Summary
Autoimmune diabetes is characterized by inflammatory infiltration; however the initiating events are poorly understood. We found that the islets of Langerhans in young non-obese diabetic (NOD) mice contained two antigen presenting cell (APC) populations: a major macrophage and a minor CD103+ dendritic cell (DC) population. By four weeks of age, CD4+ T cells entered islets coincident with an increase of CD103+ DCs. In order to examine the role of the CD103+ DCs in diabetes, we examined Batf3-deficient NOD mice that lacked the CD103+ DCs in islets and pancreatic lymph nodes. This led to a lack of autoreactive T cells in islets and, importantly, no incidence of diabetes. Additional examination revealed that presentation of major histocompatibility complex (MHC) class I epitopes in the pancreatic lymph nodes was absent with a partial impairment of MHC class II presentation. Altogether, this study reveals that CD103+ DCs were essential for autoimmune diabetes development.
Keywords: Type 1 diabetes, antigen presenting cells, islets of Langerhans, non-obese diabetic mouse, Batf3
Introduction
The progression of type 1 diabetes in non-obese diabetic (NOD) mice has been extensively investigated using a number of different approaches, with an emphasis on the involvement of different T cell subsets, effector molecules, and cytokines (Anderson and Bluestone, 2005). However, an evaluation of the role of specific antigen presenting cell (APC) subsets, particularly as it concerns the critical initial stages of diabetes, has been examined in limited ways. Normal islets of Langerhans (islets) in all species, including the prediabetic NOD mouse strain, contain APCs that are required for normal islet function (Calderon and Unanue, 2012). These APCs take up secretory granules and are instrumental in the presentation of insulin peptides, a major antigenic determinant in NOD diabetogenesis (Mohan et al., 2010, 2013). Presentation of diabetogenic antigens in the draining pancreatic lymph nodes (pLN) is also central to diabetes development, strongly pointing to a translocation of some of the islet APCs into them (Gagnerault et al., 2002; Höglund et al., 1999; Kurts et al., 1997; Levisetti et al., 2004; Zhang et al., 2002).
Diverse findings point out that CD4+ T cell responses are a major component of the islet autoreactivity, particularly to insulin-derived peptides (Brezar et al., 2011; Daniel et al., 1995; Nakayama et al., 2005; Unanue, 2014; Wegmann et al., 1994; Zhang et al., 2008). These findings agree with the dependence on alleles of class II major histocompatibility complex (MHC) genes for disease susceptibility in both mouse and human type 1 diabetes. Although there is a strong influence of class II genes in both mice and humans, there is also a strong component of reactivity to peptides presented by class I MHC molecules for disease development. Cooperativity between diabetogenic CD4+ and CD8+ T cells has been extensively studied in the NOD mouse model (Bendelac et al., 1987; Christianson et al., 1993; de Jersey et al., 2007; Krishnamurthy et al., 2006, 2008; Miller et al., 1988; Serreze et al., 2004).
In the mouse, the Batf3 transcription factor regulates the differentiation of a subset of conventional dendritic cells (cDCs) that comprises the CD8α+ DC in secondary lymphoid organs and the tissue resident CD103+ DC (Edelson et al., 2010; Hildner et al., 2008; Mashayekhi et al., 2011). This subset of cDCs is important in the presentation of class I-MHC bound peptides derived from microbes, dead cells, and cell bound antigens. This subset appears to be less involved in CD4+ T cell responses. Reports examining mice with ablation of the Batf3 gene are enlightening in showing the role of the Batf3-dependent CD8α+ and CD103+ DC subset in immune responses to viruses, tumors, and parasites (Belz et al., 2004, 2005; den Haan et al., 2000; Liu and Nussenzweig, 2010; Pooley et al., 2001; Satpathy et al., 2012). Previous studies show that the CD8α+ and CD103+ DC subsets correspond to the same lineage of DCs (Edelson et al., 2010)
We have evaluated here the effects of a Batf3 null mutation on the autoimmune diabetes of the NOD mouse. We first carried out a comprehensive cellular and gene expression analysis of the islet APCs of NOD mice, concluding that islets contained a small set of CD103+ DC together with a major component of macrophages. In NOD mice, there was an increase in the number of CD103+ DC in islets from the 4th to 12th week of age, coinciding with the entrance of CD4+ T cells. This increase required autoreactivity to insulin. Next, the absence of CD103+ DC in NOD.Batf3−/− mice changed the islet genetic profile to one that resembled NOD.Rag1−/− mice. The NOD.Batf3−/− mice lacked any evidence of autoreactivity and diabetes was absent. Therefore, a small subset of DC controls the development of diabetic autoimmunity.
Results
Batf3-deficient mice do not develop diabetes
A 129S6.Batf3−/− allele (Hildner et al., 2008) was backcrossed to the NOD background by speed congenic approaches and confirmed by microsatellite analysis (Table S1). Single nucleotide polymorphism (SNP) analysis showed that the NOD.Batf3−/− mice contained all the insulin-dependent diabetes (IDD) susceptibility alleles (Table S2). NOD.Batf3−/− mice developed normally, were resistant to diabetes, and no female mice developed disease. The cumulative incidence of diabetes in our female NOD colony is about 85% (Figure 1A). NOD.Batf3+/− mice showed a similar incidence of diabetes. Histological analysis of the NOD.Batf3−/− mice at different ages showed normal islet morphology (Figure 1B-D). The islet in Figure 1B was representative of the typical NOD profile with intra-islet insulitis and peri-insulitis. The islets shown in Figure 1C-D are representative of at least 20 NOD.Batf3−/− pancreata examined. No lymphocyte infiltration was found in more than ~400 islets examined among the various samples.
Figure 1. Absence of diabetes or immunological infiltrates in NOD.Batf3−/− mice.
