Abstract
Red blood cells, currently obtained from donors, represent the most common form of cell-based therapy. A better understanding of normal erythropoiesis is leading to improved multi-step protocols for the in vitro generation of fully mature red cells. The extensive in vitro expansion of embryonic erythroblasts and development of erythroid precursors as a potential transfusion product may help to deal with issues of scale and eventually find a place in the treatment of patients with acute and chronic anemias.
Introduction
Anemia is an extremely common disorder afflicting more than 2 billion people worldwide and 10% of the US population, with the highest incidence among the elderly. Furthermore, anemia is a common toxicity of cancer therapies, major surgery, and trauma, necessitating the delivery of more than 16 million red blood cell (RBC) transfusions every year in the United States. The need for RBC transfusions is predicted to rise as our population continues to age. Currently, RBC products are obtained from donors, which entails infectious risks and requires costly screening. In addition, donors for rare blood types are scarce, creating frequent supply bottlenecks. Given these serious concerns, alternative sources of RBCs are essential. Consequently, numerous efforts are underway to expand erythroid precursors and differentiate them in vitro into mature RBCs. Furthermore, erythroid precursors may ultimately serve as a novel cell-based therapy providing a renewable source of RBCs.
The first cell-based therapy
The transfusion of RBCs is the first documented form of cell-based therapy and its history illustrates how the translation of innovative clinical ideas into practice may entail a long and arduous path. The first successful blood transfusion, from one dog to another, was recorded in 1665, after the discovery of circulation but almost ten year before the first microscopic identification of RBCs by Antonie van Leeuwenhoek. This was followed in 1667 by a sheep to man transfusion. Despite numerous attempts, it took an additional 150 years before the first successful human-to-human blood cell transfusion occurred with the treatment of postpartum hemorrhage using a husband-to-wife transfusion (reviewed by 1). The first functional replacement therapy occurred in 1840 with whole blood transfusion treatment of haemophilia. These innovative clinical procedures, however, had random success rates and injurious consequences until the discovery of blood types by Karl Landsteiner in 1901. This discovery turned transfusion medicine into a science and earned Dr. Landsteiner a Nobel Prize for Medicine in 1930 (1). The study of the ABO and multiple minor blood group antigens led to the recognition of O-negative individuals as universal donors. Unfortunately, they represent <8% and <1% of individuals in Western and Asian nations, respectively. With the discovery of anticoagulants and the establishment of blood banks in the United States during the 1940s and 1950s, the transfusion of RBCs became widespread and common.
Currently, over 80 million RBC donations are made each year worldwide (2). This approach, with all RBC products obtained from donors, requires costly screening to minimize the inherent infectious risks. While the blood supply of industrialized countries remains adequate overall, chronic shortages of special blood units needed for alloimmunized patients are common. In addition, it is likely that blood donations may become insufficient to meet future demands. Demographic calculations based on the recent changes on the median age of the US population predict that the number of persons likely to require transfusions, primarily older individuals, will exceed that of persons who are likely to donate, primarily younger individuals, making the blood supply inadequate by 2050 (3). Furthermore, disruption of normal blood collection by natural disasters and social-political emergencies could result in blood shortages of unpredictable duration and severity at any time. One strategy to circumvent these problems, the use of synthetic hemoglobin substitutes, is associated with numerous side effects, including hypertension and increased risk of myocardial infarctions, currently obviating their clinical use (4). Another potential strategy, the production of blood cells in vitro, will be examined more closely here, following a brief background on normal RBC synthesis.
