indicative of inflammation and constrained fibrinolysis.
Methods
The BARI 2D clinical trial compared an IS strategy for treatment of hyperglycemia employing primarily metformin and/or a thiazolidinedione drug with an IP strategy employing primarily a sulfonylurea and/or a meglitinide drug. A total of 2368 patients with type 2 DM and clinically stable angiographically documented coronary artery disease without the need for immediate revascularization were randomized simultaneously to prompt revascularization or initial medical therapy alone with deferred revascularization as needed and to an IS or IP strategy targeting hemoglobin A1c (HbA1c) at <7.0%. The trial was not designed to compare specific drugs but rather to compare the 2 mechanistically different treatment strategies per se. Patients in the IS arm taking an IS drug at baseline continued their regimen with dose adjustments as necessary and had IP drugs withdrawn at a pace consistent with their HbA1c values and symptoms. Patients in the IP arm taking IS drugs at baseline had them withdrawn similarly. If a patient's HbA1c exceeded 8.0% on their assigned drugs, drugs from the opposite arm were given.
Assays of Biomarkers
As described in detail in Appendix A in the online-only Data Supplement, plasminogen activator inhibitor type 1 (PAI-1) antigen, PAI-1 activity, tissue plasminogen activator (tPA) antigen, and insulin were measured in citrated plasma in the fibrinolysis core laboratory at the University of Vermont in samples obtained at baseline and at 1, 3, and 6 months and every 6 months thereafter over 5 years. tPA complexes with excess PAI-1 in plasma; its activity was not measured because it is undetectable. In all patients who were enrolled in the BARI 2D trial during the first 3 years, an ancillary study supported by a National Institutes of Health R01 grant (B.E.S., Principal Investigator) was performed. For that study, samples were obtained over 18 months and processed and shipped to the University of Vermont BARI 2D fibrinolysis core laboratory in the same fashion as samples for the core study. The samples were assayed for fibrinopeptide A (FPA) (in heparinized plasma samples), D-dimer, fibrinogen, and C-reactive protein (CRP) (in citrated samples). HbA1c was assayed in EDTA whole blood samples by cation-exchange high-performance liquid chromatography2 in the BARI 2D biochemistry laboratory in Minneapolis or core laboratories in Brazil and Europe. The numbers of assayable samples of each type (core and ancillary study) obtained at each sampling interval are shown in Table I in Appendix A in the online-only Data Supplement.
Statistical Analyses
Cross-sectional box plots were constructed for the core laboratory– assayed analytes at baseline and 1 and 3 years and at baseline and 1 year for the ancillary study–supported measures. Means and SDs are presented for normally distributed variables; medians and interquar-tile ranges are presented for skewed variables. Spearman correlations are presented for the analytes. Wilcoxon rank sum tests and t tests were used as appropriate to determine the significance of differences observed between treatment groups. Analyte values >5 SDs above the mean were considered to be outliers and were excluded. Values of 0.0 were set to 0.01. Skewed variables are transformed with the use of natural log transformations.
Mixed models with a random intercept are used to account for the covariability among multiple measures for each patient during the course of the study. The mixed models use maximum likelihood methods, and they accommodate data that are missing at random (ie, missing data are random conditional on the variables included in the model). These models are used to estimate the effect of time on each of the analytes for each of the treatment arms. An interaction was added to each model to detect differences in the time effect between the treatment arms. Mixed models were also used to estimate the effect of the antiglycemic drugs on repeated measures of each analyte. These models were adjusted for baseline factors observed to be different among patients with comparable concentrations and activities of the analytes and concurrent HbA1c.
Kaplan–Meier estimates for death and death/MI/stroke are presented for patients in each baseline quintile of the analytes. The log-rank statistic was used to test for differences among the quintile event distributions for each analyte. Cox proportional hazard models were used to test for an interaction between each of the baseline analytes and the IP compared with the IS treatment arms.
Adjusted Cox proportional hazard models with time-varying analytes were used to estimate effects of the analytes on the outcomes of death, death/MI/stroke, and subsequent revascularization procedures and to examine the potentially mediating effects of the analytes in the causal pathway between the randomization treatments and the cardiovascular disease outcomes. These models are adjusted for baseline differences and factors known to be related to the outcomes. Values for the analytes are updated when a new measurement is obtained. For the survival end point, patients without events are censored at the last time they are known to be alive. For the other outcomes, patients not experiencing the events are censored at the time of the last mandated protocol follow-up. A subsequent revascularization procedure is defined as the first nonprotocol revascularization procedure for patients in the optimal medical treatment alone arm or a second revascularization procedure for patients in the prompt revascularization arm. The coefficients from the Cox models for the log-transformed analytes are algebraically converted to represent the hazard ratio for a 10% increase in the analyte.
Nominal P values are reported. Because our focus was on 7 specified analytes, a Bonferroni correction (0.05/7=0.007) of the P value was used to determine statistical significance.
SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all analyses.