(A) Diabetes incidence in female NOD (n=24), NOD.Batf3−/− (n=24), and NOD.Batf3+/− (n=8). Hematoxylin and eosin staining of (B) a 6 week female NOD islet, (C) a 6 week female NOD.Batf3−/− islet, and (D) a 52 week female NOD.Batf3−/− islet. Scale bars represent 100 μm. Flow cytometric profiles of CD4+ and CD8+ T cells from NOD and NOD.Batf3−/− (E) thymocytes and (F) splenocytes, respectively. Percentage of CD103+ and CD11blo to negative cells of (G) pLN and (H) mesenteric lymph nodes (gated on CD45+, CD11c+, MHC-II+). (I) Percentage of DEC205+ (CD205) and CD8α+ cells of spleen (gated on CD45+, CD11c+, MHC-II+ cells). All flow cytometry plots are representative of two or more independent experiments. See also Figure S1.
Examination of thymi, lymph nodes, and spleens from NOD.Batf3−/− showed normal percentages and ratios of CD4+ and CD8+ T cells, but the CD8α+ and CD103+ DCs were low to undetectable (Figure 1E-I, Figure S1D-F). These results were comparable to those reported in 129S6.Batf3−/− mice (Edelson et al., 2010; Hildner et al., 2008). The percentage of CD25+FoxP3+ positive T cells in the thymus, spleen, and mesenteric lymph node was identical between NOD mice and the NOD.Batf3−/− mice (Figure S1A-C). In summary, the only detectable immune cell defect in the NOD.Batf3−/− mice was the absence of the CD8α+ and CD103+ DC lineage.
NOD islets of Langerhans contain macrophages and Batf3-dependent CD103+ DCs
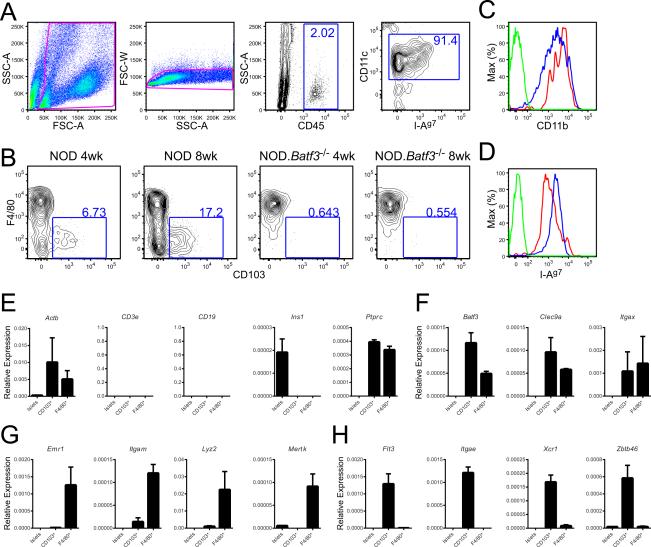
In order to place the findings related above to the events taking place in islets of NOD and NOD.Batf3−/− mice, it was necessary to examine clean islet APC profiles (Li et al., 2009). Islets from NOD contained two sets of APCs, the majority of them (80-90%) exhibited a macrophage profile when staining surface molecules: high surface expression of F4/80, CD11b, CD11c, and age-dependent surface expression of I-Ag7 (Figure 2A-D). These cells have been previously termed by us and others as islet DCs (Calderon et al., 2008; Ginhoux et al., 2009; Melli et al., 2009; Yin et al., 2012). However, a deeper analysis shows that they are macrophages.
Figure 2. Two sets of myeloid cells are identified in NOD mice islets.
(A) Gating strategy for dispersed islets. (B) F4/80+ and CD103+ staining of myeloid cells from 4 week and 8 week NOD (left) and NOD.Batf3−/− (right) islets as gated in panel (A). (C) CD11b+ and (D) MHC class II+ flow cytometry of F4/80+ (Red), CD103+ (Blue), or unstained (Green) islet myeloid cells as gated in panels (A). Representative flow cytometry plots and cumulative data from two or more independent experiments with each experiment pooling two or more mouse islets per sample.(E-H) Quantitative RT-PCR (qPCR) was performed on sorted populations of islet cells. Islets represent large and granular CD45- cells. F4/80+ and CD103+ were gated as shown in Figure S2. All qPCR is represented relative to the expression of 18s rRNA (ΔCt). All qPCRs represent data from 2 biological replicates of 10-15 pooled female NOD mouse islets each performed in duplicate (error bars, SD). All mice used for qPCR were 10-12 weeks old. See also Figure S2.
At 4 weeks of age, CD103+ DCs were found in small numbers, ranging from 2-7% of the islet myeloid cells (CD45+, CD11c+, MHC-II+) (Figure 2B). At this time, the percentage of CD45+ cells in islets ranged from 1-3% (Figure 2A). CD103+ DCs were CD11chi, expressed intermediate amounts of CD11b and I-Ag7, but did not express F4/80 (Figure 2B-D). A small number of CD45+, CD11c+, MHC-II+, F4/80−, CD103− cells were also found (Figure 2B).
To further characterize the two sets of APCs and endocrine cells, qRT-PCR was performed on cells isolated from 10-12 week old mouse islets (Figure 2E-H, Figure S2). Expression of CD3e, CD19, and Ins1 was absent from the myeloid populations ensuring the purity of our sorted populations (Figure 2E). Ins1 expression was solely found in the islet cells and Ptprc (CD45) was expressed in the myeloid populations (Figure 2E). Both the F4/80+ and CD103+ cells expressed Batf3, Clec9a, andItgax (CD11c) (Figure 2F). The sorted F4/80+ cells expressed macrophage characteristic genes (Gautier et al., 2012): Emr1 (F4/80), Itgam (Cd11b), Lyz2, and Mertk, while expressing low to undetectable DC-linked genes such as Flt3, Itgae (CD103), Xcr1, and Zbtb46 (Figure 2G-H). This expression profile contrasted with that of the CD103+ DC, which displayed high expression of Zbtb46, Xcr1, and Flt3, low expression of Itgam and Lyz2, and no detectable Emr1 (F4/80) (Figure 2G-H) (Crozat et al., 2011). Thus, the gene expression analysis together with the surface markers established the identity of the two major intra-islet myeloid cells as macrophages and CD103+ DCs.
The CD103+ DCs were absent from islets of the NOD.Batf3−/− mice that contained only the macrophages. The third set of CD45+ cells (CD45+, CD11c+, MHC-II+, F4/80−, and CD103−) shown in the lower left quadrant in Figure 2B was also absent or reduced. This cell may represent an early stage of the CD103+ lineage in the islets, but regardless it was capable of presenting antigen as shown below.