Erythropoiesis- the synthesis of RBCs
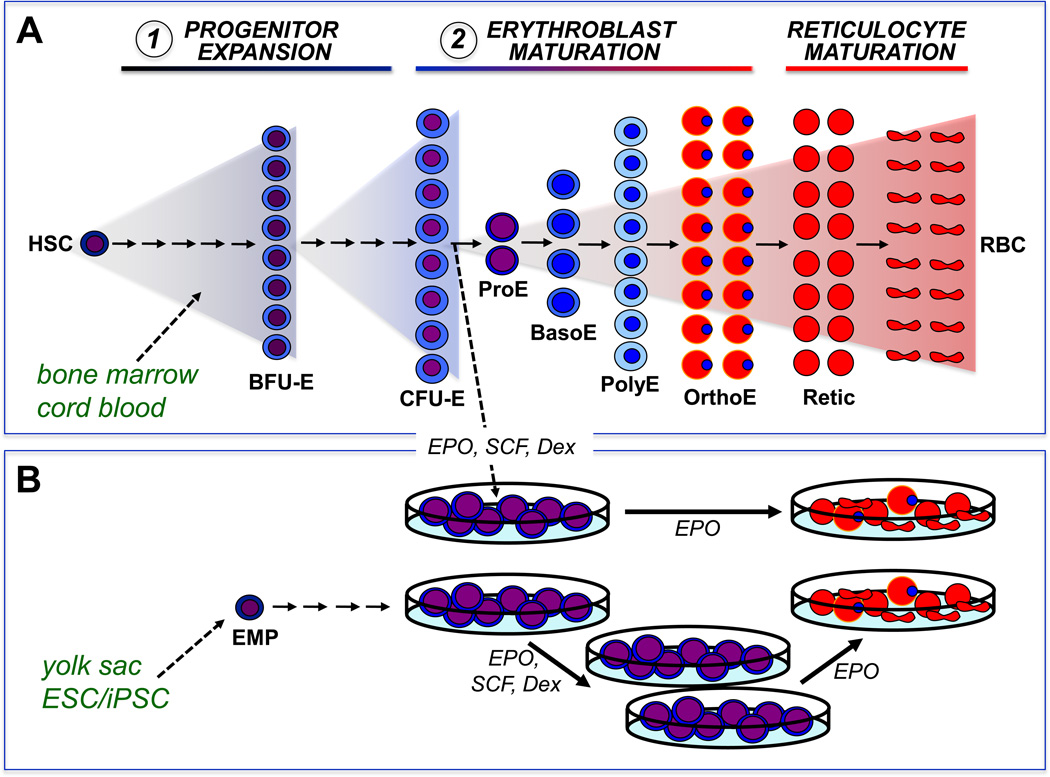
Humans synthesize more than 2 million RBCs every second to maintain a steady state red cell mass consisting of more than 2.5×1013 RBCs. As summarized in Figure 1A, these massive numbers of RBCs are ultimately derived from a small number of hematopoietic stem cells that differentiate into lineage-committed progenitors capable of forming colonies of erythroid cells in semisolid media. Immature erythroid progenitors, termed burst-forming units-erythroid (BFU-E), generate late-stage erythroid progenitors, termed colony-forming units-erythroid (CFU-E). CFU-E subsequently mature into morphologically recognizable erythroid precursors, the most immature of which is the proerythroblast. Proerythroblasts physically interact with macrophage cells in the bone marrow and undergo 3–4 maturational cell divisions as they accumulate hemoglobin, decrease cell size, and condense their nuclei (5). CFU-E and immature erythroid precursors are exquisitely dependent on the cytokine erythropoietin (EPO) for their survival (6). The most mature erythroid precursors, termed orthochromatic erythroblasts, undergo nuclear extrusion to form young RBCs (reticulocytes). Reticulocytes lose all cellular organelles, remodel their cytoskeleton to take on a biconcave shape, and enter the bloodstream to circulate as mature RBCs for 120 days.
Figure 1.
Erythropoiesis is characterized by the progressive expansion of lineage-committed cells from progenitors, to morphologically identifiable precursors, that ultimately enucleate to form mature red blood cells (RBCs).
