Abstract
Background
The CKD-EPI equation reduces bias and improves accuracy for GFR estimation compared to the MDRD Study equation. Creatinine generation differs among racial-ethnic groups but both equations only consider Blacks vs other. We developed and validated a GFR-estimating equation that includes a 4-level race variable.
Methods
Equations were developed in pooled data from 10 studies (N=8254) and validated in 17 additional studies from the US and Europe [CKD-EPI validation database (N=4014)], and in studies from China (N=675), Japan (N=248) and South Africa (N=99). Race was defined as a 2-level variable (Black vs other) and a 4-level variable (Black, Asian, Native American and Hispanic vs other).
Results
Coefficients for Black, Asian and Native American and Hispanic resulted in 15%, 5% and 1% higherlevels of estimated GFR, respectively, compared to others. The 2-level race equation had minimal bias in Blacks, Native Americans, Hispanics and others [−0.8 (−2.0,0.6), 2.3 (−2.1,5.1), and 2.8 (2.4,3.2) ml/min/1.73 m2, respectively) in the CKD-EPI validation database. The 4-level race equation improved bias in CKD-EPI Asians (0.8 (−2.2,2.6) vs 2.1 (0.3,4.4) ml/min/1.73 m2) and in Chinese (1.3 (0.6,2.2) vs 2.7 (1.9,3.7) ml/min/1.73 m2). Both equations had a large bias in Japanese [−17.8 (−0.1,−14.7) and −21.4 (−23.2,−18.2) ml/min/1.73 m2)] and South Africans [−12.4 (−18.3,−7.6) and −12.5 (−18.3,−7.6) ml/min/1.73 m2.
Conclusions
A multilevel variable for race developed in one geographic region may not be applicable in other regions. The 2-level race variable in the CKD-EPI equation can be used for all racial-ethnic groups in the US and Europe.
Introduction
Chronic kidney disease (CKD) is a common health problem among all racial and ethnic groups, both in the United States and worldwide 1. In the United States, chronic kidney failure disproportionately burdens racial and ethnic minorities. Incidence rates for chronic kidney failure treated by dialysis and transplantation are 3.6 and 1.4 times higher in Blacks and Asians, respectively, compared to Whites, and 1.5 times higher in Hispanics compared to non Hispanics2. Outside of the U.S., Asia, Taiwan and Japan have the highest prevalence rates of treated kidney failure2, 3. Data on the prevalence, etiology, and outcomes of earlier stages of kidney disease in these groups may be imprecise, in part, due to the lack of accurate GFR estimates.
The Modification of Diet in Renal Disease (MDRD) Study equation utilizes a 2-level racial variable (Black vs. White and other). The coefficient for Blacks leads to higher values for estimated GFR compared to Whites for the same level of creatinine, due to differences between Blacks vs Whites in factors other than GFR that affect the serum level of creatinine (non-GFR determinants), especially higher creatinine generation from muscle and diet 4, 5. It is widely believed there are also differences in creatinine generation in other racial, ethnic and geographic groups, which are not captured by current equations6, 7. Consistent with this assumption, introduction of coefficients for use in China and Japan improve performance of the MDRD Study equation in these populations8, 9.
We recently reported a new equation, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, based on creatinine, age, sex and a 2-level variable for race, that is more accurate than the MDRD Study equation10. We hypothesized that the performance of the CKD EPI equation could be improved in Asians and Native Americans and Hispanics by utilizing coefficients specific for these groups. Here, we report on the development of a GFR-estimating equation that includes a 4-level race variable, in a diverse population from US and Europe, and its validation in separate populations from the US and Europe as well as in populations from other countries.
Methods
Sources of Data and Measurements
CKD-EPI is a research group funded by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) to address challenges in the study and care of CKD, including development and validation of improved GFR estimating equations by pooling data from research studies and clinical populations (hereafter referred to as “studies”)10. The design and studies have been previously described and are briefly reviewed here10. We developed and internally validated the CKD-EPI equation in a database of 10 studies with a total of 8254 participants, divided randomly into separate datasets for development (n=5504) and internal validation (n=2750). The equations were then externally validated in a separate dataset of 16 other studies with a total of 3896 participants. In the current report, we use the same dataset for development and internal validation. We use the same external validation dataset, with the addition of more data from Native Americans that were not available in the original report due to absence of creatinine calibration (herein referred to as “CKD-EPI validation dataset”) (N=4014)10. In addition, we also evaluated the equations in three separate studies from outside of US and Europe; two are from Asia and one is from South Africa, each of which has been previously described9, 11, 12 (herein referred to as ‘Non-US and Europe validation datasets’). The appendix tables 1 and 2 describe the distribution and race group for each study. GFR was measured using urinary clearance of iothalamate in the development dataset and iothalamate and other filtration markers in the external validation datasets, Serum creatinine values were calibrated to standardized creatinine measurements using the Roche enzymatic method (Roche-Hitachi P-Module instrument with Roche Creatininase Plus assay, Hoffman-La Roche, Ltd., Basel, Switzerland) at the Cleveland Clinic Research Laboratory (Cleveland, OH)13, 14.
Development and validation
Methods for development and validation have been previously described in detail10. In brief, we used least squares linear regression to relate measured GFR to serum creatinine and clinical characteristics available in the development dataset. Predictor variables included serum creatinine, age, sex, and race in all equations. GFR was adjusted for body surface area (BSA)15. GFR and serum creatinine were transformed to natural logarithms to reflect their inverse relationship and to stabilize variance across the range of GFR. We tested multiple forms of creatinine and age, and the final model includes a piecewise linear spline of log serum creatinine with a knot at 0.7 mg/dl in men and 0.9 mg/dl in women, and linear age.
