Abstract
Progress in modification of conventional coronary risk factors and lifestyle behavior reduced atherosclerotic coronary artery disease incidence, nonetheless it continues to be the leading cause of mortality in the world. It might be attributed to the defective risk stratifying and prevention strategy for coronary artery disease. Atherosclerotic coronary artery disease risk is estimated based on identifying and quantifying only traditional risk factors in current clinical settings, it does not consider non-traditional risk factors. In addition, most of prevailing therapies for atherosclerosis are targeted for traditional risk factors rather than atherosclerosis itself. It is desirable to have a methodology which can directly assess the activity of atherogenesis at each moment. Endothelial function is an integrated index of all atherogenic and atheroprotective factors present in an individual including non-traditional and heretofore unknown factors, and is reported to have additional predictive value for future cardiovascular events to traditional risk factors. Moreover, endothelial function has a pivotal role in all phases of atherosclerosis, from initiation to atherothrombotic complication, and is reversible at every phase, indicating that endothelial function-guided therapies might be effective and feasible in cardiovascular practice. Thus, the introduction of endothelial function testing into clinical practice might enable us to innovate individualized cardiovascular medicine. In this review, we summarize the current knowledge on the contribution of endothelial dysfunction to atherogenesis and review methods that assess endothelial function. Finally we focus on the effects of major anti-atherosclerotic disease therapies on endothelial function, and argue the possibility of non-invasive assessment of endothelial function aiming at individualized cardiovascular medicine.
Introduction
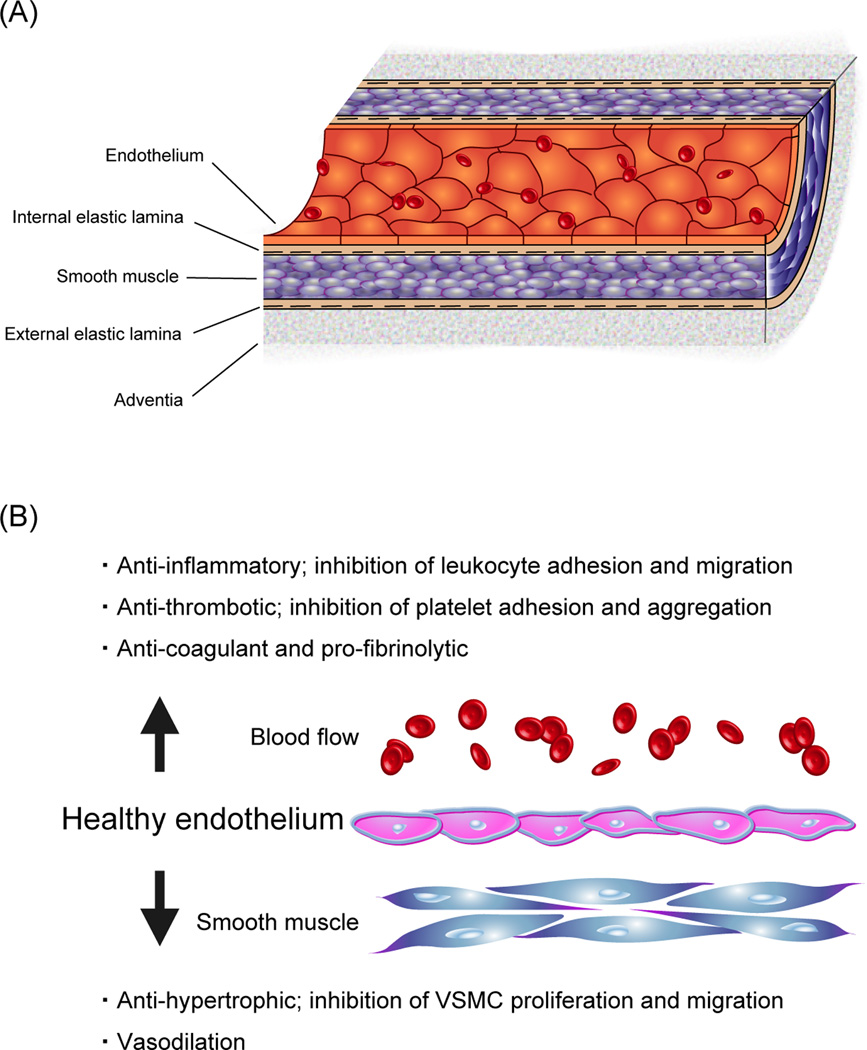
Vascular endothelium is an active monolayer of cells lining the entire circulatory system, from the heart to the smallest capillaries, separating the vascular wall from circulating blood (Fig. 1-A), and plays an essential role in almost all basic biological vascular functions, in health and disease (1,2). The endothelium not only provides a non-adhesive and highly selective physical barrier to control the vascular permeability, it also releases a large number of vasoactive substances to regulate the vascular tone and the remodeling of vessel wall (Fig. 1-B) (2). Endothelium-derived relaxing factors (EDRF) include nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factors. There are also important endothelium-derived constricting factors (EDCF) including endothelin-1 (3). Cardiovascular risk factors, which include traditional, non-traditional, and heretofore unknown factors, have potential to impair endothelial function through various complex mechanisms and lead to unfavorable physiological vascular changes such as vasomotor tone alterations, thrombotic dysfunctions, smooth muscle cell proliferation and migration, as well as leukocyte adhesion and migration (2). Overproduction of reactive oxygen species (ROS) and increased oxidative stress may serve as a common pathogenic mechanism of the effect of these risk factors (2). In addition to various systemic risk factors as shown in Table 1, local factors, including balloon angioplasty and hemodynamic forces such as shear stress, have been suggested as important modulators of endothelial function (4,5).
Figure 1. Anatomy and functions of normal endothelium.
(A) The vascular endothelium is a monolayer of cells forming an interface between the vessel lumen and the vascular smooth muscle cells. The endothelium is a dynamic autocrine and paracrine organ.
(B) Through its capacity to sense and respond to mechanical and biochemical stimuli, the endothelium plays an active and critical role in the physiologic regulation of vascular tone, cellular adhesion, vascular smooth muscle cells proliferation and migration, and resistance to thrombosis.
VSMC: vascular smooth muscle cell.