(A) In vivo, erythropoiesis proceeds from a small number of hematopoietic stem cells (HSC) that generate expanding numbers of lineage committed progenitors, termed burst-forming units erythroid (BFU-E) and colony-forming units erythroid (CFU-E). CFU-E subsequently generate erythroid precursors termed proerythroblasts (ProE), basophilic erythroblasts (BasoE), polychromatophilic erythroblasts (PolyE), and orthochromatic erythroblasts (OrthoE), that accumulate hemoglobin and condense their nucleus. Enucleation results in the generation of reticulocytes (Retic) that lose all internal organelles to become mature RBCs. Erythropoiesis has been modeled in vitro by using two or more steps of liquid culture. The first step is typically associated with the expansion of multipotential and lineage-specific erythroid progenitors, while the second step is associated with the terminal maturation of erythroblasts.
(B) In vitro, dexamethasone (Dex) synergizes with erythropoietin (EPO) and stem cell factor (SCF) to induce immature erythroblasts to undergo self-renewal cell divisions. When cultures are started from newborn or adult hematopoietic tissues, erythroblast self-renewal is ultimately restricted. In contrast, when cultures are initiated from yolk sac or early fetal liver or from differentiating embryonic stem cells that contain erythro-myeloid progenitors (EMP), erythroblasts can undergo extensive self-renewal in vitro. When the dexamethasone and SCF are removed from the cultures, these erythroblasts complete terminal maturation.
Acute hypoxia, the result of loss or destruction of RBCs or of changes in ambient oxygen tension, elicits a stress response characterized by the rapid production of new RBCs. Stress erythropoiesis is dependent not only on high levels of EPO but also on glucocorticoid signaling, since mice with restricted ability to signal through the glucocorticoid receptor have normal steady-state levels of RBCs but delayed recovery from acute chemical-induced anemia (7).
In vitro production of RBCs: the 2-step erythroid culture system
This complex process of erythropoiesis, consisting of progressive phases of 1) progenitor expansion, 2) precursor amplification and maturation, and 3) reticulocyte remodeling into terminal RBCs, has been modeled with varying success in vitro (Figure 1A). The ability to differentiate erythroid cells in vitro relies on several foundational studies, including the generation of erythroidrestricted colonies in semisolid media (8), and the cloning of erythropoietin (EPO), stem cell factor (SCF), and other cytokines (9). Twenty years ago, Fibach made the seminal observation that the conditions that promote erythroid cell maturation, including high levels of EPO, are not permissive for maintaining the cells in proliferation and developed a liquid culture system that included two sequential steps (10). The first step contained glucocorticoids and conditioned media providing cytokines to promote erythroid “progenitor” proliferation (Figure 1A). The second step contained EPO alone to promote survival of late-stage erythroid progenitors and maturation of erythroid precursors. The differentiation of erythroid cells in liquid culture using two or more “steps” has become the standard approach to generate red cells in vitro.
Since its introduction, the 2-step erythroid culture system has undergone significant improvements resulting in the synthesis of ever-increasing numbers of maturing RBCs. The first step of the 2-step culture system has been improved by the replacement of conditioned media with several defined cytokines, including SCF and low concentrations of IL3 and GM-CSF, as well as EPO, to expand the number of BFU-E and maintain the survival of late-stage erythroid progenitors, respectively (11, 12). Other investigators have divided this first step into 2 phases (Figure 1A). The first of these phases contains a cocktail of cytokines to expand multipotent progenitors, which is followed by a second phase that is more erythroid-specific and includes EPO (13, 14, 15). Immature, multipotent hematopoietic progenitors have also been expanded in vitro by culture not only with cytokines but also by using human stromal cells transduced with hTERT (16). Finally, it was also recognized that estradiol, as well as glucocorticoids, can inhibit erythroid maturation and lead to expanded numbers of erythroid “progenitors” in the first phase of erythroid culture (17). The function of glucocorticoids in the self-renewal of erythroid cells will be discussed in more detail below.