Race was defined as a 2-level variable (Black vs. White and other) and as a 4-level variable (Black, Asian, Native American and Hispanic vs. White and other). The rationale for grouping Native Americans and Hispanics together is that the majority of non-Black Hispanics in the United States are from Mexico, and they are considered to be of mixed European-Native American descent16, 17. The rationale for grouping others with White is that many of the other groups are defined as of Caucasian descent (for example, Arabs, non-Black and non-Native American Hispanics), and misclassification of this small number of non-Caucasian patients is not likely to affect the model fit. We developed models in parallel using 2-level and 4-level variables for race. The 4-level variable was forced into models, even if not all coefficients were significant. We selected specific development models for internal and then external validation based on analyses of the 2-level race variable, with models using the 4-level race variable brought along in parallel.
Models created in the development database were first validated in the internal validation database. The development and internal validation datasets were then combined and equations were refit to yield more precise final coefficients to be used in subsequent analyses. Models were then evaluated in the CKD-EPI validation dataset and a final model was selected using a pre-specified series of steps. The 4-level race variable model presented here is the final model and is compared to the previously described CKD-EPI equation using a 2-level race variable. Results are also presented in the Non-US and Europe validation dataset. For clarity of presentation, we will refer to the two equations as 2-level and 4-level race equations.
Statistical analyses
Performance of the equations was evaluated using similar metrics in both the development and two validation databases. Bias was expressed as the difference (mGFR-eGFR) and percent difference (100*[mGFR-eGFR] / mGFR) between measured and estimated GFR, with positive values indicating lower eGFR than mGFR (under-estimation). Precision was expressed as inter-quartile range (IQR) for the differences. Accuracy was expressed as the percent of estimates within 30% of the measured GFR (P30) which takes into account higher errors at higher values. In addition, we also expressed the magnitude of large errors as 1-P30.
Analyses within subgroups were defined by the following clinical characteristics: age (less than 40, 40-65, greater than 65 years); sex; race (Black, Asian, Native American and Hispanic, White and other); diabetes (yes, no), prior organ transplant (yes, no); body mass index (BMI, less than 20, 20 to 25, 26 to 30 and greater than 30 kg/m2). Level of eGFR was categorized as less than 60, 60-89 and greater than 90 ml/min/1.73 m2.
Confidence intervals were calculated by bootstrap methods (2000 bootstraps) for difference, percent difference and for P30. Significance testing between metrics for each equation was computed using the sign test on the bootstrapped estimates. Analyses were computed using R (Version 2, Free Software Foundation, Inc., Boston, MA) and SAS software (version, 9.1, Cary, NC). Smooth estimates of the mean in the figures were created using the lowess function in R.
The institutional review boards of all participating institutions approved the study.
Results
There were significant differences in the characteristics among racial and ethnic groups (Table 1). In the development dataset, measured GFR was lower in Blacks and Asians, and higher in Native Americans and Hispanics, compared to Whites and others. Blacks were older, more likely to be female, and had a larger body size compared to the other groups. In the external validation dataset, measured GFR was lower in Asians and higher in Native Americans and Hispanics compared to Whites and others. In the three datasets outside of US and Europe, measured GFR ranged between 53 and 60 ml/min/1.73 m2, and participants had lower BMI.
Table 1.
a: Clinical Characteristics of the Participants in Development Datasets
| Variable | Overall | Race-Ethnicity |
||||
|---|---|---|---|---|---|---|
| White and other | Black | Asian | Native American and Hispanic | p-values | ||
| N | 8254 | 5216 | 2585 | 100 | 353 | |
| Age, mean (SD) years | 47 (15) | 44 (15) | 53 (12) | 49 (15) | 43 (12) | <0.001 |
| Age categories N (%) | <0.001 | |||||
| < 40 years | 3076 (37) | 2464 (47) | 422 (16) | 36 (36) | 154 (44) | |
| 40-65 years | 4154 (50) | 2149 (41) | 1766 (68) | 50 (50) | 189 (54) | |