Table 1.
Traditional and non-traditional risk factors for atherosclerosis.
| Traditional risk factors from Pooled cohort equations |
Others |
|---|---|
| Male sex | Family history of coronary artery disease |
| Older age | Obesity |
| Diabetes | Homocysteine |
| Dyslipidemia | Lipoprotein (a) |
| Hypertension | Chronic kidney disease |
| Smoking | Physical inactivity |
| Race | Insulin resistance |
| Sleep apnea | |
| Low grade inflammation (Rheumatoid disease, chronic infection) |
|
| Genetic factor | |
| Mental stress | |
| Polycystic ovary syndrome | |
| Toxemia of pregnancy | |
| Atrial fibrillation | |
| Etc and unknown factors |
In current clinical settings, the risk of atherosclerotic cardiovascular disease is estimated based on identifying and quantifying the traditional risk factors (6). For example, the Pooled Cohort Equation for 10-year atherosclerotic cardiovascular disease risk is calculated with 7 variables (6), though numerous other factors are suggested to be associated with atherogenesis and endothelial dysfunction (Table 1). Many individuals with coronary artery disease have only one or none of traditional risk factors (7), and traditional risk factors might account for only a half of pathogeneses of atherosclerotic diseases (8), indicating that non-traditional and unknown risk factors have substantial role in atherogenesis. Thus the current patient-specific risk assessment may be insufficient to identify individual risk. In addition, most of prevailing therapies for atherosclerosis are targeted for traditional risk factors, not for atherosclerosis itself. It has been expectantly desired to develop a methodology which can directly assess condition of atherogenesis at each moment. Endothelial function is an integrated index of all atherogenic and atheroprotective factors present in an individual, and thus predicts cardiovascular events independent from traditional risk factors (9–12). Furthermore, endothelial function might be reversible at every phase of atherosclerosis, and has a pivotal role in all phases, from initiation to atherothrombotic complication (13–15). Taken together, a strategy based on endothelial function assessment is possibly a proper approach, and will bring the development of more tailored medicine.
In the current review, we present available data concerning the contribution of endothelial dysfunction to atherogenesis and review methods that assess endothelial function. Finally we focus on the effects of major anti-atherosclerotic therapies on endothelial function, and argue the possibility of non-invasive assessment of endothelial function aiming at individualized cardiovascular medicine. This article is novel with respect to integrating current knowledge into clinical practice.
Endothelial dysfunction in coronary artery diseases
A variety of factors may damage endothelial cells, which include physical injuries, biochemical injuries, and immune mediated damages. Among them, oxidative stress is one of the most important factor which produces endothelial dysfunction by several mechanisms. In the state of oxidative stress, enzymatic production of ROS exceeds the available antioxidant defense systems. Initially, superoxide anions react with existing NO producing peroxynitrite (ONOO−) and thus reduce the concentration of NO. In addition, the resulting ONOO−, which is itself strongly oxidizing, diminishes the production of NO via reducing endothelial NO synthase (eNOS) activity (16). Mechanistically, ONOO− oxidizes tetrahydrobiopterin (BH4), an essential cofactor for eNOS, to BH3 radical, and the resulting decrease in BH4 is likely to be the major cause for dysfunctional eNOS, “eNOS uncoupling”. Oxidation of BH4 not only reduces BH4 bioavailability, but the oxidation products such as BH2 may compete with BH4 for binding to NO synthase (17). Furthermore, NO synthase itself can be a source of superoxide. Cofactors in particular BH4 have a crucial role in this NO synthase-dependent ROS production. NO synthase reduces molecular oxygen in the lack of BH4, which results in the further production of superoxide rather than NO (16). Although it is still challenging to measure circulating biomarkers of oxidative stress, measurements of stable products released by the reaction of ROS and vascular/circulating molecular structures, such as serum lipid hydroperoxides, plasma malondialdehyde or urine F2-isoprostanes, have been reported to be a prognostic factor for cardiovascular disease (18).
Endothelial cells are constantly exposed to these milieus and endeavor to maintain a homeostasis with anti-thrombotic, anti-inflammatory, and anti-proliferative properties (19). Endothelial dysfunction leads to compensatory responses that alter the normal homeostatic properties of the endothelium, resulting in impairment or loss of its normal function, such as impaired endothelium dependent vasodilatation, the hallmark of endothelial dysfunction, due to an imbalance in the release of vasoconstrictor and vasodilator agents from the endothelium.
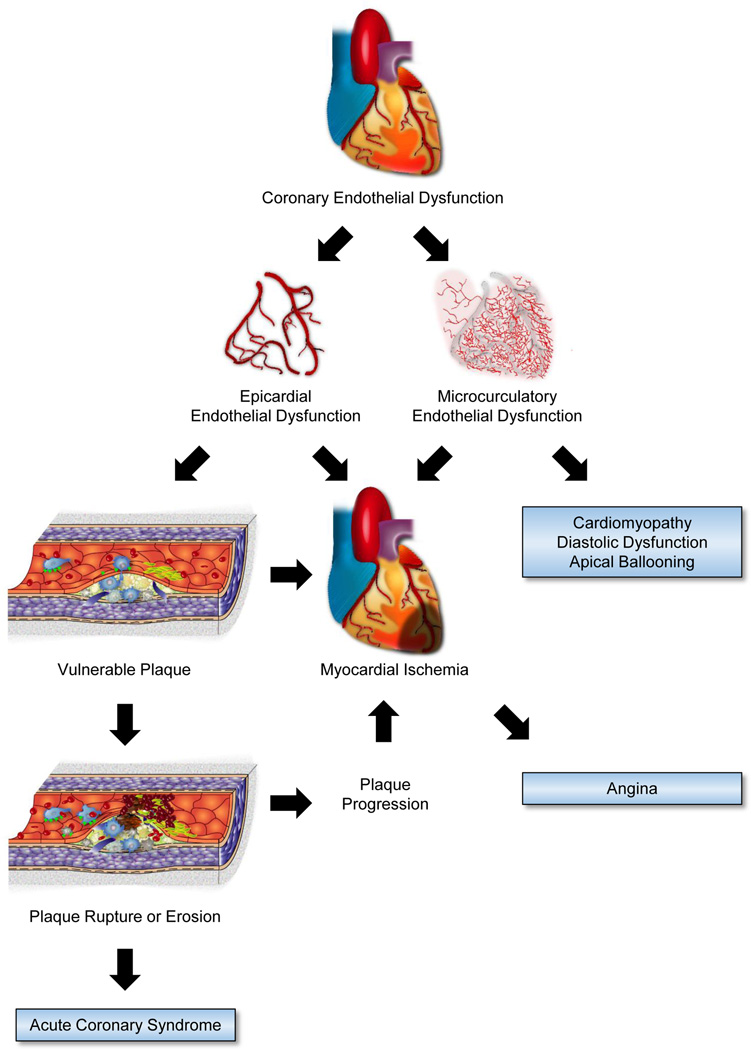
Fig. 2 shows the role of endothelial dysfunction in coronary artery diseases. Epicardial and microvascular coronary endothelial dysfunction is an independent predictor of acute cardiovascular events irrespective of presence or absence of angiographically detectable coronary lesions (20,21). Endothelial dysfunction in epicardial and/or microcirculatory coronary arteries causes myocardial ischemia (22,23). It is suggested that coronary microcirculatory endothelial dysfunction may be an important feature of the pathophysiology of apical ballooning syndrome (24,25), impaired left ventricular relaxation in patients with normal ejection fraction in the absence of occlusive coronary artery disease (26–28), and cardiomyopathy (29).