Results
Medications Used for Control of Hyperglycemia
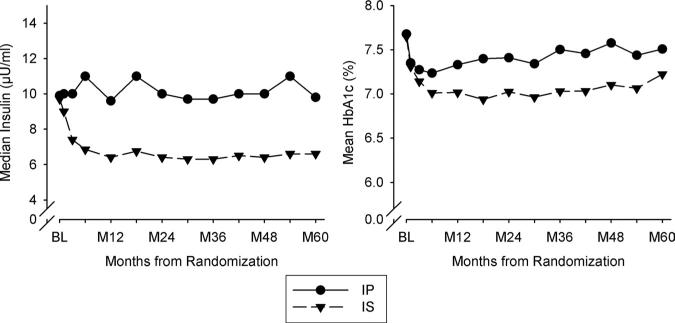
Medication use (Table 1) and HbA1c (Figure 1) at baseline were comparable in patients in the IS and IP groups. By 6 months and thereafter, patients in the IS compared with the IP treatment arm had significantly lower mean HbA1c (P<0.002 for each time point) (absolute difference=0.4%) (Figure 1). Use of IS drugs in the IP group was rare (11%). Thirty percent of patients in the IS group were taking insulin, primarily those on insulin at baseline for whom it could not be withdrawn. However, exogenous insulin exposure was only 0.19 U/kg per day in patients in the IS arm compared with 0.53 U/kg per day in those in the IP arm. Median insulin was comparable in the 2 groups at baseline (Figure 1). Subsequent values undoubtedly reflected the impact of exogenous insulin. HbA1c was <8% in most patients throughout the trial (Figure 1).
Table 1.
Drug Use for Treatment of Diabetes Mellitus at Follow-Up*
| Randomized to IS Treatment, % |
Randomized to IP Treatment, % |
|||||
|---|---|---|---|---|---|---|
| Baseline (n=1157) | Year 1 (n=1115) | Year 3 (n=988) | Baseline (n=1179) | Year 1 (n=1099) | Year 3 (n=985) | |
| Neither IS nor IP drugs | 9.2 | 4.0 | 3.9 | 8.7 | 10.7 | 9.1 |
| IS only | 17.4 | 59.9 | 53.7 | 16.4 | 0.8 | 1.0 |
| IP only | 28.2 | 4.7 | 7.1 | 33.3 | 81.0 | 80.0 |
| Both IS and IP | 45.3 | 31.4 | 35.3 | 41.7 | 7.5 | 9.9 |
| IS drug class | ||||||
| No thiazolidinedione or metformin | 37.3 | 8.7 | 10.9 | 41.9 | 91.7 | 89.1 |
| Thiazolidinedione, no metformin | 7.4 | 10.8 | 13.5 | 6.0 | 0.8 | 1.3 |
| Metformin, no thiazolidinedione | 42.4 | 21.3 | 24.8 | 40.5 | 6.1 | 7.7 |
| Both thiazolidinedione and metformin | 12.9 | 59.2 | 50.8 | 11.5 | 1.4 | 1.8 |
| IP drug class | ||||||
| No insulin or sulfonylurea | 26.5 | 64.0 | 57.6 | 25.1 | 11.6 | 10.2 |
| Sulfonylurea, no insulin | 46.2 | 11.6 | 13.0 | 46.0 | 36.3 | 29.4 |
| Insulin, no sulfonylurea | 20.4 | 22.2 | 25.4 | 22.1 | 31.9 | 36.5 |
| Both insulin and sulfonylurea | 6.9 | 2.2 | 4.1 | 6.8 | 20.2 | 24.0 |
IS indicates insulin-sensitizing; IP, insulin-providing.
86% of the 3507 person-year exposure to thiazolidinediones was with rosiglitazone; 14% was with pioglitazone.
Figure 1.
Median insulin and mean HbA1c over time of follow-up (months after randomization) in patients treated with an insulin-providing (IP) or insulin-sensitizing (IS) strategy. HbA1c indicates hemoglobin A1c; BL, baseline.
Biomarkers in the Overall Population
Baseline characteristics of all patients are shown in Table 2. Patients with higher PAI-1 activity were paradoxically younger, as reported previously.3 They were also more often non-Hispanic white and had higher body mass indexes and shorter duration of DM.
Table 2.
