Abstract
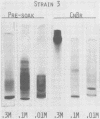
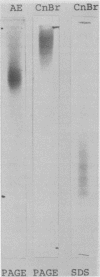
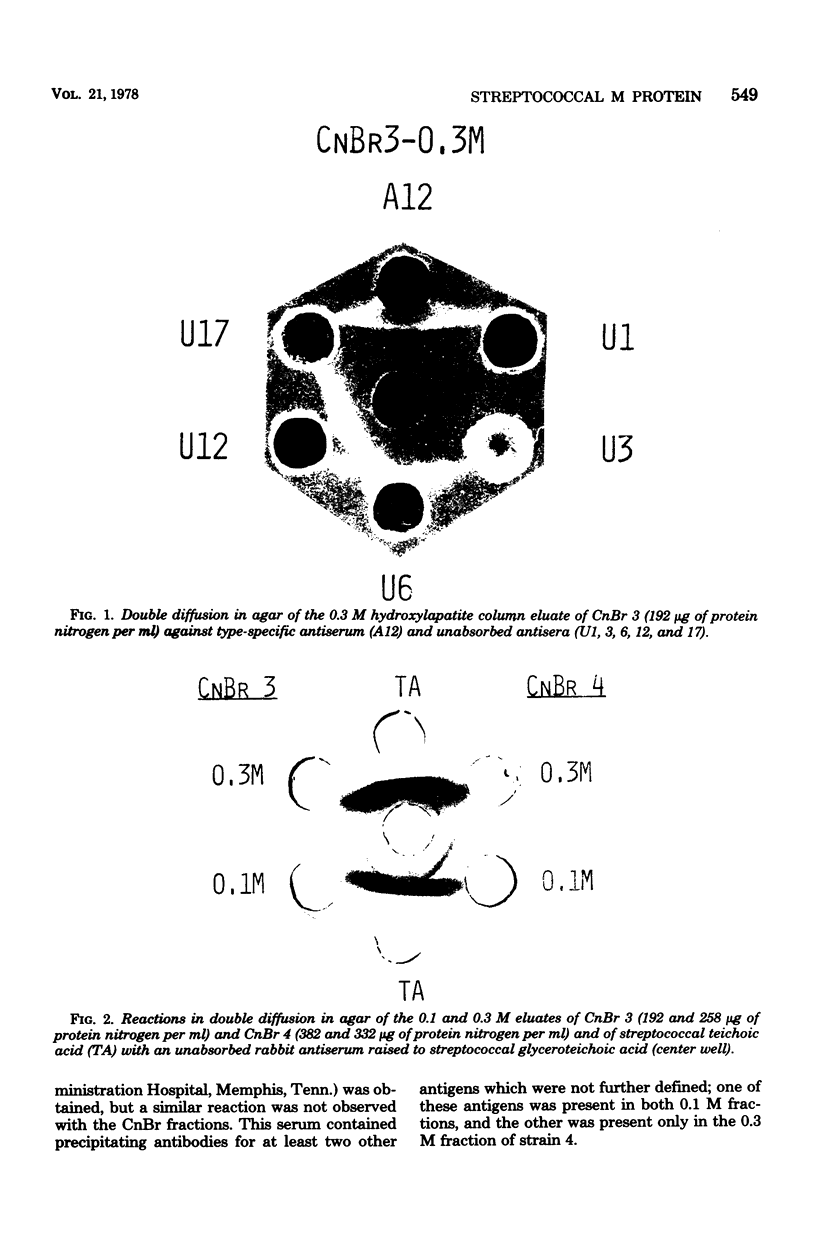
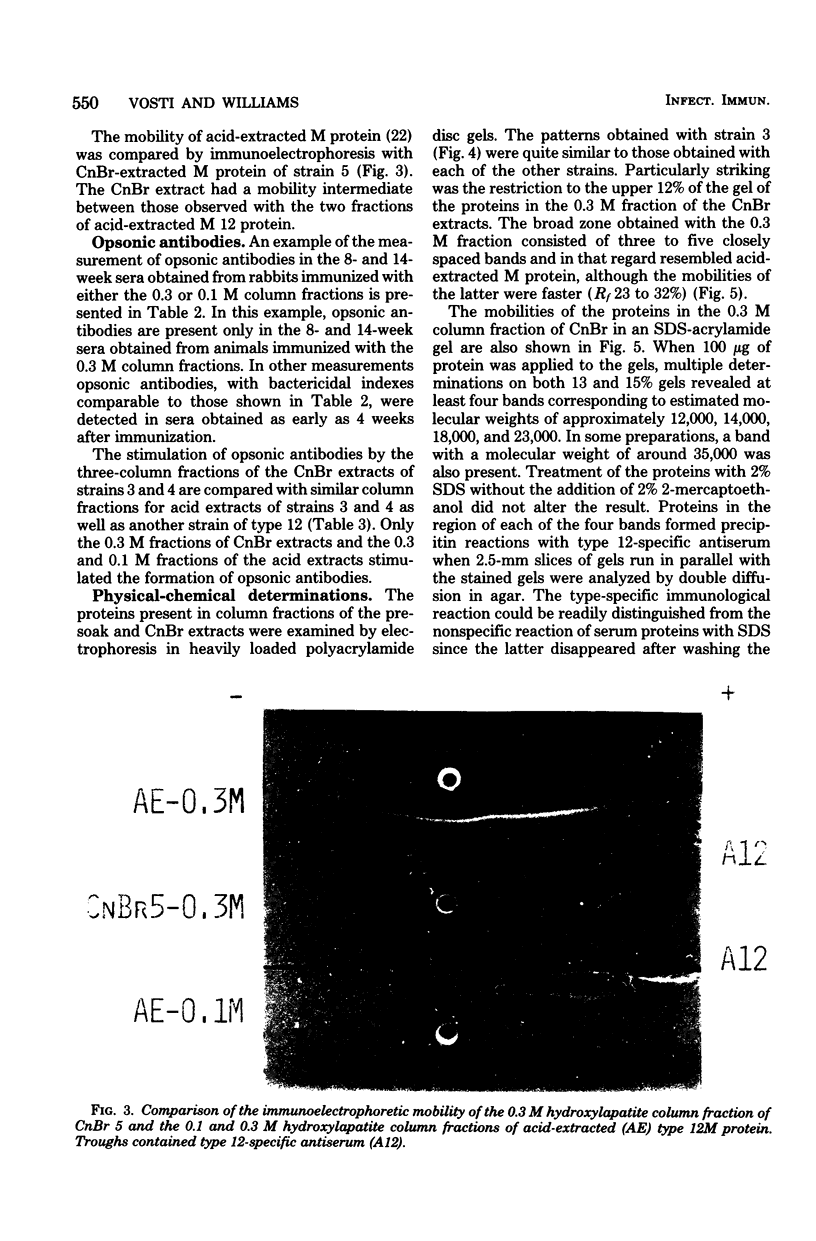
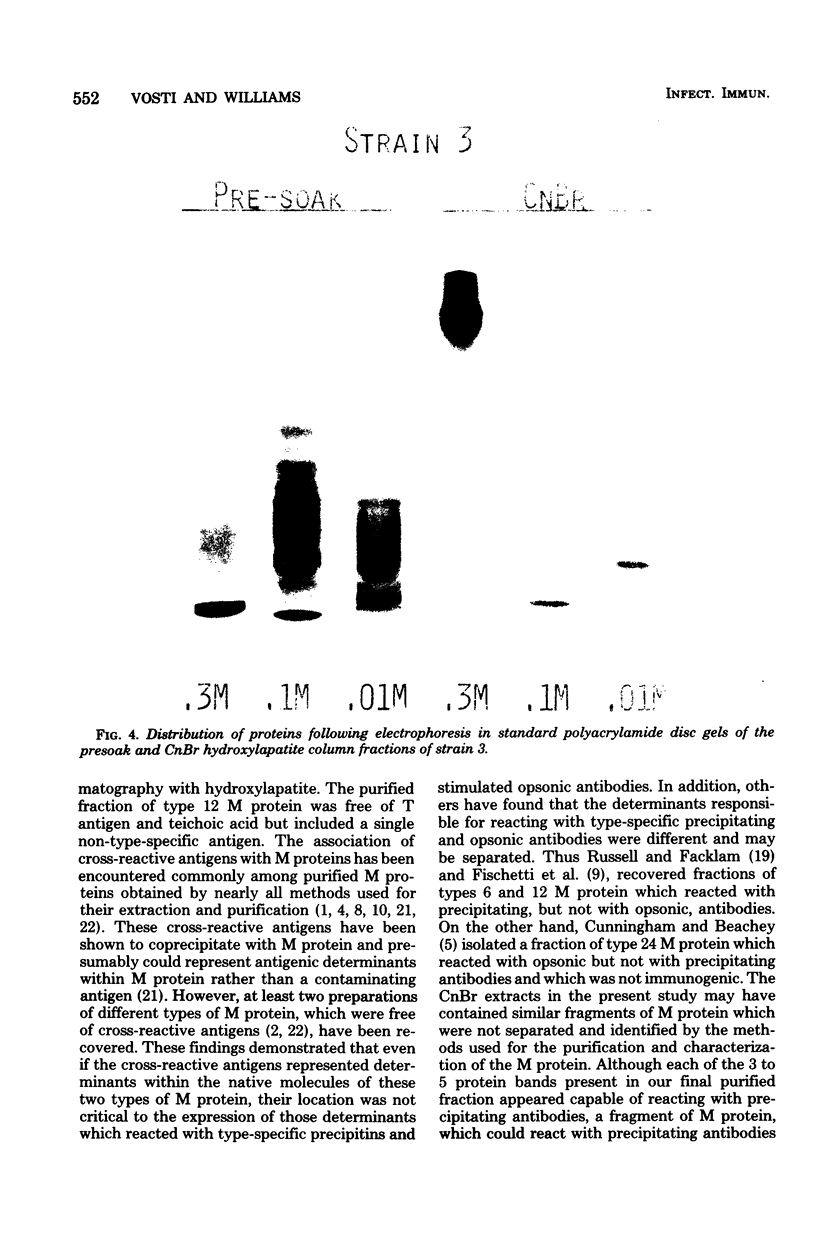
Conditions for the release of streptococcal type 12 M protein from whole cells by cyanogen bromide are described; they demonstrated that methionine is not essential to the structural arrangements which account for some of its immunological and biological properties. The released M protein was separated from other proteins by column chromatography with hydroxylapatite. The type-specific molecules which reacted with precipitating antibodies were found only in the 0.3 M eluate, formed zones with mobilities less than 12% of that of the dye front on electrophoresis in the standard acrylamide disc gel system, formed at least four bands in sodium dodecyl sulfate-acrylamide disc gels with molecular weights ranging from 12,000 to 23,000, and stimulated the formation of opsonic antibodies in rabbits. Cyanogen bromide provides a highly specific method for the release of M proteins which should prove particularly useful in analyses of structural-functional relationships among different M proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Campbell