Figure 2. Endothelial dysfunction in coronary artery diseases.
The process of atherosclerosis begins early in life, and endothelial dysfunction contributes to atherogenesis at every phase of atherosclerosis (30). The earliest atherosclerotic changes take place in endothelium prior to morphological plaque formation, including increased endothelial permeability to lipoproteins and other plasma constituents, and specific adhesion molecules formation on the surface of the endothelium that are responsible for the adherence, migration, and accumulation of monocytes and T cells (31). These adhesion molecules include vascular cell adhesion molecule 1, intracellular adhesion molecule 1, E-selectin, and P-selectin. Lipoprotein particles bind to proteoglycan in the intima, and they are retained. Lipoprotein particles bound to proteoglycan have increased susceptibility to oxidative or other chemical modifications. Subsequently, in response to the oxidized low density lipoprotein cholesterol, vascular endothelial cells and smooth muscle cells secrete monocyte chemotactic protein-1 and macrophage colony-stimulating factor, which promote monocyte chemotaxis, adhesion, and differentiation into macrophages and can augment scavenger receptors (32). These macrophages englobe and accumulate oxidized lipids. Initially fatty steaks consist of lipid-laden monocytes and macrophages (foam cells) together with T lymphocytes. Later smooth muscle cells migrate, and then fatty steaks are mainly formed by lipid accumulation in macrophage-derived foam cells and vascular smooth muscle cells (32). Thus, endothelial dysfunction plays a key role in both initiation and progression of atherosclerotic plaques.
The pathological features of the most common type of vulnerable plaque includes a thin fibrous cap, a large lipid pool inside the plaque, and increased macrophage infiltration within the cap and apoptosis, resulting in the growth of the necrotic core (33). Endothelial dysfunction also has important roles in all of these features (34). Recent clinical studies of patients with mild coronary artery disease demonstrated that coronary endothelial dysfunction was associated with vulnerable plaque characteristics including increased lipid accumulation and necrotic core formation than those with normal endothelial function (35,36). The major mechanism of coronary thrombosis is a fracture in the protective fibrous cap of the plaque. Against the impinging forces on the plaque’s cap, institutional forms of collagen, such as type-I and type-III collagens, provide most of the biomechanical resistance to disruption of the fibrous cap (37). Pro-inflammatory cytokines, in particular γ-interferon, induce the expression of enzymes capable of breaking down constituents of the arterial extracellular matrix (38). In particular, matrix metalloproteinases can degrade the collagen fibrils that lend strength to the fibrous cap (34). Impaired endothelial function is associated with an increased inflammatory response, thrombogenicity, and enhanced local expression of matrix metalloproteinases (31,39). Another mechanism of coronary thrombosis involves a superficial erosion of the intima (40). Although, the underlying molecular mechanisms of superficial erosion remain obscure, apoptosis of endothelial cells could contribute to desquamation of endothelial cells in areas of superficial erosion (41). Thus, impaired endothelial function initiates a cascade of events that permit atherogenesis to proceed, ultimately culminating in lesions that are likely to result in acute coronary syndromes (Fig. 2).
Assessment of endothelial function
Endothelial function can be assessed in humans by assaying its capacity to perform its various physiologic functions, including regulation of vasomotor tone, expression of adhesion molecules and maintenance of an anti-thrombotic microenvironment. NO is an important vasodilator and is produced from L-arginine by way of the enzyme NO synthase (42). NO is responsible for the balance of endothelium-derived contracting factors, such as endothelin-1 and thromboxane A2, thus regulating the vascular tone. In addition, it also suppresses platelet aggregation, inflammation, oxidative stress, vascular smooth muscle cell migration and proliferation, and leukocyte adhesion (43). Thereby, generally reducing endothelium-dependent vasodilation indicates a broadly dysfunctional phenotype across many properties of the endothelium. Thus, the assessment of vasodilator properties resulting from NO and other molecules have become the most widely used clinical end-point for assessment of endothelial function. These testing involves pharmacological and/or physiological stimulation of NO and other vasoactive substances release from endothelium by invasive and non-invasive methods.