The second step of the 2-step erythroid culture, composed of CFU-E and maturing erythroid precursors (Figure 1A), has also undergone several improvements during the past decade. The addition of insulin and thyroid hormone to EPO has improved the terminal maturation of erythroid precursors (18). Furthermore, molecules antagonistic to the action of glucocorticoids and estrogens have been used to overcome any inherent residual block of erythroid precursor differentiation during the second step of in vitro culture (19, 18, 20). Others have used DMSO, ferrous citrate and transferrin to increase hemoglobin synthesis (21). Humanized serum proteins have also been introduced to avoid the use of bovine-derived serum (22). The in vivo microenvironment, where erythroid precursors mature physically associated with macrophage cells in erythroblastic islands, has been partially reproduced in vitro. Fujimi, et al. expanded and differentiated macrophage cells from a starting population of cord blood cells and co-cultured these macrophages with maturing erythroid precursors to maximize their terminal differentiation into orthochromatic erythroblasts (16).
The ultimate goal of these in vitro erythroid cultures is the generation of enucleated RBCs with deformability and oxygen delivery potential similar in vivo-generated RBCs. The mechanisms responsible for enucleation remain poorly understood but have recently come under increased scrutiny (23). The 2-step liquid cultures of human erythroid cells have traditionally generated less than 50% enucleated RBCs. Enucleation rates were dramatically improved by coculture of erythroid precursors on a specific murine bone marrow (MS5) stromal cell line (24, 25). Efficient enucleation has also been facilitated using feeder-free conditions (20). This is an important issue since the production of clinically useful RBCs in vitro will require strategies to avoid exposure of cellular products to nonhuman cells. Several laboratories have investigated the functional characteristics of in vitro generated RBCs, including hemoglobin content, oxygen dissociation characteristics, membrane deformability, and in vivo lifespan when injected into immunodeficient mice (21, 24). These studies provide proof of principle evidence that fully mature and functional RBCs can be generated in vitro.
The problem of scale
Presently, several ex-vivo generated cell therapy products are in clinical trials, including stem cells for transplantation, dendritic cells as cancer vaccine, activated lymphocytes, mesenchymal stem cells to prevent -graft-versus-host disease, and chondrocytes to expedite bone repair. These cellular products require the ex-vivo generation of cell numbers ranging between 107 and 1010, which is feasible with current technologies. However, a single unit of packed RBCs contains two orders of magnitude more cells (approximately 2.5×1012 RBCs). While it is estimated that current erythroid in vitro culture protocols could generate between 3–50 units of packed RBCs from one cord blood donation, only a proof-of-principle transfusion of 1010 RBCs derived from in vitro culture has recently been published (26). The production of sufficient blood cells for clinical needs will ultimately require advances in erythroblast expansion and in bioreactor technology (27). The costs associated with ex vivo erythroid cell expansion and differentiation make it unlikely that these RBCs will be produced in clinically useful numbers in the near future.
Restricted self-renewal of fetal and adult erythroblasts
Erythroid precursors normally undergo 3–4 maturational cell divisions as proerythroblasts ultimately become mature RBCs (Figure 1A). The study of virally-induced avian erythroleukemias led to the recognition that the combined activation of tyrosine kinase receptors (SCF and ErbB) and nuclear hormone receptors (glucocorticoids or estrogens) can lead to the outgrowth of committed avian erythroid “progenitors” that can proliferate for 25–30 generations before terminally differentiating (28, 29). It was subsequently shown that the ex vivo culture of cells from mouse fetal liver and bone marrow and from human cord blood and bone marrow with EPO, SCF, and dexamethasone led to analogous populations of immature erythroid cells with a large, but ultimately restricted, capacity to proliferate (Figure 1B) (30, 31). The importance of dexamethasone in regulating the self-renewal of erythroid cells was recognized in early studies (19). Transfer of these cells into EPO-based medium lacking dexamethasone resulted in their terminal differentiation into enucleated RBCs (Figure 1B).