NOD mouse islets develop a synchronous increase in CD103+ DC and T cells
Two major changes took place in islets of NOD mice starting at 4-6 weeks of age. One was an increase in the number of CD103+ DCs peaking at 20% of the total myeloid population by the 8-12th week of age (Figure 3A,C). Second, coinciding with the increase in CD103+ DCs, was the appearance of CD3ε+ T cells within the islets (Figure 3B,D) (Carrero et al., 2013). No CD103+ myeloid cells were found in the islets of NOD.Batf3−/− mice at any time (Figure 3A,C). Islets from NOD.Batf3−/− mice did not contain intra-islet T cells but only the infrequent cells found as passenger leukocytes, their percentage never exceeded the baseline found in all NOD congenic strains (Figure 3B, D).
Figure 3. Lymphocyte infiltration is absent in NOD.Batf3−/− mice islets.
Flow cytometric analysis of (A) CD103+ myeloid cells gated as in Figure 2A and(B) CD3ε+ lymphocytes (gated on CD45+ cells) from 12 week NOD, NOD.Batf3−/−, NOD.B16A, NOD.H4, and NOD.Rag1−/− islets. (C) Representative graph of data from (A) including time course of NOD from 3 to 12 weeks of age. (D) Representative graph of data from (B) including NOD time course from 3 to 12 weeks of age. Representative flow cytometry plots and cumulative data from three or more independent experiments with each experiment pooling two or more mouse islets per sample (error bars, SD). (E) Flow cytometry of islet lymphocyte and myeloid cells from NOD, NOD.Batf3−/−, and NOD.Batf3−/− that received either NOD.Rag1−/− or NOD.Batf3−/−× Rag1−/− bone marrow 23 weeks prior to being harvested. See also Figure S3.
In order to determine the ability of the CD103+ DC to promote T cell entry into islets, we utilized bone marrow chimeras to reconstitute the missing CD8α+ and CD103+ DC lineage. NOD.Batf3−/− mice were irradiated at 3 weeks of age and NOD.Rag1−/− bone marrow was injected to reconstitute the missing DC lineage without introducing Batf3 sufficient lymphocytes. The missing CD103+ DCs were reconstituted in both the islets and the pancreatic lymph nodes of the chimeras (Figure 3E, Figure S3). Most notably, the islets of the chimeric mice displayed lymphocyte infiltration (Figure 3E). In contrast, control NOD.Batf3−/− × Rag1−/− bone marrow was incapable of reconstituting the CD103+ DCs or inducing lymphocyte infiltration (Figure 3E, Figure S3A). The pancreatic lymph node lymphocyte populations were unaffected by the bone marrow transfer (Figure S3B). This data reinforced the necessity of the CD103+ DCs for lymphocyte entry into islets and progression of type 1 diabetes, and it established that the defect in diabetogenesis in NOD.Batf3−/− mice was not intrinsic to the lymphocyte population.
Non-diabetic NOD congenic strains contain low numbers of CD103+ DC
To examine the relationship between the presence of lymphocytes and the rise in CD103+ DCs between the 4-12 week period, the NOD.Rag1−/− was examined. The myeloid (CD11c+, MHC-II+, CD45+) fraction of the islets were mostly macrophages (~95%), but also showed the low basal number of CD103+ DC (~5%). Their islets did not show the increase in CD103+ DC that took place after 4 weeks of age in NOD mice (Figure 3A, C).
In order to understand the correlation with CD103+ DC entry into islets with T cell pathogenicity, we examined the NOD.H4 mouse which has an intact lymphocyte compartment incapable of driving autoimmune diabetes (Podolin et al., 1993). Its genomic identity is derived from the NOD background except that it contains the H-2h4 MHC haplotype. The islets showed a low basal number of CD103+ DCs that never exceeded 2-5% of the islet myeloid cells (Figure 3A,C); leukocytes within their islets mostly consisted of the F4/80+ macrophages (Figure 3A). These small numbers of CD103+ DCs did not change with age. Like in other non-diabetogenic NOD congenic strains, lymphocytes were not present in the islets of NOD.H4 mice at 12 weeks, and the number of passenger T cells was about 4-6% of the very few islet CD45+ cells (Figure 3B, D).
The NOD.B16A mouse was developed to examine the importance of insulin recognition in NOD diabetes (Nakayama et al., 2005). This strain lacks Ins1 and Ins2 gene expression and has an Ins2 transgene with a mutation of tyrosine 16 to alanine within the insulin beta chain. This mutation ablates T cell recognition of a majority of insulin reactive T cells. NOD.B16A mice do not develop diabetes. These findings support insulin autoreactivity as the likely initiating event of the diabetic process (Brezar et al., 2011; Jasinski and Eisenbarth, 2005; Unanue, 2014). We have reported that the NOD.B16A strain lacks the intra-islet lymphocytes that appear by the 4-6th week of life in NOD mice. Moreover, insulin reactive CD4+ T cells from a T cell receptor transgenic mouse do not enter their islets (Mohan et al., 2013). The islets of the NOD.B16A mice contained mostly macrophages with the low basal percentage of CD103+ DCs. These islets did not have the burst of CD103+ DCs in islets and only had the small number of passenger lymphocytes (Figure 3A-D).
These findings indicate that there is a low basal percentage of CD103+ DCs within the islets of all NOD congenic strains. In NOD, there is a rapid increase in the CD103+ DCs that coincides with the infiltration of CD4+ T cells. From previous studies, the entrance of CD4+ T cells precedes that of CD8+ T cells (Carrero et al., 2013). Both this basal percentage and the burst of CD103+ DCs were absent in mice lacking the Batf3 transcription factor. The increase above baseline of CD103+ DC was dependent on the presence of lymphocytes, since it was absent in NOD.Rag1−/− mice. Moreover, the increase in CD103+ DCs also required autoreactivity to insulin, e.g. it was absent in the NOD.B16A mice that did not go on to develop diabetes. Reconstitution of the CD8α+ and CD103+ lineage in the NOD.Batf3−/− mouse led to T cell and CD103+ DC entry into islets and diabetes development. Thus, for the autoreactive process to be activated there is an absolute requirement of both insulin reactive T cells and CD103+ DCs.