Invasive methods
Initial clinical studies of endothelial function were performed in the coronary circulation and invasive assessment by cardiac catheterization is considered the reference standard for evaluating coronary endothelial function (44). This method involves intra-arterial administration of endothelium-dependent vasodilatory substances (such as acetylcholine) with measurement of changes in vessel diameter by quantitative coronary angiography and changes in coronary blood flow by Doppler flow wire. The vasodilatory agent delivered into coronary arteries activates endothelial cells and stimulates NO release, leading to measurable vasodilatation and increase in coronary blood flow in normal subjects but vasoconstriction and lack of increase in coronary blood flow as a result of direct activation of muscarinic receptors on vascular smooth muscle cells in patients with endothelial dysfunction. In 1986, Ludmer and colleagues firstly demonstrated endothelial dysfunction in the course of coronary atherosclerosis using intracoronary infusion of acetylcholine and quantitative coronary angiography (45), and the first evidence of the long-term prognostic significance of coronary endothelial vasodilator dysfunction on atherosclerotic disease progression and cardiovascular events was reported by Schächinger et al. in 2000 (46). Since these seminal studies, the concept that atherosclerosis is a purely structural disease has expanded to include the functional manifestations, such as paradoxical vasoconstriction as a consequence of dysfunctional endothelium. In addition to evidence of epicardial coronary endothelial dysfunction, microvascular coronary endothelial dysfunction as assessed by coronary flow response to intracoronary acetylcholine has been reported to independently predict cardiovascular events irrespective of presence or absence of angiographically detectable coronary lesions (20,21). Yoon et al. recently reported a randomized study of 35 patients with non-obstructive coronary artery disease (47). In this study, the left anterior descending coronary artery was divided into proximal, middle, and distal segments, and interestingly epicardial coronary endothelial function was assessed in each segment. Patients were randomized to treatment with placebo or endothelin-A receptor antagonist, and plaque volume change was evaluated by intravascular ultrasound at baseline and 6-month follow-up. They demonstrated that, in coronary artery segments with endothelial dysfunction, significant plaque progression occurred, but not in segments with normal endothelial function, and this plaque progression was attenuated by endothelin-A receptor antagonist, indicating the important role of coronary endothelial dysfunction in coronary artery disease progression. However, the undeniable disadvantage with such a method is that its invasive and costly nature; therefore its widespread use and clinical utility is limited.
Non-invasive methods
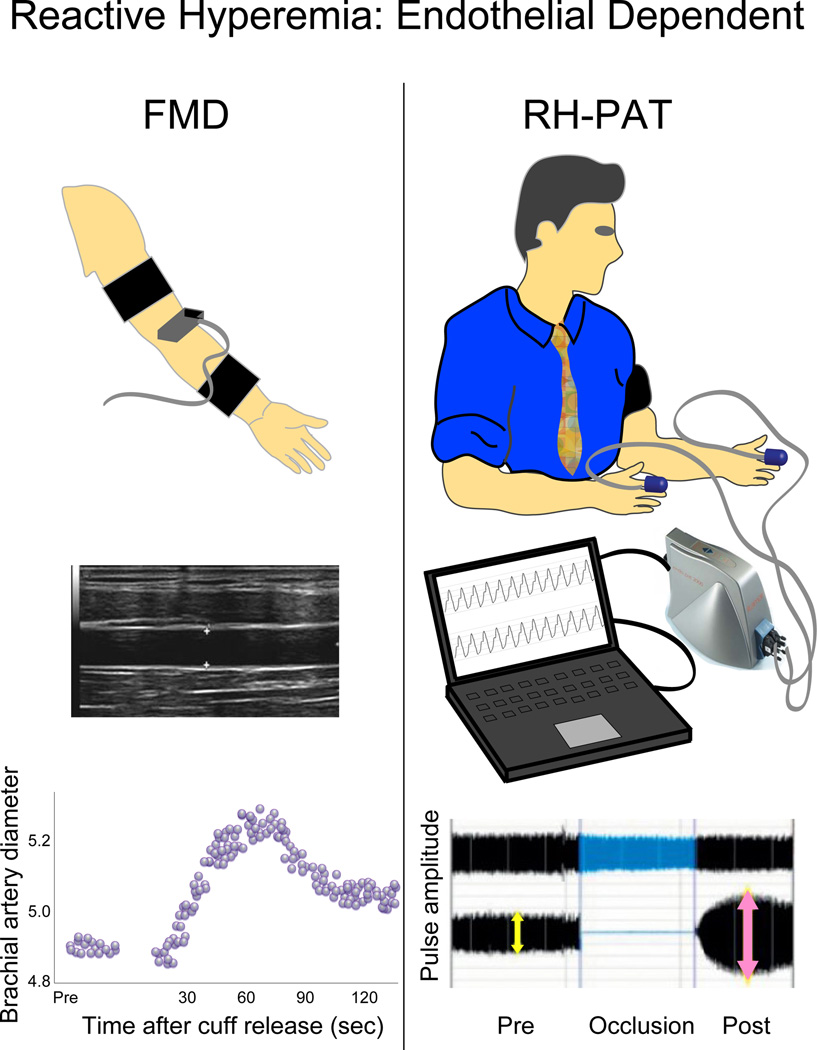
Recently, several non-invasive methods for assessment of endothelial function were introduced. Since endothelial dysfunction is a systemic process, these techniques are based on the diffuse and systemic nature of endothelial dysfunction. Most of methods to evaluate peripheral endothelial function are grounded on the same principle of reactive hyperemia which serves as an index of endothelium-dependent vasodilator function (Fig. 3). The forearm flow mediated vasodilatation (FMD) is one of these, and its measures correlate well with coronary artery endothelial function as assessed by catheterization (48). To evaluate endothelium-dependent dilation capacity, the brachial artery diameter proximal to the antecubital fossa is measured at rest and during reactive hyperemia which is achieved by rapid release of a pneumatic pressure cuff placed around the forearm and inflated to supra-systolic pressure for 5 minutes. Another major non-invasive method based on the same principle to assess peripheral endothelial function is the reactive hyperemia-peripheral arterial tonometry (RH-PAT) (2,49). Peripheral arterial tonometry probes are placed on one finger of each hand and pulse wave amplitude is assessed before and during reactive hyperemia. RH-PAT technology enables beat-to-beat plethysmographic recording of the finger arterial pulse wave amplitude which is a measure of pulsatile volume changes, and its response also well correlates with coronary artery endothelial dysfunction (49). Majority of the non-invasive peripheral endothelial function tests use reactive hyperemia after occlusion as a trigger to detect endothelium-dependent vasodilation, however, FMD represents conduit artery vasodilation, whereas RH-PAT represents microvessel vasodilation. A main advantage of RH-PAT technique is that the contralateral arm serves as its internal control that can be used to correct for any systemic changes during the test, in distinction with FMD. Moreover, RH-PAT technique is easy to use and operator independent.
Figure 3. Major non-invasive endothelial function tests.