| > 65 years | 1024 (12) | 603 (11) | 397 (16) | 14 (11) | 10 (3) | |
| SexN (%) | <0.001 | |||||
| Female | 3606 (44) | 2353 (45) | 1019 (39) | 41 (41) | 193 (55) | |
| Male | 4648(56) | 2863 (55) | 1566 (61) | 59 (59) | 160 (45) | |
| Diabetes N (%) | <0.001 | |||||
| Yes | 2406 (29) | 1885 (36) | 280(11) | 33 (33) | 208(59) | |
| No | 5848 (71) | 3331 (64) | 2305 (89) | 67 (67) | 145 (41) | |
| Transplant N (%) | <0.001 | |||||
| Yes | 360 (4) | 330 (6) | 24 (1) | 5 (5) | 1 (0.3) | |
| No | 7894(96) | 4886 (94) | 2561(99) | 95 (95) | 352 (99.7) | |
| GFR mean (SD), ml/min/1.73 m2 | 68 (40) | 73 (43) | 55 (27) | 57 (31) | 90 (45) | <0.001 |
| Serum creatinine, mean (SD), mg/dL | 1.66 (1.16) | 1.58 (1.19) | 1.87 (1.09) | 1.73 (0.91) | 1.23 (1.02) | <0.001 |
| Body surface area, mean (SD), m2 | 1.9 (0.24) | 1.90 (0.23) | 2.00 (0.25) | 1.77 (0.21) | 1.91 (0.25) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 28 (6) | 27 (5) | 31 (7) | 26 (5) | 31 (9) | <0.001 |
| BMI categories N (%) | <0.001 | |||||
| <20 kg/m2 | 287 (3) | 218(4) | 60 (2) | 4 (4) | 5 (1.4) | |
| 20-25 kg/m2 | 2447 (30) | 1896 (36) | 446 (17) | 40 (40) | 65 (18.4) | |
| 26-30 kg/m2 | 2922 (35) | 1930 (37) | 857(33) | 37 (37) | 98 (27.8) | |
| > 30 kg/m2 | 2598 (31) | 1172 (23) | 1222 (47) | 19 (19) | 185 (52.4) | |
| Table 1b. Clinical Characteristics of the Participants in Validation Datasets) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | CKD-EPI (US and Europe) |
Non-US and Europe |
||||||
| White and other | Black | Asian | Native American and Hispanic | Asian | Asian | Black | p-values | |
| N | 3378 | 384 | 67 | 185 | 248 | 675 | 99 | |
| Age, mean (SD) years | 49 (15) | 50 (15) | 51 (15) | 45 (12) | 50 (18) | 50 (15) | 47 (17) | 0.001 |
| Age categories, N (%) | <0.001 | |||||||
| < 40 years | 978 (29) | 112 (29) | 19 (28) | 68 (37) | 95 (38) | 207 (31) | 42 (42.5) | |
| 40-65 years | 1898 (56) | 224 (58) | 35 (52) | 107 (58) | 92 (37) | 333(49) | 42 (42.5) | |
| > 65 years | 502 (15) | 48 (13) | 13 (19) | 10 (5) | 61 (25) | 135 (20) | 15 (15) | |
| Sex, N (%) | 0.001 | |||||||
| Female | 1513 (45) | 184 (48) | 32 (48) | 130 (70) | 112 (45) | 328 (49) | 49 (49) | |
| Male | 1865 (55) | 200 (52) | 35 (52) | 55 (30) | 136 (55) | 347 (51) | 50 (50) | |
| Diabetes, N (%) | <0.001 | |||||||
| Yes | 975 (29) | 95 (25) | 14 (21) | 119 (64) | 35(14) | 21(3) | 6 (6) | |
| No | 2403 (71) | 289 (75) | 53 (79) | 66 (67) | 213 (86) | 654 (97) | 93 (94) | |
| Transplant, N (%) | <0.001 | |||||||
| Yes | 1072 (32) | 52 (14) | 7 (10) | 3 (2) | 0 | 0 | 0 | |
| No | 2306 (68) | 332 (86) | 60 (90) | 182 (98) | 0 | 0 | 0 | |
| GFR, mean (SD), ml/min/1.73 m2 | 69 (36) | 62 (34) | 53 (31) | 105 (47) | 53 (31) | 55 (35) | 61 (32) | <0.001 |
| Serum creatinine, mean (SD), mg/dL | 1.48 (0.94) | 1.80 (0.29) | 1.99 (1.41) | 0.9 (0.7) | 1.24 (0.56) | 2.25 (2.18) | 1.77 (1.71) | <0.001 |
| Body surface area, mean (SD), m2 | 1.90 (0.23) | 1.95 (0.23) | 1.70 (0.20) | 1.98 (0.29) | 1.62 (0.18) | 1.71 (0.18) | 1.77 (0.17) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 27 (5) | 30 (7) | 24 (4) | 34 (8) | 23 (4) | 24 (4) | 26 (5) | <0.001 |
| BMI categories, N (%) | <0.001 | |||||||
| <20 kg/m2 | 225 (7) | 17 (4) | 5 (7) | 2 (1) | 55 (22) | 107 (16) | 15 (15) | |
| 20-25 kg/m2 | 1223 (36) | 84 (22) | 34 (51) | 22 (12) | 137 (55) | 354 (52) | 44 (44) | |
| 25-30 kg/m2 | 1178 (35) | 115 (30) | 24 (36) | 49 (26) | 45(18) | 181 (27) | 20 (20) | |
| > 30 kg/m2 | 752 (22) | 168 (44) | 4 (6) | 112 (61) | 11 (4) | 33 (5) | 20 (20) | |
GFR = glomerular filtration rate. To convert GFR from mL/min per 1.73 m2 to mL/s per 1.73 m2, multiply by 0.0167.
GFR = glomerular filtration rate. To convert GFR from mL/min per 1.73 m2 to mL/s per m2, multiply by 0.0167.
The coefficients for Black, Asian and Native American and Hispanic are larger than the reference group (White and other) resulting in higher estimated GFR for the same level of creatinine for all groups compared to White and others (Table 2). For both the 2- and 4-level race equations, estimated GFR is 15% higher for Blacks than for Whites or others. In the 4-level race equation, estimated GFR is 5% higher in Asians but only 1% higher and not significant in Native Americans and Hispanics compared to Whites or others. Table 3 shows the 2-level race equation and the 4-level race equation developed using the coefficients from the combined development and internal validation datasets, expressed for specified race, sex and serum creatinine.
Table 2.