Baseline Characteristics of Patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial
| Characteristic | Total (n=2238) |
|---|---|
| Gender, % | |
| Male | 70.1 |
| Female | 29.9 |
| Age at study entry, mean (SD), y | 62.5 (8.9) |
| Race/ethnicity, % | |
| White non-Hispanic | 68.7 |
| Black non-Hispanic | 17.3 |
| Hispanic | 9.1 |
| Asian non-Hispanic | 4.3 |
| Other non-Hispanic | 0.6 |
| Body mass index, mean (SD) | 31.9 (5.6) |
| Measured ankle-brachial index, mean (SD) | 1.05 (0.23) |
| History of myocardial infarction, % | 30.7 |
| History of congestive heart failure requiring treatment, % | 6.9 |
| Peripheral or carotid artery disease, % | 24.6 |
| Chronic renal dysfunction, % | 3.1 |
| Classic angina ever, % | 72.6 |
| Anginal equivalents ever, % | 73.6 |
| Duration of diabetes mellitus, mean (SD) | 10.4 (8.7) |
| Currently taking insulin, % | 28.5 |
| Core: Hemoglobin A1c, mean (SD), % | 7.64 (1.6) |
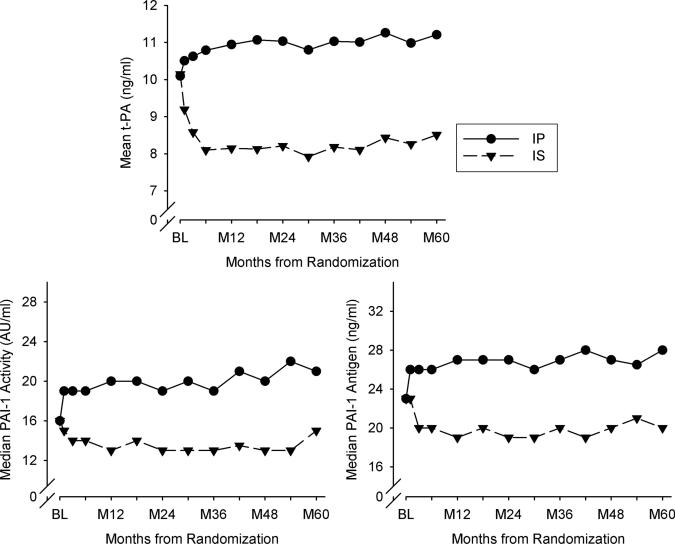
PAI-1 antigen, PAI-1 activity, tPA, and the tPA/PAI-1 antigen ratio at baseline and at 1 and 3 years for all patients were widely distributed. Means and medians in the overall population were stable over time (Figure I in Appendix B in the online-only Data Supplement). However, directionally opposite changes over time occurred in the patients in the IP compared with the IS arm (Figures 1 and 2). Fibrinogen, CRP, and FPA decreased significantly over time in the population as a whole (P<0.001 for each) (Figure II in Appendix B in the online-only Data Supplement). PAI-1 antigen and PAI-1 activity (measured with independent as-says) were highly correlated (Table I in Appendix B in the online-only Data Supplement). Of interest, correlations between analytes and HbA1c were low even when statistically significant (Table I in Appendix B in the online-only Data Supplement).
Figure 2.
Concentrations in blood or activity of biomarkers over time. tPA indicates tissue plasminogen activator; BL, baseline; IP, insulin-providing treatment strategy; IS, insulin-sensitizing treatment strategy; and PAI-1, plasminogen activator inhibitor type 1.
Time-Dependent Changes in Analytes
As shown in Figure 2, at baseline, concentrations of analytes were virtually identical in patients in the 2 treatment arms. Concentrations and activity of all 3 analytes were lower during follow-up of the trial in patients in the IS compared with the IP arm [IS versus IP estimated difference for ln(PAI-1 antigen) β=–0.26, for ln(PAI-1 activity) β=–0.25, for tPA β=–2.34; P<0.001 for each]. Concentrations of tPA are known to parallel those of PAI-1 antigen because of formation of complexes.4 tPA and PAI-1 antigen increased significantly in patients in the IP arm (P<0.001 for both). PAI-1 activity increased as well, although not significantly, probably because of attenuation by formation of complexes with the increased tPA.
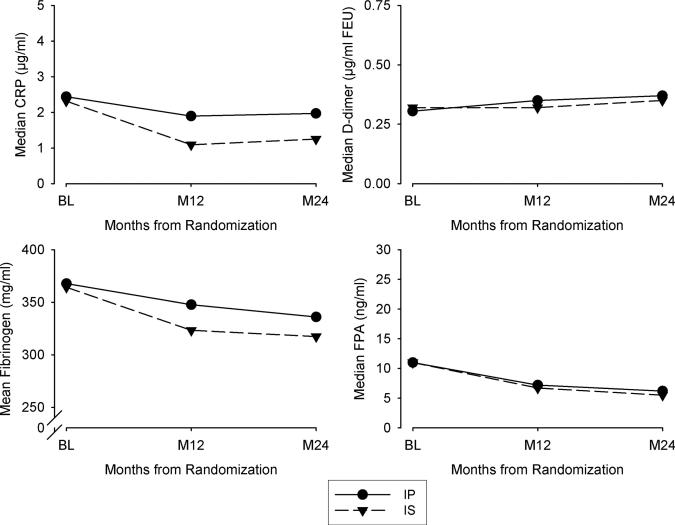
Patients in both treatment arms had similar declines in fibrinogen over time (Figure 3). However, those in the IS arm had lower values at 1 and 2 years than those in the IP arm (β=–21.71, P<0.001). Changes in D-dimer and FPA were comparable in patients in the 2 groups. Patients in the IS arm had significantly lower concentrations of CRP throughout follow-up [log(CRP) β=–0.49, P<0.001].
Figure 3.
Median or mean values of the analytes specified over 24 months. CRP indicates C-reactive protein; BL, baseline; FEU, fibrinogen equivalent unit; FPA, fibrinopeptide A; IP, insulin-providing treatment strategy; and IS, insulin-sensitizing treatment strategy.