After 5 minutes forearm occlusion, a pneumatic pressure cuff is rapidly deflated to induce reactive hyperemia. Maximal response occurs in the 60- to 90-second post-deflation interval. To detect reactive hyperemia, brachial artery diameter change is used in FMD and augmentation of fingertip pulse amplitude is used in RH-PAT.
FMD: flow mediated vasodilatation, and RH-PAT: reactive hyperemia-peripheral arterial tonometry.
Prognostic value of non-invasive endothelial function testing in primary and secondary prevention settings
Several previous studies has reported the additional value of non-invasive endothelial function assessment in predicting cardiovascular events in primary prevention settings. In 1 study of 435 and 1 study of 618 middle-aged healthy subjects without heart disease and low clinical risk, brachial artery FMD independently predicted cardiovascular events in addition to traditional risk factors, over the average follow-up 2.7 and 4.6 years (9,50). In a similar study with 270 middle-aged low risk subjects followed for 7 years, endothelial dysfunction as assessed by RH-PAT significantly predicted adverse cardiovascular events independent from traditional Framingham Risk Score (10). In addition, the impact of endothelial function to predict cardiovascular events was assessed in the elderly. The Cardiovascular Health Study, which included 2792 apparently healthy older adults aged 72 to 98 years, investigated the relationship between peripheral endothelial function and subsequent cardiovascular events over a 5-year follow-up period, and demonstrated that peripheral endothelial function is a significant predictor of future cardiovascular events even after adjustment for traditional risk factors (11). In the Multi-Ethnic Study of Atherosclerosis (12), 3026 subjects free of known atherosclerotic cardiovascular disease were enrolled. Importantly, this study sample included 34% white, 20% Chinese, 21% Black, and 25% Hispanic subjects. In this population-based cohort study, the adding peripheral endothelial function assessment to Framingham Risk Score improved the risk classification for future cardiovascular events. Furthermore, it is worthy to note that a study that included 400 postmenopausal hypertensive women without evidence of coronary artery disease demonstrated that endothelial function significantly improved after six months of antihypertensive therapy, and improvement in endothelial function clearly identified patients who possibly have a more favorable prognosis (14). In this study, women whose endothelial function had not improved 6 months after blood pressure was treated optimally, showed a nearly 7-fold increased risk for cardiovascular events over an average follow-up of 67 months.
Coronary endothelial dysfunction seems to be present in most of patients with coronary artery disease, and is reversible (51). In the setting of established coronary artery disease, patients with endothelial dysfunction have higher rates of adverse cardiovascular events compared to those with normal endothelial function (52), and impaired peripheral endothelial function has been shown to be an independent predictor of cardiovascular events among patients with established coronary artery disease. In a study of 281 stable patients with documented coronary artery disease, endothelial dysfunction predicted the risk of cardiovascular events independent from traditional risk factors, over a mean follow-up of 4.5 years (53). Additionally, in another study of patients with angiographically documented acute coronary syndrome (54), endothelial function was assessed before hospital discharge within 5 days of an episode of acute coronary syndrome. Impaired endothelial function was associated with future cardiovascular events. It is noteworthy that endothelial function was reassessed 8 weeks after an episode of acute coronary syndrome and improvement of endothelial function was significantly associated with fewer future cardiovascular events. A past report documented that overall survival in patients with coronary artery disease is largely independent of the degree of coronary luminal stenosis (55). Furthermore, it was reported that, in patients with coronary artery disease, impaired peripheral endothelial function significantly predicted future cardiovascular events independent from coronary plaque complexity as assessed by the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) Score (56). Importantly, even in patients with coronary artery disease, the improvement of peripheral endothelial function is associated with significant reduction in future cardiovascular events (13,54).
Taken together, the utility of peripheral endothelial function as assessed by non-invasive methods in predicting cardiovascular events in both primary and secondary prevention settings has been demonstrated in several studies and meta-analyses (9,11,12,14,57–60). In addition, it is worthy of note that as mentioned above patients whose peripheral endothelial dysfunction does not improve by interventions might be at higher risk for future events compared with those with improvement in endothelial dysfunction, in both primary and secondary prevention settings.
Therapeutic approaches
Pharmacologic treatment of specific risk factors and lifestyle modifications, such as smoking cessation, weight loss, diet change, and exercise, are all reportedly effective to prevent atherosclerotic diseases. Furthermore, pharmacological interventions in atherosclerotic diseases have advanced dramatically over the decades. However, clinical management of atherosclerosis is still quite difficult as there is no recognized method to prevent or improve the entire vascular bed.
As mentioned above, previous studies have demonstrated the association of improvement in endothelial dysfunction by interventions with risk reduction for future cardiovascular events in primary and secondary prevention settings (13,14). Thus, it is probably a good prognostic sign when endothelial dysfunction is reversed with treatments. In addition, most intervention studies with effects on cardiovascular risk factors also show consistent results on endothelial function and cardiovascular outcomes (Table 2). Clinical guidelines recommend (a) moderate-intensity aerobic activity, (b) weight loss, (c) smoking cessation, (d) angiotensin converting enzyme inhibitors (ACEIs), (e) statins, and (f) β-blockers, which are clearly supported by clinical evidence (61). Evidence of each intervention on endothelial function is listed below.
Table 2.
Effects on endothelial function and cardiovascular outcomes.
| Therapy | Endothelial Function | Cardiovascular Outcomes |
|---|---|---|
| Physical activity | + | + |
| Weight loss | + | + |
| Smoking cessation | + | + |
| ACEIs | + | + |
| Statins | + | + |
| 3rd generation β-blockers | + | + |
| CPAP for OSAS | + | + |
| Glucose lowering therapy | ± | ± |
| CETP inhibitor, Torcetrapib | − | − |
+ indicates improvement, ± indicates controversial, and – indicates worsening. ACEI: angiotensin converting enzyme inhibitors, CETP: cholesterol ester transfer protein, CPAP: continuous positive airway pressure, and OSAS: obstructive sleep apnea syndrome.