Race-Ethnicity Coefficients (95% Confidence Intervals)*
| Equation | White and other | Black | Asian | Native American and Hispanic |
|---|---|---|---|---|
| 2 level race | 1.0 (ref) | 1.157 (1.144, 1.170) | - | - |
| 4 level race | 1.0 (ref) | 1.160 (1.146, 1.173) | 1.052 (1.004, 1.102) | 1.010 (0.984, 1.037) |
Corresponds to percent increase in estimated GFR for the same level of serum creatinine.
Table 3.
CKD EPI Equation for Estimating GFR on the Natural Scale Expressed for Race, Sex and Range of Serum Creatinine.
| 2-Level Race Equation | |||
|---|---|---|---|
| Race | Sex | Serum Creatinine | eGFR (ml/min/1.73 m2) |
| Black | Female | ≤0.7 mg/dl | 161 × (0.993)Age × (Scr/0.7)−0329 |
| Black | Female | >0.7 mg/dl | 161 × (0.993)Age × (Scr/0.7)−1.209 |
| Black | Male | ≤ 0.9 mg/dl | 163 × (0.993)Age × (Scr/0.9)−0.411 |
| Black | Male | >0.9 mg/dl | 163 × (0.993)Age × (Scr/0.9)−1.209 |
| White and other | Female | ≤0.7 mg/dl | 139 × (0.993)Age × (Scr/0.7)−0.329 |
| White and other | Female | >0.7 mg/dl | 139 × (0.993)Age × (Scr/0.7)−1209 |
| White and other | Male | ≤ 0.9 mg/dl | 141 × (0.993)Age × (Scr/0.9)−0.411 |
| White and other | Male | >0.9 mg/dl | 141 × (0.993)Age × (Scr/0.9)−1.209 |
| 4-Level Race Equation | |||
|---|---|---|---|
| Race | Sex | Serum Creatinine | eGFR (ml/min/1.73 m2) |
| Black | Female | ≤0.7 | 167 × (0.993)Age × (Scr/0.7)−0.328 |
| Black | Female | >0.7 | 167 × (0.993)Age × (Scr/0.7)−1.210 |
| Black | Male | ≤ 0.9 | 164 × (0.993)Age × (Scr/0.7)−0.415 |
| Black | Male | >0.9 | 164 × (0.993)Age × (Scr/0.7)−1.210 |
| Asian | Female | ≤0.7 | 151 × (0.993)Age × (Scr/0.7)−0.328 |
| Asian | Female | >0.7 | 151 × (0.993)Age × (Scr/0.7)−1.210 |
| Asian | Male | ≤ 0.9 | 149 × (0.993)Age × (Scr/0.7)−0.415 |
| Asian | Male | >0.9 | 149 × (0.993)Age × (Scr/0.7)−1.210 |
| Hispanic and Native American | Female | ≤0.7 | 145 × (0.993)Age × (Scr/0.7)−0.328 |
| Hispanic and Native American | Female | >0.7 | 145 × (0.993)Age × (Scr/0.7)−1.210 |
| Hispanic and Native American | Male | ≤ 0.9 | 143 × (0.993)Age × (Scr/0.7)−0.415 |
| Hispanic and Native American | Male | >0.9 | 143 × (0.993)Age × (Scr/0.7)−1.210 |
| White and other | Female | ≤0.7 | 144 × (0.993)Age × (Scr/0.7)−0.328 |
| White and other | Female | >0.7 | 144 × (0.993)Age × (Scr/0.7)−1.210 |
| White and other | Male | ≤ 0.9 | 141 × (0.993)Age × (Scr/0.7)−0.415 |
| White and other | Male | >0.9 | 141 × (0.993)Age × (Scr/0.7)−1.210 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration. To convert GFR from mL/min/1.73 m2 to mL/s/1.73 m2, multiply by 0.0167. To convert serum creatinine from mg/dL to μmol/L, multiply by 88.4. CKD-EPI equation coefficients derived from pooled development and internal validation datasets. CKD-EPI 2 level equation expressed as a single equation: GFR = 141 × min(Scr/k, 1)α × max(Scr/k, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black] where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/k or 1, and max indicates the maximum of Scr/k or 1. 4 level equation, GFR = 141 × min(Scr/k, 1)α × max(Scr/k, 1)−1.210 × 0.993Age × 0.993 [if female] × 1.16 [if Black] × 1.05 [if Asian] × 1.01 [if Hispanic and Native American] where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is −0.412 for females and −0.328 for males, min indicates the minimum of Scr/k or 1, and max indicates the maximum of Scr/k. In the table, the multiplication factors for race and sex are incorporated into the intercept, resulting in different intercepts for age and sex combinations.
Tables 4 and 5 show the performance of the two models in the external validation datasets. In the CKD-EPI validation dataset, performance of the equation with the 2-level and 4-level race terms was similar in Blacks and Whites (Table 4). In Asians, there was a significant difference in bias [0.8 (−2.2, 2.6) ml/min/1.73 m2 for the 4-level race equation vs. 2.1 (0.3, 4.4) ml/min/1.73 m2 for the 2-level race equation (p <0.005)], IQR 12.3 (9.0,16.1) vs 10.5 (8.0,14.6) ml/min/1.73 m2, p=0.001] and RMSE 0.293(0.178,0.424) vs 0.302(0.188,0.436), p=0.003) but no significant difference in P30. There were no significant differences in performance between the two equations for Native Americans and Hispanics. In the Chinese dataset, as in the Asians in the CKD-EPI validation dataset, there was an improvement in performance with the 4-level race equation compared to the 2-level race equation in bias [1.3 (0.6,2.2) vs. 2.7 (1.9,3.7) ml/min/1.73 m2 (p <0.0001)], IQR 15.5 (14.4,17.4) vs 16.7 (15.0,18.5) ml/min/1.73 m2, p<0.0001], RMSE 0.318 (0.295,0.343) vs 0.325 (0.302,0.348) ml/min/1.73 m2, p=0.002), as well as in P30 [72.1 (68.7,75.7) vs 73.2 (69.9,76.6), p=0.01]. In the Japanese datasets, performance for 2-level race equation was substantially worse than in the Asians in the CKD-EPI validation dataset and not improved with the use of the 4-level race equation. In the South African dataset, performance of both the 2-level and 4-level race equations was substantially worse than in the Blacks in the CKD-EPI validation dataset. Performance was better for the South African dataset when the Black coefficient was not used [bias of −12.4 (−18.3, −7.6) with the use of the Black term vs. −4.9 (−7.0,−0.5) ml/min/1.73 m2 without the use of the Black term].