Table 3 shows estimates of the independent effect of medications used to treat hyperglycemia on the concentrations or activities of the analytes after adjustment for baseline imbalances and changes in drug use and concentrations of HbA1c during the trial. PAI-1 antigen, PAI-1 activity, and tPA decreased more with the use of insulin sensitizers (thiazolidinediones or metformin). Thiazolidinedione use was associated with a 1.57-ng/mL lower concentration of tPA and a 15% lower concentration of PAI-1 antigen. Thus, the IS compared with the IP strategy was associated with a marked and directionally different change in the biomarker profile indicative of a shift in the balance between fibrinolysis and thrombosis favoring fibrinolysis. The same was true with respect to reduction of intensity of inflammation reflected by CRP in patients in the IS arm (Table 3).
Table 3.
Estimates of Effects of Drugs Used in IS and IP Treatment Strategy on Repeated Measures of Analytes*
| IP Drugs |
IS Drugs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Insulin | P | Sulfonylurea | P | Thiazolidinedione | P | Biguanide | P |
| tPA, ng/mL | 0.37 | <0.0001 | 0.24 | <0.0001 | –1.57 | <0.0001 | –0.83 | <0.0001 |
| log(PAI-1 antigen) | 0.031 | 0.011 | 0.078 | <0.0001 | –0.16 | <0.0001 | –0.070 | <0.0001 |
| log(PAI-1 activity) | 0.029 | 0.060 | 0.11 | <0.0001 | –0.16 | <0.0001 | –0.062 | <0.0001 |
| log(CRP) | 0.17 | 0.0011 | 0.022 | 0.64 | –0.33 | <0.0001 | –0.17 | 0.0033 |
| log(FPA) | 0.062 | 0.27 | –0.061 | 0.25 | –0.09 | 0.15 | –0.12 | 0.07 |
| log(D-dimer) | 0.031 | 0.44 | –0.024 | 0.52 | 0.004 | 0.93 | –0.21 | <0.0001 |
| Fibrinogen, mg/dL | 16.6 | <0.0001 | 9.33 | 0.019 | –5.02 | 0.30 | –13.4 | 0.006 |
IP indicates insulin-providing; IS, insulin-sensitizing; tPA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor type 1; CRP, C-reactive protein; and FPA, fibrinopeptide A. The P value indicates the significance level of the estimated drug effect on the analyte.
Analysis also includes indicators for follow-up period of analyte measure, IP/IS randomization, age, gender, race/ethnicity, baseline body mass index, duration of diabetes mellitus, and concurrent hemoglobin A1c.
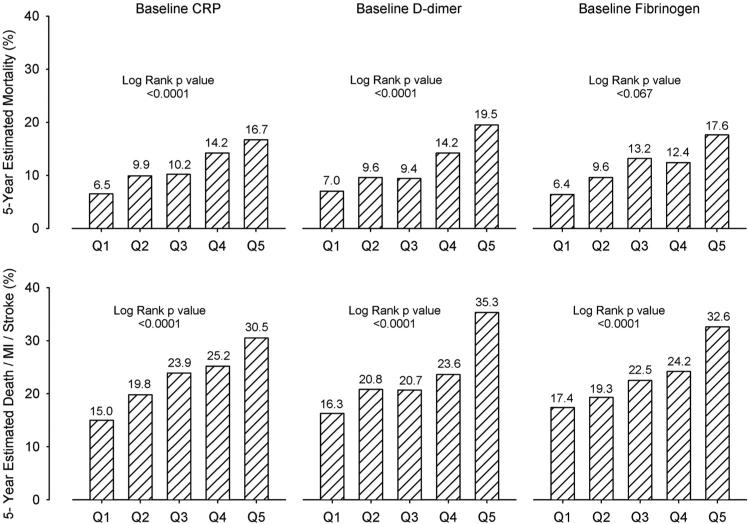
The baseline values for tPA, PAI-1 antigen and activity, and FPA were not significantly associated with the primary or principal secondary end points in the BARI 2D trial as a whole (log-rank P values >0.05) (Figure III in Appendix B in the online-only Data Supplement). However, there was striking predictive power for high baseline D-dimer, fibrinogen, and CRP for mortality and the incidence of death/MI/stroke (P<0.0001) in patients in both arms (all interaction P values >0.20), as shown in Figure 4.
Figure 4.
Kaplan–Meier 5-year rate estimates for mortality and major cardiovascular events stratified by baseline values of the analytes specified. CRP indicates C-reactive protein; Q, quintile; and MI, myocardial infarction.
The adjusted hazard ratio estimates shown in Table 4 indicate that increased D-dimer (P<0.001) was significantly associated with greater all-cause mortality, as were increased fibrinogen (P<0.001) and CRP (P<0.001). A 10% increase in D-dimer was associated with a hazard ratio for death of 1.04. The hazard ratio for a 10% increase in fibrinogen was 1.08. Similar associations were seen for the incidence of death/MI/stroke (Table 4). Furthermore, higher concentrations of CRP (P<0.001) and PAI-1 activity (P<0.005) were associated with a greater need for subsequent revascularization procedures. When multivariate analyses including all of the analytes were considered and results were adjusted for concurrent HbA1c, higher CRP and D-dimer were independently and significantly associated with the risk of death/MI/stroke (Table 5). Thus, the hazard ratios were 1.02 for both, with P values of 0.0002 and <0.0001, respectively. Consistent with the results in the primary outcome article from BARI 2D published previously,1 the randomized glycemic control strategy (IP compared with IS) was not associated with a difference in clinical outcomes with or without adjustment for the analytes.