(a) Physical activity
A recent meta-analysis provided evidence that physical activity improves endothelial function in persons at increased cardiovascular risk (62). Additionally, a meta-analysis of patients with type 2 diabetes mellitus also reported a beneficial effect of increasing physical activity on endothelial function (63). A study of 43 obese patients demonstrated that exercising training improved RH-PAT, and improvement in insulin resistance was independently correlated with improvement in endothelial function (64).
(b) Weight loss
Several intervention studies have investigated short and long term effects of weight loss on endothelial function and suggest that endothelial dysfunction could be reversible by medical and surgical weight loss (65–67). One study with 29 severely obese subjects (body mass index 48±9 kg/m2) reported that long-term sustained weight loss significantly improved endothelial dysfunction at 1 year (67). In another study with 41 obese subjects, 17 patients were treated with medical therapy alone and 24 patients underwent gastric bypass surgery (65). Gastric bypass surgery was associated with more dramatic weight loss, greater metabolic changes, and more pronounced improvement in endothelial function than medical therapy alone. On the other hand, in a study of 70 Japanese patients with metabolic syndrome (body mass index 26±4 kg/m2, waist 93±7 cm in male and 96±11 cm in female) demonstrated that reduction in waist circumference, rather than reduction in body mass index, was associated with improvement in endothelial function (15).
(c) Smoking cessation
In 1993, Celermajer et al. reported that cigarette smoking is associated with dose-ralated and potentially reversible impairment of endothelial function (68). Furthermore, in 1996, passive smoking was also reported to be associated with dose-related endothelial dysfunction (69). In a recent prospective study of 1504 current smokers (70), smoking intensity was independently associated with endothelial dysfunction at baseline. Despite that body mass index and waist circumference increased after smoking cessation, endothelial function significantly improved after 1 year in those who quit smoking, but did not change in those who continued to smoke.
(d) ACEIs
ACEIs improve endothelial function by reducing the production of angiotensin II by inhibiting angiotensin converting enzyme, a key enzyme affecting the transformation from angiotensin I to angiotensin II and increasing bradykinin production (71,72). A meta-analysis of randomized controlled trials demonstrated that ACEIs improve peripheral endothelial function and are superior to other anti-hypertensive drugs including calcium channel blockers and β-blockers (73).
(e) Statins
The beneficial effect of statins on endothelial function are attributed partly to their anti-inflammatory and anti-oxidant properties, in addition to lowering cholesterol levels (74). The significant beneficial effect of statin therapy on endothelial function in coronary and peripheral artery was demonstrated by a recent meta-analysis of 46 randomized clinical trials (75).
(f) β-blockers
The 1st and 2nd generation β-blockers have no significant benefit on endothelial function. However, different efficacy has been suggested for the 3rd generation β-blockers, such as nebivolol and carvedilol. Both two drugs have been reported to improve endothelial function, which is mediated by β3 receptor activation by nebivolol and anti-oxidant effect by carvedilol (76,77). A recent randomized controlled study reported that carvedilol was able to improve endothelial function compared with metoprolol in patients with hypertension and diabetes mellitus (78).
In addition to the above, obstructive sleep apnea syndrome is associated with endothelial dysfunction, and its severity is related to the degree of endothelial dysfunction (79). Continuous positive airway pressure therapy may improve cardiovascular outcomes (80,81), and some small clinical studies have reported the beneficial effects of treatment for obstructive sleep apnea on endothelial dysfunction (82,83). The evidence of efficacy of blood glucose lowering therapy on cardiovascular disease outcomes from large clinical trials is conflicting (84). Consistently, it was reported that brachial artery endothelial function was not influenced by reduction in hemoglobin A1c (85). Fluctuations in blood glucose levels might have important role in endothelial dysfunction in type 2 diabetes, rather than hemoglobin A1c level (86). Similarly, the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) study reported the increased risk for cardiovascular events and mortality in patients treated with torcetrapib, the cholesterol ester transfer protein (CETP) inhibitor (87), and it is suggested that sustained and marked impairment of endothelial function may at least in part explain the increased mortality associated with torcetrapib treatment in the trial (88).
These are indicating that a clinical strategy with endothelial function assessment potentially useful to improve cardiovascular disease outcomes. Assessment of endothelial function may allow us to treat atherosclerosis itself, rather than risk factor control.
Perspectives
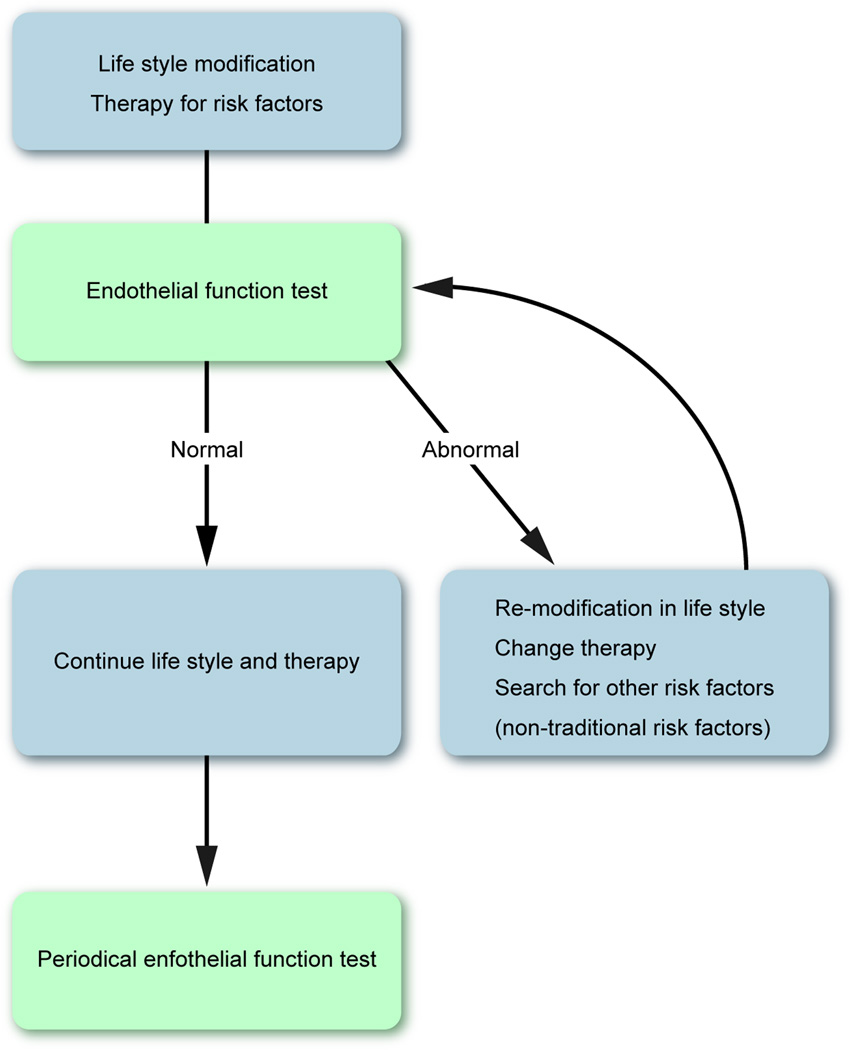
Fig. 4 shows a potential strategy for cardiovascular disease prevention with endothelial function assessment. As demonstrated by the studies discussed in the current review, non-invasive endothelial function assessment has several advantages, and it seems to be feasible and reasonable to manage patients according to endothelial function-guided strategy. However there are still several points in controversy (Table 3). For example, data indicates the significant predictive value of endothelial function for future cardiovascular events in addition to traditional risk factors, but nonetheless, endothelial function measurements are not yet recommended by the latest guidelines for risk assessment (6). It might be attributed to the poorly standardized methodology of non-invasive endothelial function assessment. With technical modifications and more accurate analysis software, the variability of FMD measurements and thus the specificity can be shown to be further improved. The methodology of RH-PAT measurement is well standardized and this technique has the advantage of the automatic and operator independent feature. However, the evidence of RH-PAT is still scarce. Although, the endothelial function-guided prevention strategy, with pharmacological therapies, lifestyle modification, etc., might be beneficial to provide a tailored treatment according to the specificities of atherosclerosis in a given patient, we cannot conclude so far, therefore the question of whether endothelial function-guided therapies will provide benefits in improving outcomes in patients with risk factors and in patients with established atherosclerotic cardiovascular disease should be tested with large scale randomized studies.
Figure 4. Potential strategy for cardiovascular disease prevention with non-invasive endothelial function testing.
Table 3.
Advantages and remaining questions regarding non-invasive peripheral endothelial function tests.