Table 4.
Performance in CKD-EPI External Validation Dataset (US and Europe) by Race-Ethnicity
| Measures | Equation | Total | White and other | Black | Asian | Native American and Hispanic |
|---|---|---|---|---|---|---|
| N | 4014 | 3378 | 384 | 67 | 185 | |
| Bias ml/min/1.73 m | 2-level | 2.5 (2.1,2.9) | 2.8 (2.4,3.2) | −0.8 (−2.0,0.6) | 2.1 (0.3,4.4) | 2.3 (−2.1,5.1) |
| 4-level | 2.5 (2.1,2.9) | 2.9 (2.5,3.4) | −0.9 (−2.0,0.6) | 0.8 (−2.2,2.6) | 1.6 (−3.0,4.2) | |
| IQR, ml/min/1.73 m2 | 2-level | 17.0 (16.1,17.6) | 16.8 (16.0,17.6) | 15.1 (12.6,17.6) | 10.5 (8.0,14.6) | 25.6 (20.8,32.0) |
| 4-level | 17.0 (16.2,17.6) | 16.8 (16.0,17.6) | 15.1 (12.6,17.6) | 12.3 (9.0,16.1) | 26.1 (20.8,32.2) | |
| P30, % | 2-level | 84 (83,85) | 84 (83,86) | 82 (78,85) | 85 (76,93) | 80 (74,85) |
| 4-level | 84 (83,85) | 84 (83,85) | 82 (80,85) | 85 (76,93) | 81 (76,87) | |
| RMSE | 2-level | 0.250(0.242,0.259) | 0.250 (0.240,0.258) | 0.242(0.221,0.265) | 0.302(0.188,0.436) | 0.265(0.223,0.310) |
| 4-level | 0.250(0.242,0.259) | 0.250 (0.240,0.259) | 0.243(0.221,0.266) | 0.293(0.178,0.424) | 0.264(0.222,0.310 | |
Bias is calculated as measured GFR - estimated GFR; Interquartile range (IQR) refers to the 25–75th percentile; P30 refers to the percentage of GFR estimates that are within 30% of measured GFR; RSME is the root mean square error. Numbers in brackets are 95% confidence intervals.
To convert GFR from mL/min/1.73 m2 to mL/s/1.73 m2, multiply by 0.0167.
Table 5.
Performance in Non US and Europe External Validation Dataset by Country and Race-Ethnicity
| Measures | Equation | China (Asian) | Japan (Asian) | South Africa (Black) |
|---|---|---|---|---|
| N | 675 | 248 | 99 | |
| Bias, ml/min/1.73 m2 | 2-level | 2.7 (1.9,3.7) | −17.8 (−20.1,−14.7) | −12.4 (−18.3,−7.6) |
| 4-level | 1.3 (0.6,2.2) | −21.4 (−23.3,−18.2) | −12.5 (−18.3,−7.6) | |
| IQR, ml/min/1.73 m2 | 2-level | 16.7 (15.0,18.5) | 21.0 (18.5,23.9) | 28.0 (20.8,33.3) |
| 4-level | 15.5 (14.4,17.4) | 23.5 (20.4,26.0) | 28.0 (20.8,33.4) | |
| P30, % | 2-level | 73.2 (69.9,76.6) | 29.4 (23.8,35.1) | 55.6 (46.5,64.6) |
| 4-level | 72.1 (68.7,75.7) | 36.3 (30.6,42.3) | 55.6 (46.5,64.6) | |
| RMSE | 2-level | 0.325 (0.302,0.348) | 0.469 (0.424,0.515) | 0.326 (0.292,0.361) |
| 4-level | 0.318 (0.295,0.343) | 0.507 (0.463,0.553) | 0.327 (0.292,0.362) | |
Bias is calculated as measured GFR - estimated GFR; Interquartile range (IQR) refers to the 25–75th percentile; P30 refers to the percentage of GFR estimates that are within 30% of measured GFR; RSME is the root mean square error. Numbers in brackets are 95% confidence intervals. To convert GFR from mL/min/1.73 m2 to mL/s/1.73 m2, multiply by 0.0167.