The NOD. Batf3−/− mice display a transcriptional signature identical to the non-diabetic NOD.Rag1−/− mice
We performed gene expression analyses on RNA from NOD, NOD.Batf3−/− and NOD.Rag1−/− islets to determine if the block in diabetogenesis seen in the NOD.Batf3−/− mice had a unique gene expression signature. Previous studies show that the islets of 4-6 week old NOD mice had an upregulation of inflammatory gene signatures enriched in interferon responsive genes (Carrero et al., 2013).
The typical autoreactive gene signature of the early 6-8 week NOD mouse was absent from the islets of NOD.Rag1−/− and NOD.Batf3−/− mice (Figure 4). The gene expression differences by 6 weeks were represented as response to stress, immune response, and antigen processing and presentation signatures (Figure 4A,C). By 8 weeks of age, statistically significant changes included inflammatory signatures compatible with immune system process and immune effector process signatures (Figure 4B,C). By 8 weeks of age, all major leukocyte subsets implicated in diabetes were represented in NOD mice (Carrero et al., 2013) but not in the islets of NOD.Batf3−/− or NOD.Rag1−/− (Figure 4B).
Figure 4. Gene expression analysis reveals a quiescent state in NOD.Batf3−/− mice islets.
(A-B) Microarray analysis of the islets of NOD, NOD.Batf3−/− and NOD.Rag1−/− mice. Scatter-plots of the normalized probe intensity of all annotated microarray signals are shown for whole islet preparations. Numbers labeled in red are those that are at least 2-fold different at a 99% confidence interval by moderated t test. Data are plotted at a log2 scale. (A) 6 week and (B) 8 week comparisons of NOD vs. NOD.Rag1−/− (left), NOD vs. NOD.Batf3−/− (middle), and NOD.Batf3−/− vs. NOD.Rag1−/− (right). Several statistically significantly different genes are highlighted in green. (C) Gene Ontology (GO) analysis of the differentially expressed genes between NOD and either NOD.Rag1−/− or NOD.Batf3−/− at 6 or 8 weeks of age. Corrected hypergeometric p values were calculated using the genes selected in the scatter plots shown in panels A-B. Each analysis represents the mean of 3-6 independent biological replicates.
Islets from 6 week old NOD.Rag1−/− and NOD.Batf3−/− mice had virtually identical gene expression profiles. The only genes that were more expressed in 6 week old NOD.Rag1−/− mice when compared with NOD or NOD.Batf3−/− included the Mela, Reg3a, Reg3b, and Reg3g genes as well as a handful of islet-specific genes that did not fit a statistically significant grouping (Figure 4C). These genes appear to be negatively influenced by the systemic presence of lymphocytes in mice, an issue that remains unresolved.
In addition to microarray analyses, we performed qRT-PCR on cDNA from the islets of NOD and NOD.Batf3−/− mice. Transcripts that demonstrated inflammation of the islets, including Cxcl9, Gbp2, Icam1, and Vcam1 were all upregulated during the progression of diabetes (Carrero et al., 2013). These genes were upregulated as early as 4 weeks of age in NOD but not in NOD.Batf3−/− mice (Figure 5A). T cell associated genes (Cd3e; Figure 5A) were present starting at 4 weeks of age. No such increase was found in the islets from NOD.Batf3−/− mice. Coincident with T cell entry, we detected upregulation of Xcl1 and Xcr1 (Figure 5A). XCR1 is highly expressed on CD103+ DCs while XCL1 is produced by activated T cells (Crozat et al., 2010, 2011; Dorner et al., 2009). This likely reflects the burst of CD103+ DCs found by flow cytometry in the experiments reported in Figure 3. Furthermore, there was upregulation of Ifng (Figure 5A) when both Cd3e and Xcl1 were upregulated, indicating the activation of T cells. These findings are consistent with our data showing the lack of increase of CD103+ DCs in conditions where anti-insulin T cells are not becoming activated or infiltrating islets (see Figure 3). The expression of interleukin-12 is one of the important functional hallmarks of the CD103+ DC (Mashayekhi et al., 2011). Il12b transcript expression was higher in the NOD than in NOD.Batf3−/− mice after 6 weeks of age (Figure 5A). We were unable to reliably detect Il12a transcripts in our preparations (Figure 5A shows sporadic detection in ~2 out of 6-9 samples per time point). Finally, we analyzed canonical markers of myeloid cells (Lyz2) and B cells (Cd19). Consistent with our previous publication, Lyz2 (Figure 5A) and Cd19 (Figure 5A) expression increased at 8 weeks of age in NOD but not in NOD.Batf3−/− mice (Carrero et al., 2013).
Figure 5. Real time PCR shows progression of inflammation in NOD but not NOD.Batf3−/− mice islets.
(A) Taqman qPCR quantification of the indicated genes. Bars represent the mean of the ΔCt value of the gene of interest normalized to an Actb control. All values were adjusted by multiplying by a factor of 105 to facilitate visualization. Bars represent the mean +/− S.D. of 6 biological replicates performed and tested in duplicate. P values were calculated using Mann-Whitney U test. (B-D) The indicated populations were sorted from (B) NOD.Rag1−/− and NOD.Batf3−/− as shown previously, (C-D) NOD mouse islets as in Figure S4A, and (D) CD11c+ MHC-II+ cells from mediastinal lymph nodes. Taqman or SYBR Green I qPCR was used to quantify the indicated genes. Bars represent the mean+/− S.D. of 2 biological replicates performed and tested in duplicate. See also Figure S4.
Cytokine and chemokine transcript production was evaluated by qPCR on sorted islet cells from NOD.Rag1−/−, NOD.Batf3−/− (Figure 5B), and NOD (Figure 5C, S4A,C) mice at 10-14 weeks of age. Cells from NOD.Rag1−/− mice were used to determine chemokine patterns in an uninflamed setting when compared to the NOD.Batf3−/− mice. No major differences were found. Purity was confirmed by gene transcript expression of Cd3e, Emr1, Ins1, and Xcr1 (Figure S4B). The CD45− component expressed Ccl2, Ccl19, Ccl21, and Ccl25 (Figure 5B). These correspond to ligands for CCR2, CCR5, CCR7, and CCR9. The islet macrophage expressed Ccl2, Ccl5, Cxcl9, and Cxcl10. These correspond to ligands for CCR1, CCR2, CCR3, CCR5, and CXCR3. No Xcl1 transcript was detectable (Figure 5B). Overall, this chemokine pattern corresponds to dendritic cell and T cell homing.