|
Advantages | |
| Related to cardiovascular risks | |
| Predict cardiovascular events | |
| Reversible with interventions | |
|
Remaining questions | |
| For whom? | (suitable subjects in the primary prevention setting) |
| How? | (a standardized method and a reference value) |
| How often? | (a proper frequency) |
| How effective? | (effectiveness of endothelial function-guided strategy) |
Thus, current evidence is definitely not enough in this area in order to establish the endothelial function-based prevention strategy. Further evidence is required to determine appropriate subjects for endothelial function testing, a standardized method, a reference value, and a proper frequency of tests, in addition to verification of the benefit of the endothelial function-guided strategy (Table 3). Such further studies may lead us into a new era of individualized medicine by endothelial function assessment in cardiology.
Summary
Although progress in modification of conventional coronary risk factors and lifestyle behavior reduced atherosclerotic coronary artery disease incidence, it continues to be one of the most common cause of death and disability in the United States and the developed world. It might be attributed to the defective current risk stratifying and prevention strategy for coronary artery disease. In current clinical settings, atherosclerotic cardiovascular disease risk is estimated based on identifying and quantifying the traditional risk factors, and it does not consider non-traditional risk factors. Thus, the current patient-specific risk assessment may be insufficient to identify individual risk. In addition, most of prevailing therapies are targeted for traditional risk factors rather than atherosclerosis itself.
Endothelial function is an integrated index of all atherogenic and atheroprotective factors present in an individual including non-traditional and unknown factors, and has the significant predictive value for future cardiovascular events in addition to traditional risk factors. Moreover, endothelial function has a pivotal role in all phases of atherosclerosis, from initiation to atherothrombotic complication, and is reversible at every phase, indicating the utility of endothelial function-guided therapies. Further researches, including large-scale randomized controlled trials, are necessary to determine whether non-invasive endothelial function assessment can be useful to guide treatment, change outcomes.
Acknowledgements
A.L. is supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750), the Mayo Foundation.
Footnotes
Statement of conflict of interest
Author A.L. declared consulting for Itamar Medical.
References
- 1.Reriani MK, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circulation journal : official journal of the Japanese Circulation Society. 2012;76:778–783. doi: 10.1253/circj.cj-12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 3.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss medical weekly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 4.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Kipshidze N, Dangas G, Tsapenko M, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. Journal of the American College of Cardiology. 2004;44:733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA : the journal of the American Medical Association. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 8.Reriani MK, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel Functional Risk Factors for the Prediction of Cardiovascular Events in Vulnerable Patients Following Acute Coronary Syndrome. Circulation Journal. 2012;76:778–783. doi: 10.1253/circj.cj-12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shechter M, Issachar A, Marai I, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. International journal of cardiology. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 11.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 12.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. Journal of the American College of Cardiology. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 14.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. Journal of the American College of Cardiology. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circulation journal : official journal of the Japanese Circulation Society. 2013;77:2807–2815. doi: 10.1253/circj.cj-13-0549. [DOI] [PubMed] [Google Scholar]
- 16.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? European heart journal. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 17.Rochette L, Lorin J, Zeller M, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacology & therapeutics. 2013;140:239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Current medicinal chemistry. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 21.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 22.Summers MR, Lerman A, Lennon RJ, Rihal CS, Prasad A. Myocardial ischaemia in patients with coronary endothelial dysfunction: insights from body surface ECG mapping and implications for invasive evaluation of chronic chest pain. European heart journal. 2011;32:2758–2765. doi: 10.1093/eurheartj/ehr221. [DOI] [PubMed] [Google Scholar]
- 23.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 24.Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) European heart journal Acute cardiovascular care. 2013;2:147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. Journal of the American College of Cardiology. 2010;56:1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elesber AA, Redfield MM, Rihal CS, et al. Coronary endothelial dysfunction and hyperlipidemia are independently associated with diastolic dysfunction in humans. American heart journal. 2007;153:1081–1087. doi: 10.1016/j.ahj.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Prasad A, Higano ST, Al Suwaidi J, et al. Abnormal coronary microvascular endothelial function in humans with asymptomatic left ventricular dysfunction. American heart journal. 2003;146:549–554. doi: 10.1016/S0002-8703(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roura S, Bayes-Genis A. Vascular dysfunction in idiopathic dilated cardiomyopathy. Nature reviews Cardiology. 2009;6:590–598. doi: 10.1038/nrcardio.2009.130. [DOI] [PubMed] [Google Scholar]
- 30.Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Liao JK. Endothelium and acute coronary syndromes. Clinical chemistry. 1998;44:1799–1808. [PubMed] [Google Scholar]