At the transcriptional level, erythroid precursor maturation is regulated by a GATA2/GATA1 switch (32). In immature erythroid cells, Gata2 regulates genes that activate proliferation and cell cycle progression. Gata2 also activates the expression of GATA1, which, in turn, suppresses GATA2 and activates erythroblast maturation. These concepts suggest that agents that reduce GATA1 expression may retain erythroid cells in a GATA2-dependent self-replication state. Glucocorticoids reduce GATA1 levels by activating Gata1 degradation through an HSP70-dependent caspase pathway (33). Estrogens, instead, reduce the transcriptional activity of GATA1 by inducing formation of transcriptionally inactive estrogen receptor-Gata1 complexes (34). In addition, estrogens upregulate telomerase activity and prevent chromosomal telomere losses during extensive cellular proliferation (35). These data suggest that the massive amplification observed during the first step of erythroid cultures and in the erythroid “progenitor” cultures (described above) may be due to the fact that glucocorticoids and estrogen may confer self-renewal ability to committed erythroid precursors. This hypothesis was recently tested by sequential purification/re-culture experiments. When immature human erythroblasts from the first step of 2-step cultures were sorted and recultured for two days, they generated additional immature cells as well as mature erythroblasts (36). These immature erythroblasts retained their self-replication potential through two additional sorting/re-culture procedures. Cell losses during sorting prevented assessment of whether this self-renewal property could be retained for long periods of time.
Extensive self-renewal of embryonic erythroblasts
Erythroid cells derived from the murine yolk sac and early fetal liver are capable, not only of restricted, but also extensive (106–1060-fold) proliferation ex vivo when cultured in EPO, SCF, and dexamethasone (Figure 1B) (37). This proliferative capacity is far greater than previously found in cultures derived from late fetal liver- or postnatal bone marrow. Despite prolonged culture with daily cell divisions extending for many months, these immature erythroblasts preserve the potential to mature into 8–16 enucleated RBCs when transferred into media lacking dexamethasone. Since proerythroblasts normally generate 8–16 RBCs following 3–4 maturational cell divisions (Figure 1A), these findings provide further evidence that dexamethasone acts in concert with EPO and SCF to induce self-renewal cell divisions in immature erythroid precursors. These extensively self-renewing erythroblasts (ESRE) remain dependent on EPO, SCF, and dexamethasone despite prolonged culture (37).
Upon maturation, ESREs upregulate adult globins, consistent with a definitive erythroid identity. In contrast, primitive erythroid cells derived from the early yolk sac are incapable of either restricted or extensive self-renewal (37). Thus, the capacity for extensive ex vivo self-renewal is associated temporally and spatially in the murine embryo with the transient wave of definitive erythro-myeloid progenitors that emerge in the yolk sac and migrate to the fetal liver prior to the appearance of hematopoietic stem cells (38). Of note, an analogous wave of definitive erythroid potential (BFU-E) first emerges at 3–4-weeks gestation in the yolk sac of the human embryo and is subsequently found in the developing fetal liver (39), suggesting that these waves of hematopoietic potential are found both in murine and human embryos.
Overlapping waves of primitive and definitive erythroid progenitors are found not only in the early yolk sac of mouse embryos but also during the in vitro differentiation of murine embryonic stem (ES) cells (40). An initial study suggested that immature erythroblasts derived from differentiating murine ES cells can proliferate for longer periods of time in vitro than their fetal liver-derived counterparts (41). Recently, erythroblasts with extensive self-renewal potential and definitive erythroid identity have also been derived from murine ES cells (37). The generation of RBCs from ES cell-derived erythroid cell lines have been shown to protect mice from experimentally induced lethal anemia (42). Taken together, these studies raise the possibility that ESREs derived from human ES cells and iPS cells may ultimately serve as an in vitro source of RBCs for use in transfusion therapy (43). Using enucleated RBCs as a cell-based therapeutic minimizes the inherent risks of malignancy associated with ES cell-derived cellular products.