To determine chemokine and cytokine transcript expression in an inflamed setting, NOD islet cells were sorted. To ensure cleanliness, Cd3e, Cd19, Emr1, Ins1, and Xcr1 gene transcripts expressed in T cells, B cells, macrophages, beta cells, and CD103+ DCs, respectively, were assayed and each gene transcript was solely expressed in the corresponding cell lineage (Figure S4C). The most notable changes in cytokine and chemokine patterns was the T cell production of Ifng, the T cell production of Ccl5 and Xcl1, particularly by the CD8+ subset, and the increase in production of chemokine transcripts by the macrophage component (Ccl2 and Cxcl9) and CD45− component (Ccl19 and Cxcl10) (Figure 5C).
Figure 5D shows qPCR expression of genes for retinoic acid (RA) production: Aldh1a1 and Aldh1a2. RA production is associated with CD103+ CD11b+ DCs of the intestine, mesentery, and lung and linked to the conversion of T cells to the T regulatory cell lineage (Agace and Persson, 2012; Grainger et al., 2014). The expression of Aldh1a1 was undetectable in any of the APC populations in the islet while the expression of Aldh1a2 on the CD103+ DC component was 5% of the mediastinal lymph node DC (lung DC) positive control (Figure 5D). This data affirmed that the Islet CD103+ DC was not of the CD103+CD11b+ gut regulatory lineage.
Antigen presentation in the islets and the pLNs of NOD.Batf3−/− mice is reduced
To determine if the block in diabetogenesis was due to an absence of peptide presentation or T cell priming, antigen presentation was tested with two diabetogenic CD4+ and one CD8+ T cell receptor (TCR) transgenic lines in the pLN and the islet proper. The BDC2.5 TCR transgenic T cell (Katz et al., 1993) recognizes a beta cell antigen thought to be derived from chromogranin A (Stadinski et al., 2010).The CD4+ T cell 8F10 recognizes the insulin B chain peptide segment 12-20 (Mohan et al., 2013). The CD8+ TCR transgenic 8.3 recognizes a peptide from the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (Lieberman et al., 2003). To determine if the two APC of the islets in NOD could present diabetogenic peptides, we examined macrophages and CD103+ DCs sorted from islets of NOD mice. Intra-islet APCs contain peptide-MHC complexes derived from beta cells and stimulate the BDC2.5 T cells as well as the 8F10 T cells (Calderon et al., 2008; Mohan et al 2010). Both APCs presented equally to either T cell (Figure S5A). There was no presentation to 8.3 (Figure S5A), which is an expected result (Tsai et al., 2008).
CFSE labeled splenocytes from BDC2.5 TCR transgenic mice proliferated in the pLN of NOD mice but the degree of proliferation in the NOD.Batf3−/− mice was reduced to about half of that found in NOD mice (Figure 6A). A percentage of the injected T cells entered the islets of NOD mice where proliferation also took place (Figure 7A-B). However, there was no entrance of the labeled T cells into islets of NOD.Batf3−/− mice (Figure 7A). Lastly, islets were isolated, dispersed and their presentation to BDC2.5 was tested. The BDC2.5 T cell reacted equivalently to NOD and NOD.Batf3−/− dispersed islet cells (Figure 7C).
Figure 6. TCR transgenic T cells proliferate more effectively in the pancreatic lymph nodes of NOD than NOD.Batf3−/− mice.
CFSE dilution of transferred (A) BDC2.5 NOD CD4+ T cells and (B) 8.3 NOD CD8+ T cells isolated from the pancreatic lymph nodes and inguinal lymph nodes from recipient 8-12 week NOD or NOD.Batf3−/− mice. Cells were assayed 3 days post transfer. Dots represent individual mice. P values were calculated using Mann-Whitney U test (*** P < 0.005).
Figure 7. TCR transgenic T cell entrance into NOD or NOD.Batf3−/− islets and splenocyte transfer into recipients.
(A) Percentage entering islets and (B) CFSE dilution of CD45.1+ and CD45.2+ BDC2.5 NOD CD4+ T cells (both at day 3 post transfer). (D) Percentage entering islets and (E) CFSE dilution of CD45.1+ and CD45.2+ 8F10 NOD CD4+ T cells transferred into 6-12 week NOD or NOD.Batf3−/− mice (both at day 7 post transfer). Dispersed islet cell presentation to (C) BDC2.5 hybridoma and (F) 8F10 hybridoma as measured by CTLL-2 assay for IL-2. Each dot represents an independent T cell assay. Representative flow cytometry plots and cumulative data from two or more independent experiments. P values were calculated using Mann-Whitney U test (** P<0.005). (G) Diabetic NOD splenic T cells were isolated using CD90.2 magnetic beads and then 107 cells were transferred to the indicated irradiated (600 cGy) recipients. (H) 10-12 week old NOD or NOD.Batf3−/− splenocytes (107) were transferred into NOD.Rag1−/− recipients. Recipient mice in (G) and (H) were then monitored for diabetes. Diabetes incidence was monitored as indicated in the main methods section.
The 8F10 TCR transgenic T cell does not proliferate in the pLN, but rather in the islets bypassing the pLN (Mohan et al., 2013). The T cells entered and proliferated in the islets of NOD mice but did not enter the islets of the NOD.Batf3−/− mice (Figure 7D-E). The presentation from isolated islet cells of NOD.Batf3−/− was statistically significantly reduced when compared to NOD (Figure 7F). We presume presentation came from the islet macrophages since there were no CD103+DC in their islets. Thus, despite the macrophages containing the relevant peptide-MHC complexes, in the absence of the CD103+ DC, T cell localization was impaired.