- 33.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 35.Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. European heart journal. 2013;34:2047–2054. doi: 10.1093/eurheartj/eht132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends in cardiovascular medicine. 2007;17:253–258. doi: 10.1016/j.tcm.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. Journal of internal medicine. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajavashisth TB, Liao JK, Galis ZS, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 40.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 41.Mayranpaa MI, Heikkila HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coronary artery disease. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 42.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods in enzymology. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 43.Tousoulis D, Simopoulou C, Papageorgiou N, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England journal of medicine. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 46.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 47.Yoon MH, Reriani M, Mario G, et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. International journal of cardiology. 2013;168:1316–1321. doi: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 49.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 50.Shechter M, Shechter A, Koren-Morag N, Feinberg MS, Hiersch L. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. The American journal of cardiology. 2014;113:162–167. doi: 10.1016/j.amjcard.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 51.Elbaz M, Carrie D, Baudeux JL, et al. High frequency of endothelial vasomotor dysfunction after acute coronary syndromes in non-culprit and angiographically normal coronary arteries: a reversible phenomenon. Atherosclerosis. 2005;181:311–319. doi: 10.1016/j.atherosclerosis.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 53.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 54.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the "vulnerable" patient. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 55.Little WC, Downes TR, Applegate RJ. The underlying coronary lesion in myocardial infarction: implications for coronary angiography. Clinical cardiology. 1991;14:868–874. doi: 10.1002/clc.4960141103. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. Journal of the American Heart Association. 2013;2:e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirsch L, Shechter A, Feinberg MS, Koren-Morag N, Shechter M. The impact of early compared to late morning hours on brachial endothelial function and long-term cardiovascular events in healthy subjects with no apparent coronary heart disease. International journal of cardiology. 2011;151:342–347. doi: 10.1016/j.ijcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 58.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. The international journal of cardiovascular imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 59.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. International journal of cardiology. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. European heart journal cardiovascular Imaging. 2014;15:736–746. doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 61.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 62.Brockow T, Conradi E, Ebenbichler G, Michalsen A, Resch KL. The role of mild systemic heat and physical activity on endothelial function in patients with increased cardiovascular risk: results from a systematic review. Forschende Komplementarmedizin. 2011;18:24–30. doi: 10.1159/000323632. [DOI] [PubMed] [Google Scholar]
- 63.Montero D, Walther G, Benamo E, Perez-Martin A, Vinet A. Effects of exercise training on arterial function in type 2 diabetes mellitus: a systematic review and meta-analysis. Sports medicine. 2013;43:1191–1199. doi: 10.1007/s40279-013-0085-2. [DOI] [PubMed] [Google Scholar]
- 64.Kurose S, Tsutsumi H, Yamanaka Y, et al. Improvement in endothelial function by lifestyle modification focused on exercise training is associated with insulin resistance in obese patients. Obesity research & clinical practice. 2014;8:e106–e114. doi: 10.1016/j.orcp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Gokce N, Vita JA, McDonnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. The American journal of cardiology. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 67.Bigornia SJ, Mott MM, Hess DT, et al. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity. 2010;18:754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 69.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. The New England journal of medicine. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 70.Johnson HM, Gossett LK, Piper ME, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. Journal of the American College of Cardiology. 2010;55:1988–1995. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alreja G, Joseph J. Renin and cardiovascular disease: Worn-out path, or new direction. World journal of cardiology. 2011;3:72–83. doi: 10.4330/wjc.v3.i3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe T, Barker TA, Berk BC. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 2005;45:163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]
- 73.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216:7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 74.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering--are they clinically relevant? European heart journal. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 75.Reriani MK, Dunlay SM, Gupta B, et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18:704–716. doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 76.Broeders MA, Doevendans PA, Bekkers BC, et al. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102:677–684. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- 77.Feuerstein GZ, Ruffolo RR., Jr Carvedilol, a novel multiple action antihypertensive agent with antioxidant activity and the potential for myocardial and vascular protection. European heart journal. 1995;16(Suppl F):38–42. doi: 10.1093/eurheartj/16.suppl_f.38. [DOI] [PubMed] [Google Scholar]
- 78.Bank AJ, Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. American journal of hypertension. 2007;20:777–783. doi: 10.1016/j.amjhyper.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Siarnik P, Carnicka Z, Krizova L, et al. Predictors of impaired endothelial function in obstructive sleep apnea syndrome. Neuro endocrinology letters. 2014;35:142–148. [PubMed] [Google Scholar]
- 80.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 81.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 82.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. American journal of respiratory and critical care medicine. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 83.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clinical cardiology. 2012;35:231–236. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avitabile NA, Banka A, Fonseca VA. Glucose control and cardiovascular outcomes in individuals with diabetes mellitus: lessons learned from the megatrials. Heart failure clinics. 2012;8:513–522. doi: 10.1016/j.hfc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 85.Reboussin DM, Goff DC, Jr, Lipkin EW, et al. The combination oral and nutritional treatment of late-onset diabetes mellitus (CONTROL DM) trial results. Diabetic medicine : a journal of the British Diabetic Association. 2004;21:1082–1089. doi: 10.1111/j.1464-5491.2004.01289.x. [DOI] [PubMed] [Google Scholar]
- 86.Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovascular diabetology. 2013;12:1. doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 88.Simic B, Hermann M, Shaw SG, et al. Torcetrapib impairs endothelial function in hypertension. European heart journal. 2012;33:1615–1624. doi: 10.1093/eurheartj/ehr348. [DOI] [PubMed] [Google Scholar]