An alternative approach to erythroid cell-based therapy
Immature erythroblasts might serve as an alternative erythroid cell transfusion product. The use of immature erythroblasts, with their capacity to generate 8–16 RBCs upon maturation, would reduce the number of cells needed to provide a potentially useful transfusion product. Furthermore, erythroblasts may be more advantageous than RBCs in the treatment of patients with chronic anemia, since erythroblast maturation would, unlike transfused RBCs, use the iron pool and reduce, rather than increase, iron overload. In addition, RBCs generated in vitro by the transfused erythroblasts would be on average younger than those contained in a unit of blood and therefore survive longer in vivo, reducing the frequency of transfusion episodes necessary to sustain adequate hemoglobin levels. However, this potential therapeutic approach raises significant concerns. It would need to be determined if in vitro-derived erythroblasts, like their normal counterparts, express appropriate levels of the adhesion molecules necessary to home to and establish proper cell-cell interactions within the bone marrow niche, rather than being cleared by the spleen upon transfusion (44). Finally, the generation of erythroblasts from ES cell and induced pluripotent stem (iPS) cell sources carries inherent safety risks that would need to be rigorously addressed prior to the institution of clinical trials.
Summary and Conclusions
RBC transfusions are the most common form of cell-based therapy, delivered to millions of patients worldwide each year. Currently, all of these blood cells are obtained from donors, with entailed infectious risks. A better understanding of the complex process of normal erythroid cell differentiation is leading to improved protocols for the in vitro differentiation of hematopoietic progenitors into fully mature RBCs using two or more steps of liquid culture. Thus, the stage is being set for the in vitro production of RBCs, which offers the potential for creation of universal donor RBCs. A proof-of-principle transfusion of in vitro-generated RBCs has recently been reported. However, the technology to generate the massive number of RBCs necessary for transfusion therapy in a cost-effective fashion remains to be developed. The extensive in vitro expansion of immature erythroblasts and the development of erythroid precursors as a potential transfusion product may help to deal with issues of scale and eventually find a place in the treatment of patients with chronic anemias. In the meantime, ex-vivo generated RBCs will likely find uses in drug discovery as well as for drug delivery.
Acknowledgements
This study was supported by a grant from the NY-STAR foundation (C-06066), from Centro Nazionale Sangue, Rome, Italy and by institutional funds from the Mount Sinai School of Medicine and Istituto Superiore Sanità, Italy (ARM), as well as from NIDDK/NIH and NYSTEM (JP). Drs. Carolyn Whitsett, Giovanni Migliaccio, Samantha England, and Ah Ram Kim are gratefully acknowledged for insights and helpful discussions. We thank Scott Peslak with assistance for Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna Rita Migliaccio, Tisch Cancer Center, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1079, New York, N.Y. 10029, Tel: (212) 241-6974, Fax: (212) 876-5276, annarita.migliaccio@mssm.edu.
James Palis, Center for Pediatric Biomedical Research, Department of Pediatrics, University of Rochester Medical Center, 601 Elmwood Ave., Rochester, N.Y. 14642, Tel: (585) 275-5098, Fax: (585) 276-0232, james_palis@urmc.rochester.edu.
References
- 1.Diamond LK. A history of blood transfusions. In. In: Wintrobe MM, editor. Blood, Pure and Eloquent. McGraw-Hill Book Company; 1980. pp. 659–688. [Google Scholar]
- 2.Glynn SA. Blood supply safety: an NHLBI perspective. Transfusion. 2008;48:1541–1544. doi: 10.1111/j.1537-2995.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 3.Ali A, et al. The aging population poses a global challenge for blood services. Transfusion. 2010;50:548–588. doi: 10.1111/j.1537-2995.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Greenberg AG. Artificial oxygen carriers as red blood cell substitutes: a selected review and current status. Artif Organs. 2004;28:813–828. doi: 10.1111/j.1525-1594.2004.07345.x. [DOI] [PubMed] [Google Scholar]
- 5.Bessis M, et al. Erythropoiesis: comparison of in vivo and in vitro amplification. Blood Cells. 1978;4:155–174. [PubMed] [Google Scholar]
- 6.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 7.Bauer A, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson JR, et al. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci USA. 1971;68:1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs K, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 10.Fibach E, Rachmilewitz EA. A two-step liquid culture--a novel culture procedure for studying erythroid cell development. Haematologia. 1991;24:211–220. [PubMed] [Google Scholar]