CFSE labeled 8.3 T cells proliferated in the pLN of NOD mice, as expected (Lieberman et al., 2003). However, there was no proliferation in the lymph nodes of the NOD.Batf3−/− mice indicating an absence in presentation of this class I peptide (Figure 6B). The 8.3 CD8+ T cells did not migrate into NOD or NOD.Batf3−/− islets (data not shown). We were only able to detect presentation of IGRP peptides to the 8.3 hybridoma when sorted APCs were given exogenous peptide (Figure S5A,B).
The islets of the NOD.Batf3−/− mice were not receptive to the entrance of two CD4+ T cell lines despite having presentation in the islets, albeit less efficiently in the case of the insulin epitope. The findings with the BDC2.5 CD4+ T cells indicated that the presentation in the local draining node was reduced. With regards to MHC-I presentation, the NOD.Batf3−/− mice showed a major deficit in CD8+ T cell presentation and priming. These findings support previous studies showing that cross-priming is required in diabetes (Yamanouchi et al., 2003).
Finally, we examined the ability of T cells harvested from diabetic NOD mice to cause diabetes in NOD.Batf3−/− mice. Spleens from diabetic NOD mice contain activated diabetogenic T cells (Wicker et al., 1986). These splenocytes were transferred into irradiated NOD or NOD.Batf3−/− mice. While about half of recipient NOD mice developed diabetes by the first 10 days, it took 100 days for the NOD.Batf3−/− mice to become diabetic, about 10 fold longer (Figure 7G). Thus, the results confirm that the NOD.Batf3−/− islets are less receptive to diabetogenic T cells. To determine if NOD.Batf3−/− mice had diabetogenic T cells we transferred splenocytes into NOD.Rag1−/− recipients. After 200 days post transfer, 40% of the recipient NOD.Rag1−/− mice became diabetic demonstrating that the defect in diabetogenesis in the NOD.Batf3−/− mouse is not intrinsic to the lymphocytes (Figure 7H). In sum, the lack of diabetes in NOD.Batf3−/− mice is associated with the absence of the CD103+DC and not with a lymphocyte defect. We find defects in antigen presentation in islets and the pLN with a block in the entrance of diabetogenic T cells.
Discussion
This work has established the importance of the CD8α+ and CD103+ DC lineage in the initiation of type 1 diabetes. The absence of Batf3, and consequently this lineage, prevented the induction and subsequent immune infiltration of islets during diabetogenesis. Complementation of NOD.Batf3−/− mice with NOD.Rag1−/− bone marrow was sufficient to reconstitute the CD8α+ and CD103+ DC lineage and corrected the deficit. From this and other experiments we concluded that: (i) the CD8α+ and CD103+ DC lineage was required, and (ii) Batf3 deficiency had no effect on the diabetogenic capacity of lymphocytes.
Islets of NOD mice contain a population of CD103+ DCs evident by the 3rd to 4th week of age along with resident macrophages, the latter representing the largest number of the islet APCs. For these findings on CD103+ DC to be meaningful, it was important to critically define the islet APC as well as the initial events at the time of the first entrance of lymphocytes. After carefully isolating the islets making sure to eliminate lymphoid aggregates, only two sets of islet APCs were identified during the critical initiation of diabetes. Cell surface markers and gene expression analysis supported the separation of these resident myeloid cells into these two distinct lineages.
Several crucial events in the islets characterize the initiation of diabetes. At about 3-4 weeks of age, and only in NOD mice, three events took place: (i) an increase in the CD103+ DCs, (ii) the localization of CD4+ T cells inside islets, presumably insulin reactive, and (iii) changes in gene expression. Notably, the installment of the diabetic process required two interconnected cells: the presence of the CD103+ DCs and of CD4+ T cells that recognized immunogenic insulin. The absence of the CD103+ DC lineage in the NOD.Batf3−/− mouse resulted in a quiescent islet preventing the autoreactive diabetic process for the life of the mouse. Islets of NOD.Batf3−/− mice were comparable with those of the NOD.Rag1−/− mice that lacked the gene signature that characterized the initiation of diabetes.
An important issue is the process whereby CD103+ DCs localize to the islets. Previous reports show that CD8α+ and CD103+ DCs bear the XCR1 chemokine receptor and respond to the chemokine XCL1 (Crozat et al., 2010, 2011; Dorner et al., 2009). Our data showed that the islet CD103+ DCs were XCR1 positive and that XCL1 was expressed by both CD4+ and CD8+ T cells but particularly high by the latter. It is possible that the expression of Xcl1 resulted in the increase in the CD103+ DCs. However, no Xcl1 was detectable in islets of mice that expressed the baseline number of CD103+ DCs, such as in NOD.Rag1−/− mice. Analysis of NOD.Rag1−/− islet demonstrated the production of transcripts for chemoattractants responsible for DC and T cell homing. Previous studies show that these transcripts are specific to the pancreas of NOD and not in other strains of mice (Bouma et al., 2005). However, their role remains unresolved. Expression of chemokine transcripts was low and, despite their presence, the islets of the NOD.Batf3−/− mice lacked DC or lymphocytes suggesting a non-chemokine driven process that drives early lymphoid and myeloid entry into islets
Our data suggests that there are two processes that are influenced by the CD103+ DCs. The first centers on early T cell localization to islets. Because there is absence of T cells in the islets of the NOD.B16A mice, the anti-insulin CD4+ T cells are likely the initiating autoimmune T cells. These anti-insulin CD4+ T cells enter islets of NOD mice in the absence of the pLN (Mohan et al., 2013), but were not found in the NOD.Batf3−/− mice. The lack of entrance was confirmed in the experiments transferring CFSE-labeled anti-insulin CD4+ T cells into the NOD.Batf3−/− mice. The absence of T cells in islets from NOD.Batf3−/− mice occurred despite the presentation of diabetic peptides by the resident macrophages and the presence of diabetogenic T cells in the spleen. Thus, the CD103+ DC is essential for entrance of anti-insulin CD4+ T cells that initiate diabetogenesis. The CD103+ DC may be necessary for the islet to be receptive to T cell entrance through ways that we are now investigating.