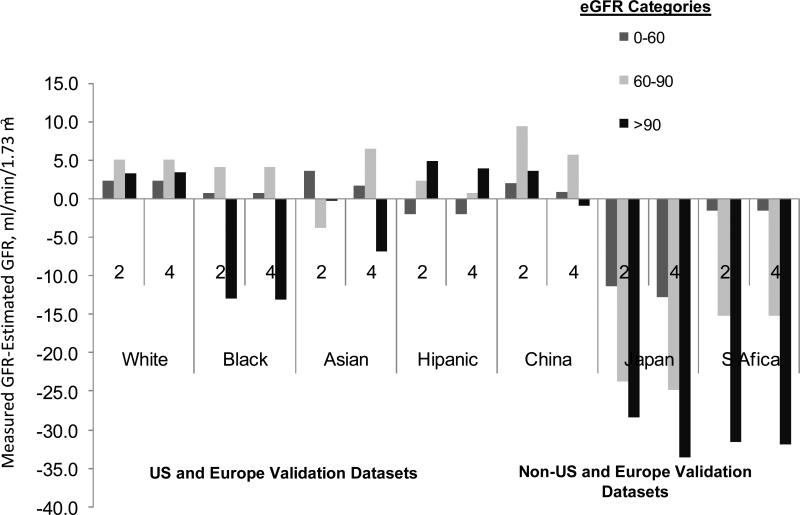
The figure shows the bias by level of eGFR. In the CKD-EPI validation dataset, using the 2- and 4-level race equation, bias was less than approximately 5 ml/min/1.73 m2 except for Blacks with eGFR greater than 90 ml/min/1.73 m2, as we reported previously. In the non-US and Europe datasets, using both equations, bias was less than 5 ml/min/1.73 m2 across the range of GFR, but improved in China with the use of the 4-level race equation and varied substantially throughout the GFR range in Japan and South Africa.
Figure. Performance by level of estimated GFR.
Comparison of performance of Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (2-level race equation) to the 4-level race equation by estimated GFR in the external validation datasets by level of eGFR.
Discussion
Different relationships between serum creatinine and measured GFR primarily reflect variation in creatinine generation due to muscle mass or diet. The definition of race as Black vs other in the current equations cannot account for differences in creatinine generation among other racial and ethnic groups. Our goal was to develop an equation that more accurately estimated GFR in racial, ethnic and geographic groups than the currently available equation. The developed equation has coefficients greater than 1.0 for all racial and ethnic groups compared to Whites and others. The larger coefficient translates into higher GFR estimates for these groups compared to Whites and others for a given serum creatinine level. Compared to the 2-level race equation, the 4-level equation is more accurate in Asians in the CKD-EPI validation dataset as well as in the Chinese dataset. Both the 2-level or 4-level race equation are as accurate in Native Americans and Hispanics as in Blacks and Whites and others in the CKD-EPI validation dataset, but neither equation resulted in accurate estimates in the Japanese and South African datasets. Based on the heterogeneous results among these populations, we concluded that 4-level race equation that we derived is not accurate enough to be implemented in clinical practice. Nevertheless, these results are informative for use of the 2-level race CKD-EPI equation in these groups and also suggest future research directions to derive generalizable racial and ethnic coefficients for GFR estimating equations based on creatinine.
The coefficient for Blacks in the 2-level and 4-level race term yielded a 15% higher estimated GFR for Blacks than for Whites at a given serum creatinine level, which is consistent with physiological data showing greater skeletal muscle mass than otherwise equivalently-matched subjects18, 19. Similarly, African Black athletes also have greater lean body mass compared to Whites20. Using these coefficients, the eGFR for Blacks in the CKD-EPI validation dataset accurately estimated measured GFR. In contrast, use of the Black coefficient in the South African data leads to an overestimation of measured GFR by 12 ml/min/1.73 m2, which was substantially reduced when the Black coefficient was not used, indicating a different relationship between serum creatinine and GFR for Black South Africans vs. US and European Blacks, as shown previously for the MDRD Study equation using these data21. This difference may be due to lower muscle mass in South African Blacks compared to African Americans, potentially secondary to poorer diet or overall health, related to HIV infection or other chronic diseases. Indeed, the mean BMI in the South African population was lower than in the Blacks in the CKDEPI validation dataset, and 15% of the South African cohort had a BMI level less than 20 kg/m2 compared to 2% for Blacks in the development dataset. In a prior publication, we showed that the CKD-EPI equation overestimates measured GFR in people with low BMI5. These data raise important questions about the appropriateness of use of the Black coefficient for GFR estimation in Blacks outside the US and Europe.
The Asian coefficient in the 4-level race equation translates into a 5% higher GFR for every serum creatinine value compared to Whites and others. This is unexpected given that prior physiological and epidemiological data suggest that Asians have less muscle mass and lower dietary intake than Whites. For example, in an analysis of people in Pakistan, participants had lower mean creatinine excretion rates than those estimated for age and gender-matched white individuals22. In other studies, Asians have been shown to have a higher percent body fat for the same level of BMI than Whites, suggesting lower levels of muscle mass23. The direction of the Asian coefficient is consistent with the modification of the MDRD Study equation for Chinese by Ma and colleagues, which was previously published, and the data which are included in the current publication12. Although the increase in GFR of 5% was substantially lower than the 23% reported by Ma et al 12, both are in contrast to the Japanese coefficient for the modification of the MDRD Study equation of 0.8124, which translates to a 19% lower GFR for every serum creatinine value. Both the Chinese and Japanese cohorts had a greater proportion of people with BMI less than 20 kg/m2 than the CKD-EPI development and validation datasets, but were similar to each other, suggesting that the overestimation of measured GFR in the Japanese cohort is not related to differences in levels of BMI. The difference between the Chinese and Japanese coefficients may be due to factors other than muscle mass and diet, such as differences in GFR measurement methods and the accuracy of creatinine calibration25. Our findings of a coefficient in the same direction in a separate group of Asians may provide some support for the validity of the Chinese coefficient, as well as, more generally, variation within Asians of the determinants of creatinine. The specific origins for the Asians in the CKD-EPI validation dataset are not known, and therefore we are not able to ascertain whether the higher GFR seen in the CKD-EPI population is because they are of Chinese origin. If future analyses establish that creatinine generation varies among Asian groups, then coefficients for subgroups of Asians in creatinine based equations will need to reflect this variation.