- 11.Migliaccio AR, et al. Long-term generation of colony-forming cells in liquid culture of CD34+ cord blood cells in the presence of recombinant human stem cell factor. Blood. 1992;79:2620–2627. [PubMed] [Google Scholar]
- 12.Malik, et al. An in vitro model of human red blood cell production from hematopoietic progenitor cells. Blood. 1998;91:2664–2671. [PubMed] [Google Scholar]
- 13.Freyssinier JM, et al. Purification, amplification and characterization of a population of human erythroid progenitors. Br J Haematol. 1999;106:912–922. doi: 10.1046/j.1365-2141.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- 14.Douay L. Experimental culture conditions are critical for ex vivo expansion of hematopoietic cells. J Hematother Stem Cell Res. 2001;10:341–346. doi: 10.1089/152581601750288948. [DOI] [PubMed] [Google Scholar]
- 15.Neildez-Nguyen, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 16.Fujimi A, et al. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008;87:339–350. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- 17.Migliaccio G, et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- 18.Leberbauer C, et al. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105:85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- 19.Wessely O, et al. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO Journal. 1997;16:267–280. doi: 10.1093/emboj/16.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miharada K, et al. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nature Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 21.Maggakis-Keleman C, et al. Biological and mechanical quality of red blood cells cultured from human umbilical cord blood stem cells. Med Biol Eng Comput. 2003;41:350–356. doi: 10.1007/BF02348442. [DOI] [PubMed] [Google Scholar]
- 22.Migliaccio G, et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 2010;19:453–469. doi: 10.3727/096368909X485049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giarratana MC, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 25.Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: what Is the future in transfusion? Transfusion Medicine Reviews. 2007;21:91–100. doi: 10.1016/j.tmrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Giarratana MC, et al. Proof of principle for transfusion of in vitro generated red blood cells. Blood. 2011 doi: 10.1182/blood-2011-06-362038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmins NE, Nielsen LK. Manufactured RBC - Rivers of blood, or an oasis in the desert? Biotechnol Adv. 2011;29:661–666. doi: 10.1016/j.biotechadv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Hayman MJ, et al. Self-renewal and differentiation of normal avian erythroid progenitor cells: regulatory roles of the TGF alpha/c-ErbB and SCF/c-kit receptors. Cell. 1993;74:157–169. doi: 10.1016/0092-8674(93)90303-8. [DOI] [PubMed] [Google Scholar]
- 29.Steinlein P, et al. Primary, self-renewing erythroid progenitors develop through activation of both tyrosine kinase and steroid hormone receptors. Curr Biol. 1995;5:191–204. doi: 10.1016/s0960-9822(95)00040-6. [DOI] [PubMed] [Google Scholar]
- 30.Panzenbock B, et al. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658-–3668. [PubMed] [Google Scholar]
- 31.von Lindern M, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- 32.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeil JA, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- 34.Blobel GA, Orkin SH. Estrogen-induced apoptosis by inhibition of the erythroid transcription factor GATA-1. Mol Cell Biol. 1996;16:1687–1694. doi: 10.1128/mcb.16.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calado RT, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migliaccio G, et al. Under HEMA conditions, self-replication of human erythroblasts is limited by autophagic death. Blood Cells Mol Dis. 2011 doi: 10.1016/j.bcmd.2011.06.001. in press. [DOI] [PubMed] [Google Scholar]
- 37.England SJ, et al. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palis J, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio G, et al. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac----liver transition. J Clin Invest. 1986;78:51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller G, et al. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carotta S, et al. Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood. 2004;104:1873–1880. doi: 10.1182/blood-2004-02-0570. [DOI] [PubMed] [Google Scholar]
- 42.Hiroyama T, et al. Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS One. 2008;3:e1544. doi: 10.1371/journal.pone.0001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapillonne H, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hintze C, et al. Erythrocytic precursor cells show potent shear stress resistant adhesion and home to hematopoietic tissue in vivo. Transfusion. 2009;49:2122–2130. doi: 10.1111/j.1537-2995.2009.02241.x. [DOI] [PubMed] [Google Scholar]