The second process influenced by the CD103+ DCs is the priming of CD8+ T cells in the pLN. An absence of presentation of class I MHC epitopes in the NOD.Batf3−/− mice was evident in the draining pLN as determined by transferring IGRP CD8+ reactive T cells. Therefore, the absence of CD103+ DC also results in the ablation of the CD8+ T cell response, which constitutes the effector T cell in diabetes. The involvement of CD8+ T cell in NOD autoimmunity has been recognized since early studies in which spleen cells transferred diabetes into non-diabetic NOD mice, although these required the presence of both CD4+ and CD8+ T cells. The requirement of CD8+ T cells was also noted in mice lacking beta 2-microglobulin and in studies showing that CD8+ T cells directed to beta cell antigens can participate in the diabetic process (de Jersey et al., 2007; Kay et al., 1996; Serreze et al., 1994, 2004; Wang et al., 1996; Wicker et al., 1994). We posit that the CD103+ DC is the essential APC for diabetogenic class I MHC epitopes. This is based on the strong findings with the CD8+ TCR transgenic T cell recognizing a peptide of IGRP. There was no presentation in the pLN, a site thought to be crucial for the selection and activation of CD8+ T cells (Höglund et al., 1999; Kurts et al., 1997; Zhang et al., 2002).
In summary, the data allows us to posit a model for the initiating events of the autoreactive process in islets of Langerhans. The first set of CD4+ T cells that enter islets are the insulin reactive T cells, those akin to the 8F10 TCR transgenic mouse (Mohan et al., 2013). These T cells initiate the autoimmune process, enter islets spontaneously without the need for priming in the pLN, and require both the display of immunogenic insulin and the presence of CD103+ DC [e.g., the findings in the NOD.B16A and NOD.Batf3−/− mice (Mohan et al., 2013; Nakayama et al., 2005)]. Once insulin reactive T cells encounter CD103+ DC, an amplification loop begins: a burst of CD103+ DCs enters islets, antigen presentation increases in the pLN, additional inflammatory cells enter islets, and, importantly, the autoreactive CD8+ T cells are primed. This scenario takes place starting at about the fourth week of life and increases very rapidly during a limited time period, after which the diabetogenic process is in full operation and difficult to control. The pLNs are not required beyond three weeks of age as their removal does not halt the diabetic process (Gagnerault et al., 2002). This working scenario sets the base for future investigations.
Materials and Methods
Mice
NOD/ShiLtJ (NOD), NOD.129S7(B6)-Rag1tm1Mom/J (NOD.Rag1−/−), NOD.Cg-Tg(Ins2*Y16A)1EllIns1tm1JjaIns2tm1Jja/GseJ (NOD.B16A), NOD.Cg-H2h4/DilTacUmmJ (NOD.h4), NOD.Cg-Tg(TcraBDC2.5,TcrbBDC2.5)1Doi/DoiJ (BDC 2.5), NOD.Cg-Tg(TcraTcrbNY8.3)1Pesa/DvsJ (8.3) mice were obtained from the Jackson Laboratory. NOD.Batf3−/− mice were generated by breeding the 129S-Batf3tm1Kmm/J mouse obtained from Dr. Kenneth Murphy to NOD. Speed congenics was utilized selecting for the highest percentage of NOD microsatellite markers at each generation resulting in 100% NOD microsatellite markers at the sixth generation of backcross. SNP analysis by the Jackson Laboratory confirmed the IDD loci were of the NOD background. NOD.Batf3−/−xRag1−/− were generated by intercrossing NOD.Batf3−/− with NOD.129S7(B6)-Rag1tm1Mom/J. For bone marrow reconstitution, donor NOD.Rag1−/− and NOD.Batf3−/−xRag1−/− were administered 150 mg/kg 5-fluorouracil (Sigma-Aldrich, St. Louis, MO) by intraperotineal injection. The bone marrow was harvested 5 days post 5-fluorouracil injection and transferred intravenously into 300 cGY treated 3 week old NOD.Batf3−/− recipients. All mouse experiments were approved by the Division of Comparative Medicine of Washington University School of Medicine (Association for Assessment & Accreditation of Laboratory Animal Care [AAALAC] accreditation number A3381-01).
Histology and islet Isolation
Pancreata were sectioned and stained by conventional techniques. For islet isolation, the pancreata were perfused (5 mL HBSS without calcium supplemented with collagenase), removed, and digested in a 37 °C waterbath for 15 min. After shaking for 90 seconds, the pancreata were washed 3 times and passed through a 70 μm filter. The remains were flushed from the filter and islets were handpicked using the zinc-chelating dye dithizone (Sigma-Aldrich; 200 μg/mL 10% DMSO PBS) as a marker of islets.
Flow Cytometry and Cell Sorting
Flow cytometry was performed using a FACSCanto II (BD Biosciences, Franklin Lakes, New Jersey) and data was analyzed using FlowJo software (Tree Star Software, Ashland, OR). See Supplemetal Information for complete antibody list. Islets were harvested as described previously and dispersed via Cell Dissociation Solution Non-Enzymatic (Sigma-Aldrich) for 5 min at 37°C. Single cell suspensions were treated with 2.4G2 conditioned media (PBS, 1% bovine serum albumin,and 50% 2.4G2 in DMEM) at 4 °C for 15 min to block FC receptors. Cells were then stained with fluorescent antibodies and sorted via FACSAria II (BD Biosciences).
Diabetes Monitoring
Blood glucose was monitored daily or weekly and after two consecutive readings of ≥250 mg/dL mice were considered diabetic (Chemstrip 2GP, Roche Diagnostics, Indianapolis, IN).
RNA Isolation, Real Time PCR, and Micro Array Analysis
RNA isolation and qRT-PCR was performed as in (Carrero et al., 2013) with minor modifications (see supplemental materials and methods). RNA (50 ng) was amplified using Ovation PicoSL WTA System V2 (NuGEN, San Carlos, CA, USA) following the manufacturer's instructions. Amplified RNA (100 ng) was labeled using Affymetrix GeneChip Whole Transcript Sense Target Labeling Assay following the manufacturer's instructions (Affymetrix, Santa Clara, CA, USA). Labeled RNA was hybridized to Mouse Gene 1.0 ST microarrays using a GeneChip Fluidics Station 450 (Affymetrix). Microarrays were scanned using a GeneChip Scanner 3000 7G (Affymetrix). All GeneChip processing steps were performed by the Laboratory for Clinical Genomics at Washington University School of Medicine.