Table 4.
Estimates of Time-Varying Effects of Single Analytes on Clinical Outcomes
| Death* |
Death/MI/Stroke* |
Subsequent Procedure* |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio per 10% Increase of Analyte (95% CI) | P | Hazard Ratio per 10% Increase of Analyte (95% CI) | P | Hazard Ratio per 10% Increase of Analyte (95% CI) | P | |
| tPA, ng/mL | 1.03 (1.0–1.05) | 0.033 | 1.01 (0.99–1.03) | 0.26 | 1.001 (0.999–1.003) | 0.23 |
| PAI-1 antigen | 1.01 (0.99–1.30) | 0.25 | 1.00 (0.99–1.02) | 0.66 | 1.01 (1.00–1.03) | 0.052 |
| PAI-1 activity | 0.99 (0.98–1.0) | 0.095 | 0.99 (0.98–1.00) | 0.24 | 1.02 (1.01–1.03) | 0.0032 |
| CRP | 1.03 (1.02–1.04) | <0.0001 | 1.03 (1.02–1.03) | <0.0001 | 1.01 (1.01–1.02) | <0.0001 |
| FPA | 1.01 (1.0–1.05) | 0.13 | 1.01 (1.0–1.01) | 0.10 | 1.00 (0.99–1.01) | 0.73 |
| D-Dimer | 1.04 (1.02–1.05) | <0.0001 | 1.03 (1.02–1.04) | <0.0001 | 1.00 (0.99–1.01) | 0.83 |
| Fibrinogen, mg/dL | 1.08 (1.04–1.13) | <0.0001 | 1.07 (1.04–1.11) | <0.0001 | 1.03 (1.00–1.06) | 0.016 |
CI indicates confidence interval; tPA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor type 1; CRP, C-reactive protein; and FPA, fibrinopeptide A. Hazard ratio is presented for each 10% increase in the analyte. Each row reports a separate Cox regression analysis for each analyte to determine its relationship with the outcomes.
Death analysis includes insulin-providing randomization, age, sex, race/ethnicity, history of congestive heart failure, baseline body mass index, hemoglobin A1c, renal disease, ankle-brachial index category, peripheral or carotid artery disease, and number of significant lesions. Death/MI/stroke analysis includes insulin-providing randomization, baseline factors of sex, age, race/ethnicity, baseline body mass index, hemoglobin A1c, congestive heart failure, myocardial infarction history, renal disease, ankle-brachial index category, and number of significant lesions. Subsequent procedure analysis includes insulin-providing randomization, age, sex, race/ethnicity, baseline factors of body mass index, hemoglobin A1c, angina status, and Myocardial Jeopardy Index.
Table 5.
Estimates of Time-Varying Effects of Multiple Analytes on Death/Myocardial Infarction/Stroke*
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| PAI-1 antigen (per 10% increase) | 1.01 (0.99–1.04) | 0.28 |
| PAI-1 activity (per 10% increase) | 0.98 (0.97–1.00) | 0.056 |
| CRP (per 10% increase) | 1.02 (1.01–1.03) | 0.0002 |
| D-Dimer (per 10% increase) | 1.02 (1.01–1.04) | <0.0001 |
| Fibrinogen (per 10% increase) | 1.02 (0.98–1.06) | 0.42 |
| IP vs IS | 1.01 (0.84–1.23) | 0.90 |
CI indicates confidence interval; PAI-1, plasminogen activator inhibitor type 1; CRP, C-reactive protein; IP, insulin-providing; and IS, insulin-sensitizing. Estimates of hazard ratios from a Cox regression model including all 5 analytes are shown. P value for overall test of significance of the 5 analytes <0.0001.
Analysis includes baseline factors of sex, age, race/ethnicity, congestive heart failure, myocardial infarction history, renal disease, baseline body mass index, ankle-brachial index category, number of significant lesions, and concurrent hemoglobin A1c.
Discussion
As published previously, clinical outcomes were comparable with the IS and IP treatment strategies.1 However, our results show that the 2 had differential effects on biomarker profiles. We found that (1) baseline values for all analytes were similar with IS and IP; (2) high baseline D-dimer, fibrinogen, and CRP portended decreased survival with both; (3) fibrinogen, FPA, and CRP decreased significantly over time with both IS and IP; (4) an IS compared with an IP strategy led to lower concentrations of plasma insulin, tPA antigen, PAI-1 antigen and activity, CRP, and fibrinogen; (5) with IS, insulin, PAI-1 antigen, PAI-1 activity, tPA antigen, and CRP decreased over time from baseline values (by contrast, all except CRP increased with IP); and (6) fibrinogen was significantly lower at each interval with IS. Thus, IS compared with IP decreased compensatory hyperinsulinemia and led to a change in biomarker profiles indicative of constrained fibrinolysis. The directional differences in biomarker profiles with IS compared with IP were consistent over time.
Although IS was associated with slightly lower HbA1c beginning within 6 months, the difference is unlikely to account for a difference in biomarker profiles with IS compared with IP for several reasons, including (1) insulin increases synthesis and elaboration of PAI-15; (2) concentrations of PAI-1 increased significantly over time with IP (Figure 2); and (3) correlations between analyte values and HbA1c were low.