The Hispanic coefficient resulted in a 1% higher estimated GFR for every serum creatinine value compared to Whites and others, but the coefficient was not significant and did not improve GFR estimation. There are minimal data on muscle mass in both the Native American and Hispanic populations. Data from NHANES, shows a 5.3% lower mean level of serum creatinine for young healthy Mexican American men compared to Whites, which was interpreted to indicate lower creatinine generation4. In contrast, our results suggest a similar relationship between GFR and serum creatinine among Native Americans and Hispanics compared to Whites and others. There are only a small number of Native Americans and Hispanics in the CKD-EPI development dataset and we do not have information on the country of origin of the Hispanics.
The strengths of this study include the large diverse population with and without kidney diseases; calibration of the creatinine assays in each study to standardized values; and rigorous statistical techniques for equation development including testing of all transformations, and evaluation of the equations in a separate dataset of multiple studies, which maximized external generalizability. There are several limitations. First, there are a small number of non-Blacks and non-Whites included in both the CKD-EPI development and validation datasets. Nonetheless, the confidence intervals for the Asian and Native American and Hispanic coefficients were narrow, suggesting little variability among these groups in non-GFR determinants of serum creatinine. Second, differences in methods to measure GFR may have led to the observed results. Finally, in the CKD-EPI datasets we grouped some Native Americans and Hispanics and do not have information on country of origin for Asians and Hispanics. Comparison of equations in a separate validation dataset overcomes some of the limitations of differences among studies in patient characteristics and methods for measurement of GFR and serum creatinine.
This study has several implications for clinical practice and research. First, the results demonstrate that the current CKD-EPI equation performs well, with minimal bias across the range of eGFR in the Native Americans and Hispanics, Asian-Americans and Chinese populations who were included in this dataset, but has a large bias in Japanese and South Africans. To our knowledge, this is the first demonstration of performance of GFR estimating equations in Hispanics. As such, the equation can be applied to all populations in the US and Europe but should be used only after evaluation compared to measured GFR in other geographic regions. In addition, this study draws attention to the inadequacy of current equations in identifying disease prevalence and severity across groups and geographic regions. To further understand the effect of race on non-GFR determinants of serum creatinine and its implications in determining GFR, there is a need for studies that measure GFR in representative samples of racial and ethnic groups with and without CKD in the US and globally. In particular, data from the present study emphasize the importance of including sufficient representation of subgroups within a particular racial or ethnic group. Future studies should evaluate the effects of race-ethnicity on non-GFR determinants of filtration markers. Finally, emphasis should be placed on investigation of filtration markers that may be less affected than creatinine by race, such as cystatin C and other novel markers.
Appendix
APPENDIX A.
Development and Internal validation Race/ ethnic group N (%)
| Studies | GFR Measurement Method | Race |
|||
|---|---|---|---|---|---|
| White and other | Black | Native American and Hispanic | Asian | ||
| MDRD Study 26 | Iothalamate | 1317 (25) | 197 (8) | 97 (27) | 17 (17) |
| AASK 27 | Iothalamate | 0 (0) | 1807 (70) | 0 (0) | 0 (0) |
| DCCT28 | Iothalamate | 1138 (22) | 21 (1) | 14(4) | 3 (3) |
| DRDS 29 | Iothalamate | 0 (0) | 0 (0) | 190 (54) | 0 (0) |
| CSG 30 | Iothalamate | 355 (7) | 32 (1) | 11 (3) | 1 (1) |
| CRIC 31 | Iothalamate | 306 (6) | 289 (11) | 19(5) | 55 (55) |
| CCF CKD 32 | Iothalamate | 850 (16) | 169 (7) | 8 (2) | 10 (10) |
| CCF Donors 32 | Iothalamate | 380 (7) | 63 (2) | 10(3) | 4 (4) |
| Mayo CKD 33 | Iothalamate | 312 (6) | 0 (0) | 1 (0.2) | 5 (5) |
| Mayo donors 33 | Iothalamate | 558 (11) | 7 (0.3) | 3 (1) | 5 (5) |
Abbreviations: MDRD Study, Modification of Diet in Renal Disease Study; AASK, African American Study of Kidney Diseases and Hypertension; DCCT, Diabetes Control and Complications Trial; DRDS, Diabetic Renal Disease Study; CSG, Collaborative Study Group: Captopril in Diabetic Nephropathy Study; CRIC, Chronic Renal Insufficiency Cohort Study; CCF, Cleveland Clinic Foundation
APPENDIX B.