G. L., Ofek I. Peptic digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974 May;9(5):891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Beachey E. H. Peptic digestion of streptococcal M protein. I. Effect of digestion at suboptimal pH upon the biological and immunochemical properties of purified M protein extracts. Infect Immun. 1974 Feb;9(2):244–248. doi: 10.1128/iai.9.2.244-248.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M., Beachey E. H. Immunochemical properties of streptococcal M protein purified by isoelectric focusing. J Immunol. 1975 Oct;115(4):1002–1006. [PubMed] [Google Scholar]
- Daniels J. R., Chu G. H. Basement membrane collagen of renal glomerulus. J Biol Chem. 1975 May 10;250(9):3531–3537. [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein extracted by nonionic detergent. II. Analysis of the antibody response to the multiple antigenic determinants of the M-protein molecule. J Exp Med. 1977 Oct 1;146(4):1108–1123. doi: 10.1084/jem.146.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H. Characterization of group A streptococcal R-28 antigen purified by hydroxyapatite column chromatography. Infect Immun. 1975 Oct;12(4):901–909. doi: 10.1128/iai.12.4.901-909.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H., Vosti K. L. Purification of two fragments of M protein from a strain of group A, type 12 streptococcus. J Immunol. 1968 Sep;101(3):381–391. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957 Oct 1;106(4):525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Russell H., Facklam R. R. Guanidine extraction of streptococcal M protein. Infect Immun. 1975 Sep;12(3):679–686. doi: 10.1128/iai.12.3.679-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Vosti K. L. Characterization of a non-type-specific antigen(s) associated with group A streptococcal type 12 M protein. Infect Immun. 1975 Jun;11(6):1300–1306. doi: 10.1128/iai.11.6.1300-1305.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti K. L., Johnson R. H., Dillon M. F. Further characterization of purified fractions of M protein from a strain of group A, type 12 Streptococcus. J Immunol. 1971 Jul;107(1):104–114. [PubMed] [Google Scholar]
- WILSON A. T., WILEY G. G. THE CELLULAR ANTIGENS OF GROUP A STREPTOCOCCI; IMMUNOELECTROPHORETIC STUDIES OF THE C, M, T, PGP, E4, F, AND E ANTIGENS OF SEROTYPE 17 STREPTOCOCCI. J Exp Med. 1963 Oct 1;118:527–556. doi: 10.1084/jem.118.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]