A priori, it was recognized that an association with clinical outcomes of differences in biomarker profiles might not be evident for several reasons. Thus, the biomarkers might not be determinants in the pathogenesis of diabetic vasculopathy. In addition, the ancillary study of D-dimer, FPA, CRP, and fibrinogen was not powered to delineate effects on outcomes. Numerous factors in addition to the IS and IP treatment strategies can affect biomarker profiles. Both IS and IP treatment improved glycemic control, thereby potentially modulating adverse effects associated with specific bio-marker profiles. Diverse processes besides coagulation, fibrinolysis, and inflammation can alter progression of diabetic vasculopathy, and an imbalance between fibrinolysis and thrombosis and an increased intensity of inflammation are likely to affect clinical outcomes only over very prolonged intervals. The intensive monitoring and care in BARI 2D probably diminished event rates and may therefore have attenuated adverse outcomes associated with specific bio-marker profiles. Furthermore, the number of subsets in the BARI 2D population was large, limiting the power for detection of differences in clinical outcomes. Nevertheless, as shown in Table 4, the hazard ratios associated with several analytes were indicative of increased risk for death/MI/stroke and the need for subsequent revascularization procedures. For example, the risk of death/MI/stroke was 3% greater for every 10% increase in D-dimer and CRP. For total mortality, the increases were 4% and 3% for each 10% increase, respectively.
Several observations pertinent to biomarker profiles may merit consideration. Thus, the incidence of nonfatal, nonprocedurally related acute MI is significantly lower with IS than with IP.6 Furthermore, in patients at very high risk (those in the coronary artery bypass grafting stratum prespecified by the BARI 2D protocol2) who underwent prompt revascularization, clinical outcomes may have been more favorable with IS compared with IP, although the trends observed did not reach statistical significance (P=0.07).1 In addition, the trends reflected by the hazard ratios we observed are consistent with clinical benefit with IS compared with IP.
The design of the BARI 2D trial and the present study was influenced by several considerations. Traditionally, hyperglycemia refractory to management with diet and exercise was treated with sulfonylureas and, if necessary, insulin. Recently, metformin has been favored as initial treatment followed by addition of newer agents, including thiazolidinediones, along with other strategies to enhance sensitization to insulin.7 Insulin resistance intensifies the systemic inflammatory state, is prothrombotic, and constrains fibrinolysis (reviewed in Trost et al8). Treatment with metformin decreases insulin resistance and PAI-1 activity9 and the incidence of cardiovascular events. Thiazolidinediones lower PAI-1 and CRP10 and may slow progression of atherosclerosis.11
Increased expression of PAI-1 is a hallmark of insulin resistance. It shifts the balance between thrombosis and fibrinolysis, favoring thrombosis.12,13 It portends an increased risk of MI.14 Insulin increases synthesis and concentrations of PAI-1 in blood,15–20 as do mediators of inflammation.21
Increased expression of PAI-1 in vessel walls can accelerate development of atheroma prone to rupture and to precipitate acute coronary syndromes.22–25 It can increase proliferation of mural cellular elements and restenosis after percutaneous coronary intervention.26 Increased expression of PAI-1 in vessel walls has been associated with vascular inflammation.27,28 Insulin sensitizers attenuate the increased expression of PAI-1 seen with insulin resistance10 and may slow atherogenesis.29 Furthermore, insulin resistance increases the intensity of inflammation.30–35
BARI 2D was designed to elucidate mechanisms through which treatment of DM might modify cardiovascular risk.2 It compared 2 mechanistically different treatment strategies. Selection of a specific medication for a given patient must, of course, be individualized. Renal failure is a contraindication for use of metformin. Heart failure is a contraindication for use of thiazolidinediones in view of their peroxisome proliferator-activated receptor-γ–mediated sodium-retaining effects on the kidney.36 Our results show that despite the use of various combinations of agents in each of the IS and IP treatment arms, a consistent difference in their effects on the biomarker profiles evaluated was evident.
Summary
The analytes we evaluated were chosen because changes in their concentrations in blood have been implicated as markers of or determinants of risk of cardiovascular disease. Our study was performed to determine whether 2 different approaches for treatment of type 2 DM with the same glycemic target would affect the profiles of these biomarkers differentially. The results showed that an IS as opposed to an IP strategy led to changes in biomarker profiles indicative of a profibrinolytic, antithrombotic, and anti-inflammatory state.
Our study was not designed or powered sufficiently to determine whether the changes in biomarker profiles observed would portend different clinical outcomes. However, the IS strategy has been associated with a previously reported decreased rate of nonfatal MI and a more favorable outcome in high-risk patients in the BARI 2D coronary artery bypass grafting stratum undergoing prompt revascularization as well as with the trend of reduced hazard ratios for clinical events that we observed. However, the possibility that the biomarker profiles portend clinical outcomes will require comparisons of large numbers of patients over prolonged intervals stratified with respect to changes in biomarker profiles induced by specific treatment strategies.
Supplementary Material
CLINICAL PERSPECTIVE.