External validation
| Studies | GFR Measurement Method | Race/ethnic group N (%) |
|||
|---|---|---|---|---|---|
| White and other | Black | Native American and Hispanic | Asian | ||
| CKD-EPI Validation Dataset | |||||
| Baylor 34 | Iothalamate | 651 (19) | 47 (10) | 3 (2) | 7 (1) |
| CCF P CKD | Iothalamate | 92 (3) | 9 (2) | 1 (0.5) | 1 (0.1) |
| CCF P donors | Iothalamate | 83 (2) | 10 (2) | 2 (1) | 1 (0.1) |
| CRIC 31 | Iothalamate | 156 (5) | 127 (26) | 3 (2) | 12 (1) |
| CRISP 35 | Iothalamate | 172 (5) | 21 (4) | 3 (2) | 2 (0.2) |
| DNA donor | Iothalamate | 60 (2) | 19 (4) | 28 (15) | 2 (0.2) |
| DNA Tx | Iothalamate | 116 (3) | 61 (13) | 27 (15) | 5 (0.5) |
| DRDS 29 | Iothalamate | 0 (0) | 0 (0) | 118 (64) | 0 (0) |
| Groningen 36 | Iothalamate | 418 (12) | 4 (1) | 0 (0) | 0 (0) |
| Groningen donors 37 | Iothalamate | 43 (1) | 0 (0) | 0 (0) | 0 (0) |
| Lund 38 | Iohexol | 387 (11) | 0 (0) | 0 (0) | 0 (0) |
| Lund donors 38 | Iohexol | 7 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| NephroTest CKD 39 | EDTA | 371 (11) | 37 (8) | 30 (3) | 0 (0) |
| NephroTest donors 39 | EDTA | 332 (10) | 43 (9) | 7 (1) | 0 (0) |
| RASS Study 40 | Iothalamate, Iohexol | 245 (7) | 6 (1) | 0 (0) | 0 (0) |
| Steno Diabetes Center 41-45 | EDTA | 245 (7) | 0 (0) | 0 (0) | 0 (0) |
| Non-US and Europe Validation Dataset | |||||
| China | DTPA | 0 (0) | 0 (0) | 675 (68) | 0 (0) |
| Japan | Inulin | 0 (0) | 0 (0) | 248 (25) | 0 (0) |
| South Africa | EDTA | 0 (0) | 99 (21) | 0 (0) | 0 (0) |
Abbreviations: CCF P, Cleveland Clinic Foundation Prospective; CKD, Chronic Kidney Disease; CRIC, Chronic Renal Insufficiency Cohort; CRISP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease; DNA, Dallas Nephrology Associates; DRDS, Diabetic Renal Disease Study; RASS, Renin Angiotensin System Study.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System . USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2008. [Google Scholar]
- 3.U.S. Renal Data System . USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 4.Jones CY, Jones CA, Wilson IB, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis. 2008;51:914–924. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR- estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25:449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi G, Del Fabbro M, Lippi G. Relation between serum creatinine and body mass index in elite athletes of different sport disciplines. Br J Sports Med. 2006;40:675–678. doi: 10.1136/bjsm.2006.026658. discussion 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai E, Matsuo S. Chronic kidney disease in Asia. Lancet. 2008;371:2147–2148. doi: 10.1016/S0140-6736(08)60928-9. [DOI] [PubMed] [Google Scholar]
- 12.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau, Hispanic Population of the United States [April 11, 2010]; Available from http://www.census.gov/population/www/socdemo/hispanic/tables.html.
- 17.U.S. Census Bureau, The Hispanic Population [April 23, 2010];Census 2000 Brief. Available from http://www.census.gov/prod/2001pubs/c2kbr01-3.pdf.
- 18.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 19.He Q, Heo M, Heshka S, et al. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr. 2003;78:72–77. doi: 10.1093/ajcn/78.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Holden C. Peering under the hood of Africa's runners. Science. 2004;305:637–639. doi: 10.1126/science.305.5684.637. [DOI] [PubMed] [Google Scholar]
- 21.van Deventer HE, George JA, Paiker JE, et al. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem. 2008;54:1197–1202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]
- 22.Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- 23.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 24.Imai E, Horio M, Nitta K, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927–937. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? Am J Kidney Dis. 2009;53:932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38:744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim H, Mondress M, Tello A, et al. An alternative formula to the Cockcroft-Gault and the modification of diet in renal diseases formulas in predicting GFR in individuals with type 1 diabetes. J Am Soc Nephrol. 2005;16:1051–1060. doi: 10.1681/ASN.2004080692. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 30.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 31.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 32.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 33.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 34.Gonwa TA, Jennings L, Mai ML, et al. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10:301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 35.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 36.Bosma RJ, Doorenbos CR, Stegeman CA, et al. Predictive performance of renal function equations in renal transplant recipients: an analysis of patient factors in bias. Am J Transplant. 2005;5:2193–2203. doi: 10.1111/j.1600-6143.2005.00982.x. [DOI] [PubMed] [Google Scholar]
- 37.Rook M, Hofker HS, van Son WJ, et al. Predictive capacity of pre-donation GFR and renal reserve capacity for donor renal function after living kidney donation. Am J Trans. 2006;6:1653–1659. doi: 10.1111/j.1600-6143.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- 38.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 39.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 40.Mauer M, Zinman B, Gardiner R, et al. ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2002;3:262–269. doi: 10.3317/jraas.2002.048. [DOI] [PubMed] [Google Scholar]
- 41.Hansen HP, Tauber-Lassen E, Jensen BR, et al. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62:220–228. doi: 10.1046/j.1523-1755.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant. 2002;17:1019–1024. doi: 10.1093/ndt/17.6.1019. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63:1874–1880. doi: 10.1046/j.1523-1755.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 44.Mathiesen ER, Hommel E, Giese J, et al. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. Brit Med J. 1991;303:81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarnow L, Rossing P, Jensen C, et al. Long-term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2000;23:1725–1730. doi: 10.2337/diacare.23.12.1725. [DOI] [PubMed] [Google Scholar]