In Vivo T Cell Proliferation and Antigen Presentation Assays
8F10, BDC2.5, and 8.3 TCR transgenic mouse spleens were harvested and dispersed into single cell suspensions. CD4 or CD8 positive cells were selected via magnetic microbead cell separation (Miltenyi Biotech, San Diego, CA) according to the manufacturer's protocol. The cells were then stained with 1 μM CFDA SE (Life Technologies) for 15 min at 37 °C and immediately quenched with 4 °C DMEM supplemented with 10% FCS. 5-10 × 106 cells were transferred via intravenous injection into recipient mice. After 3-7 days, islets, draining pancreatic lymph nodes, and inguinal lymph nodes were harvested, dispersed into single cell populations, and stained with fluorescent antibodies. Flow cytometry was performed and analyzed as described above. For antigen presentation assays, T cell hybridomas (5 × 104 per well) were cultured with dispersed islet cells (5 × 104 per well) and with peptide pulsed APC (5 × 104 or 105 per well) as controls. After incubating for 18 hours, the culture supernatant of each well was assayed for IL-2 production through use of the IL-2 dependent cell line CTLL-2. Proliferation of CTLL-2 was assessed by [3H] thymidine incorporation.
Statistical Analysis
Mann–Whitney U test was used to determine significant differences between samples. All data was plotted using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Supplementary Material
Acknowledgements
We thank all the members of our laboratory for many helpful discussions and assistance, but in particular to Katherine E. Frederick, Xiaoxiao Wan, Lindsay Moore, and Anthony N. Vomund. This research was supported by the National Institutes of Health grants DK058177 and DK020579, Juvenile Diabetes Research Foundation (JDRF) 17-2012-141, and the Kilo Diabetes and Vascular Disease Foundation (Kilo). Experimental support was provided by the Rheumatic Diseases Core Center For Speed Congenics and supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P30AR048335). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, JDRF, or the Kilo foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2012;33:42–48. doi: 10.1016/j.it.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 1950. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J. Exp. Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Coppens JMC, Mourits S, Nikolic T, Sozzani S, Drexhage HA, Versnel MA. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur. J. Immunol. 2005;35:2386–2396. doi: 10.1002/eji.200526251. [DOI] [PubMed] [Google Scholar]
- Brezar V, Carel J-C, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr. Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- Calderon B, Unanue ER. Antigen presentation events in autoimmune diabetes. Curr. Opin. Immunol. 2012;24:119–128. doi: 10.1016/j.coi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PloS One. 2013;8:e59701. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre C-A, Ventre E, Vu Manh T-P, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J. Exp. Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat K, Tamoutounour S, Vu Manh T-P, Fossum E, Luche H, Ardouin L, Guilliams M, Azukizawa H, Bogen B, Malissen B, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α+ type. J. Immunol. 1950. 2011;187:4411–4415. doi: 10.4049/jimmunol.1101717. [DOI] [PubMed] [Google Scholar]
- Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnerault M-C, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J. Exp. Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Askenase MH, Guimont-Desrochers F, da Fonseca DM, Belkaid Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol. Rev. 2014;259:75–87. doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski JM, Eisenbarth GS. Insulin as a primary autoantigen for type 1A diabetes. Clin. Dev. Immunol. 2005;12:181–186. doi: 10.1080/17402520500078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jersey J, Snelgrove SL, Palmer SE, Teteris SA, Mullbacher A, Miller JFAP, Slattery RM. Beta cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1295–1300. doi: 10.1073/pnas.0610057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Kay TW, Parker JL, Stephens LA, Thomas HE, Allison J. RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null nonobese diabetic mice. J. Immunol. 1950. 1996;157:3688–3693. [PubMed] [Google Scholar]
- Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, Santamaria P, Thomas HE, Kay TWH. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J. Immunol. 1950. 2008;180:4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisetti MG, Suri A, Frederick K, Unanue ER. Absence of lymph nodes in NOD mice treated with lymphotoxin-beta receptor immunoglobulin protects from diabetes. Diabetes. 2004;53:3115–3119. doi: 10.2337/diabetes.53.12.3115. [DOI] [PubMed] [Google Scholar]
- Li D-S, Yuan Y-H, Tu H-J, Liang Q-L, Dai L-J. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 2009;4:1649–1652. doi: 10.1038/nprot.2009.150. [DOI] [PubMed] [Google Scholar]
- Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol. Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, et al. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli K, Friedman RS, Martin AE, Finger EB, Miao G, Szot GL, Krummel MF, Tang Q. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J. Immunol. 1950. 2009;182:2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Appel MC, O'Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J. Immunol. 1950. 1988;140:52–58. [PubMed] [Google Scholar]
- Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat. Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J. Exp. Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. IE+ nonobese diabetic mice develop insulitis and diabetes. J. Exp. Med. 1993;178:793–803. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8-dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 1950. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Holl TM, Marron MP, Graser RT, Johnson EA, Choisy-Rossi C, Slattery RM, Lieberman SM, DiLorenzo TP. MHC class II molecules play a role in the selection of autoreactive class I-restricted CD8 T cells that are essential contributors to type 1 diabetes development in nonobese diabetic mice. J. Immunol. 1950. 2004;172:871–879. doi: 10.4049/jimmunol.172.2.871. [DOI] [PubMed] [Google Scholar]
- Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat. Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv. Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Antigen Presentation in the Autoimmune Diabetes of the NOD Mouse. Annu. Rev. Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur. J. Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- Wicker LS, Miller BJ, Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986;35:855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- Yamanouchi J, Verdaguer J, Han B, Amrani A, Serra P, Santamaria P. Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J. Immunol. 2003;171:6900–6909. doi: 10.4049/jimmunol.171.12.6900. 1950. [DOI] [PubMed] [Google Scholar]
- Yin N, Xu J, Ginhoux F, Randolph GJ, Merad M, Ding Y, Bromberg JS. Functional specialization of islet dendritic cell subsets. J. Immunol. 1950. 2012;188:4921–4930. doi: 10.4049/jimmunol.1103725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, O'Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP. In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J. Immunol. 1950. 2002;168:1466–1472. doi: 10.4049/jimmunol.168.3.1466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.