Type 2 diabetes mellitus is associated with a 2- to 4-fold increase in the incidence and prevalence of coronary artery disease. Comparable increases occur also with respect to cardiovascular events including death, myocardial infarction, and stroke. Insulin resistance has been implicated strongly in accelerating the pathogenesis of coronary artery disease. Two mechanisms potentially linking insulin resistance to poor prognosis include constraint of fibrinolysis and intensification of inflammation. In this study, 2368 patients with type 2 diabetes mellitus and clinically stable angiographically documented coronary artery disease were treated with either an insulin-sensitizing or insulin-providing strategy for glycemic control targeting hemoglobin A1c to <7.0%. Changes in biomarker profiles during the targeted 5 years of follow-up were consistent with reduction of constraints on fibrinolysis and diminution of the intensity of the inflammatory state with the insulin-sensitizing strategy. These changes were not seen with the insulin-providing strategy. On the contrary, the insulin-providing strategy was associated with an apparently increased constraint of fibrinolysis. Thus, the use of insulin-sensitizing drugs for glycemic control may confer benefit with respect to the balance between fibrinolysis and thrombosis, favoring fibrinolysis in patients with type 2 diabetes mellitus.
Acknowledgments
We thank Lori Dales, Dagnija Neimane, and Michaelanne Rowen for their expert administrative and technical assistance throughout the study.
Sources of Funding
The BARI 2D trial was funded by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, Nos. U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804, and R01 HL71306. Significant supplemental funding was provided by GlaxoSmithKline, Collegeville, PA; Lantheus Medical Imaging, Inc (formerly Bristol-Myers Squibb Medical Imaging, Inc), North Billerica, MA; Astellas Pharma US, Inc (formerly Fujisawa Pharmaceutical Co, Ltd), Deerfield, IL; Merck & Co, Inc, Whitehouse Station, NJ; Abbott Laboratories, Inc, Abbott Park, IL; and Pfizer, Inc, New York, NY. Generous support was given by Abbott Laboratories Ltd, MediSense Products, Mississauga, Canada; Bayer Diagnostics, Tarrytown, NY; Becton, Dickinson and Company, Franklin Lakes, NJ; J.R. Carlson Labs, Arlington Heights, IL; Centocor, Inc, Malvern, PA; Eli Lilly and Company, Indianapolis, IN; LipoScience, Inc, Raleigh, NC; Merck Sante, Lyon, France; Novartis Pharmaceuticals Cor poration, East Hanover, NJ; and Novo Nordisk, Inc, Princeton, NJ. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
Footnotes
Clinical Trial Registration—http://www.clinicaltrials.gov. Unique identifier: NCT00006305.
Disclosures
None.
References
- 1.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones T, Molitich LZ, Nesto RW, Sako EY, Sobel BE, the BARI 2D Study Group A randomized clinical trial of treatment strategies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ. BARI 2D Trial Investigators. Hypothesis, design and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97(suppl):9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 3.McBane RD II, Hardison RM, Sobel BE. BARI 2D Study Group. Comparison of plasminogen activator inhibitor-1, tissue-type plasminogen activator antigen, fibrinogen, and D-dimer levels in various age decades in patients with type-2 diabetes mellitus and coronary artery disease (from the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial). Am J Cardiol. 2010;105:17–24. doi: 10.1016/j.amjcard.2009.08.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordenhem A, Wiman B. Tissue plasminogen activator (tPA) antigen in plasma: correlation with different tPA/inhibitor complexes. Scand J Clin Lab Invest. 1998;58:475–483. doi: 10.1080/00365519850186274. [DOI] [PubMed] [Google Scholar]
- 5.Schneider DJ, Sobel BE. Augmentation of synthesis of plasminogen activator inhibitor type 1 by insulin and insulin-like growth factor type I: implications for vascular disease in hyperinsulinemic states. Proc Natl Acad Sci U S A. 1991;88:9959–9963. doi: 10.1073/pnas.88.22.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaitman BR, Hardison RM, Adler D, Bebhart S, Grogan M, Ocampo S, Sopoko G, Ramires JA, Schneider D, Frye RL. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–2540. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(suppl):S11–S24. doi: 10.1016/j.amjmed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Trost S, Pratley R, Sobel B. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr Diabetes Rep. 2006;6:47–54. doi: 10.1007/s11892-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 9.Nagi DK, Yudkin JS. Effects of metformin on insulin-resistance for cardiovascular disease, and plasminogen activator inhibitor in NIDDM. Diabetes Care. 1993;16:621–629. doi: 10.2337/diacare.16.4.621. [DOI] [PubMed] [Google Scholar]
- 10.Kruszynska YT, Yu JG, Olefsky JM, Sobel BE. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes. 2000;49:633–639. doi: 10.2337/diabetes.49.4.633. [DOI] [PubMed] [Google Scholar]
- 11.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Tataon J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 12.Matsuno H, Kozawa O, Niwa M, ueshima S, Matsuo O, Collen D, Uematsu T. Differential role of components of the fibrinolytic system in the formation and removal of thrombus induced by endothelial injury. Thromb Haemost. 1999;81:601–604. [PubMed] [Google Scholar]
- 13.Izuhara Y, Takahashi S, Nangaku M, Takizawa S, Ishida H, Kurokawa K, van Ypersele de Strihou C, Hirayama N, Miyata T. Inhibition of plasminogen activator inhibitor-1: its mechanism and effectiveness on coagulation and fibrosis. Arterioscler Thromb Vasc Biol. 2008;28:672–677. doi: 10.1161/ATVBAHA.107.157479. [DOI] [PubMed] [Google Scholar]
- 14.Hamsten A, Wiman B, de Faire U, Blombäck M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med. 1985;313:1557–1563. doi: 10.1056/NEJM198512193132501. [DOI] [PubMed] [Google Scholar]
- 15.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 16.Juhan-Vague I, Alessi MC, Vague P. Increased plasma plasminogen activator inhibitor 1 levels: a possible link between insulin resistance and atherothrombosis. Diabetologia. 1991;34:457–462. doi: 10.1007/BF00403280. [DOI] [PubMed] [Google Scholar]
- 17.McGill JB, Schneider DJ, Arfken CL, Lucore CL, Sobel BE. Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes. 1994;43:104–109. doi: 10.2337/diab.43.1.104. [DOI] [PubMed] [Google Scholar]
- 18.Nordt TK, Sawa H, Fujii S, Sobel BE. Induction of plasminogen activator inhibitor type-1 (PAI-1) by proinsulin and insulin in vivo. Circulation. 1995;91:764–770. doi: 10.1161/01.cir.91.3.764. [DOI] [PubMed] [Google Scholar]
- 19.Auwerx J, Bouillon R, Collen D, Geboers J. Tissue-type plasminogen activator antigen and plasminogen activator inhibitor in diabetes mellitus. Arteriosclerosis. 1988;8:68–72. doi: 10.1161/01.atv.8.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Gough SC, Rice PJ, McCormack L, Chapman C, Grant PJ. The relationship between plasminogen activator inhibitor-1 and insulin resistance in newly diagnosed type 2 diabetes mellitus. Diab Med. 1993;10:638–642. doi: 10.1111/j.1464-5491.1993.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 21.Macfelda K, Weiss TW, Kaun C, Breuss JM, Zorn G, Oberndorfer U, Voegele-Kadletz M, Huber-Beckmann R, Ullrich R, Binder BR, Losert UM, Maurer G, Pacher R, Huber K, Wojta J. Plasminogen activator inhibitor 1 expression is regulated by the inflammatory mediators interleukin-1alpha, tumor necrosis factor-alpha, transforming growth factor-beta and oncostatin M in human cardiac myocytes. J Mol Cell Cardiol. 2002;34:1681–1691. doi: 10.1006/jmcc.2002.2117. [DOI] [PubMed] [Google Scholar]
- 22.Schneider DJ, Hayes M, Wadsworth M, Taatjes H, Rincon M, Taatjes DJ, Sobel BE. Attenuation of neointimal vascular smooth muscle cellularity in atheroma by plasminogen activator inhibitor type-1 (PAI-1). J Histochem Cytochem. 2004;52:1091–1099. doi: 10.1369/jhc.4A6260.2004. [DOI] [PubMed] [Google Scholar]
- 23.Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;3:1979–1989. doi: 10.1161/01.ATV.0000091250.53231.4D. [DOI] [PubMed] [Google Scholar]
- 24.Sobel BE. Increased PAI-1 and vasculopathy: a reconcilable paradox. Circulation. 1999;99:2496–2498. doi: 10.1161/01.cir.99.19.2496. [DOI] [PubMed] [Google Scholar]
- 25.Sobel BE. Accelerated atherosclerosis, increased intramural PAI-1, and diabetes. Proc Assoc Am Physicians. 1999;111:313–318. doi: 10.1046/j.1525-1381.1999.99231.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Kelm RJ, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspace-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 27.Katsaros KM, Speidl WS, Kastl SP, Zorn G, Huber K, Maurer G, Glogar D, Wojta J, Crhist G. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. J Thromb Haemost. 2008;6:508–513. doi: 10.1111/j.1538-7836.2007.02884.x. [DOI] [PubMed] [Google Scholar]
- 28.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type-1 in coronary artery atherectomy specimens from type 2 diabetic compared with non-diabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 29.Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–1820. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 30.Yeh ET. A new perspective on the biology of C-reactive protein. Circ Res. 2005;97:609–611. doi: 10.1161/01.RES.0000186188.38344.13. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 32.Tabák AG, Kivimäki M, Brunner EJ, Lowe GD, Jokela M, Akbaraly TN, Singh-Manoux A, Ferrie JE, Witte DR. Changes in C-reactive protein levels before type 2 diabetes and cardiovascular death: the Whitehall II study. Eur J Endocrinol. 2010;163:89–95. doi: 10.1530/EJE-10-0277. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SE, Haffner SM, Viberti G, Herman WH, Lachin JM, Kravitz BG, Yu D, Paul G, Golman RR, Zinman B, ADOPT Study Group Rosiglitazone decreases C-reactive protein to a greater extent relative to glyburide and metformin over 4 years despite greater weight gain: observations from a Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2010;33:177–183. doi: 10.2337/dc09-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, Palumbo I, D'Angelo A, Cicero AF. Fenofibrate, simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr Med Res Opin. 2009;11:1973–1983. doi: 10.1185/03007990903073159. [DOI] [PubMed] [Google Scholar]
- 35.Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302:1186–1194. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 36.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARγ stimulation of EnaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.