Abstract
A series of three linear and two trivalent aminooxy-containing hydrophilic linkers and cores were synthesized. The five molecules contain from one to three aminooxy groups, and all but one contain an ether for enhanced aqueous solubility. These unique and versatile molecules can be utilized in the chemoselective conjugation of aldehyde/ketone-containing molecules, including reducing sugars, under mild aqueous conditions, and give rise to oxime-containing conjugates useful in a wide variety of applications and studies. The value of these aminooxy-based molecules and the ease and speed of preparation of both monovalent and multivalent oxime-linked molecules is demonstrated in two examples using the disaccharide cellobiose; one with a linear linker, and the second with a trivalent core.
Keywords: aminooxy, oxime, hydrophilic linkers, trivalent cores
Introduction
Aminooxy functional groups are useful for the chemoselective attachment of aldehyde-and ketone-containing molecules, such as reducing carbohydrates. These conjugation reactions can be conducted under mild aqueous conditions, which generally include the use of aniline1,2 or other aromatic amines such as m-phenylenediamine (mPDA)3 as a catalyst, and yield the oxime linkage (Figure 1). The oxime has been shown to be stable to hydrolysis, and is more stable than simple hydrazones, making it an attractive functional group for bioconjugation reactions involving aldehydes and ketones.4,5 In particular, where carbohydrates and amino acids are employed, the chemoselective nature of the oxime-forming reaction allows for the use of unprotected building blocks, even in the presence of a variety of other functional groups. To this end, many reports have been made of diverse aminooxy-containing molecules, including linkers,6–9 polymers,8,10 amino acids/peptides/proteins,9,11 carbohydrates,12,13 nanoparticles,14 multivalent scaffolds,15–18 etc. Molecules such as these have been utilized to create a variety of oxime-containing bioconjugates such as glycopeptides/glycoproteins,19,20 drug conjugates,21,22 and biopolymers.18,23 Oxime bioconjugates have been incorporated into studies that include: protein-carbohydrate interactions,14 improvement of drug activity,21 targeted delivery of chemotherapeutic agents to tumors,22 polymeric drug carriers,8 labeling studies of living cells,24,25 and surface-based investigations using surface plasmon resonance (SPR) and microarrays.26–28
Figure 1.
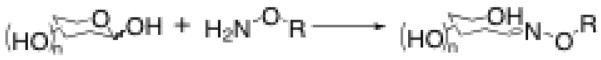
Oxime-forming reaction with a carbohydrate.
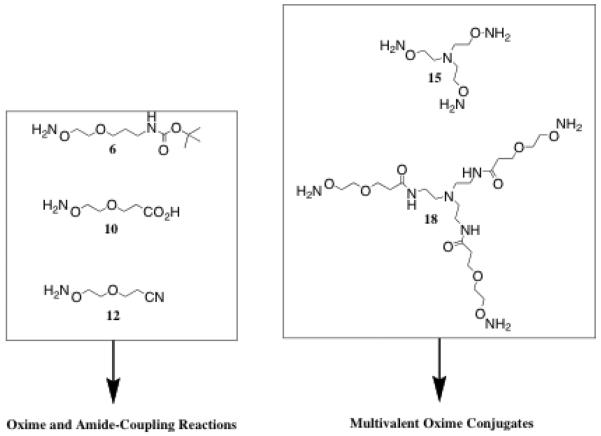
Based on the versatility of the aminooxy functional group as described above, we set out to synthesize a series of hydrophilic linear and trivalent aminooxy-containing molecules using a straightforward synthetic pathway.29 Our intention was to utilize these molecules to create a diverse array of multivalent glycoconjugates for use in the study of carbohydrate-protein binding interactions, in particular the interaction between the glycoconjugates and the HIV-1 surface glycoprotein, gp120. We previously showed that amide-linked sialic acid based-glycodendrimers were micromolar inhibitors of HIV-1 infection.30 In the present study, we desired to create the building blocks necessary to synthesize glycoconjugates utilizing any reducing sugar, not just sugars bearing carboxylic acids/amines. Additionally, it was anticipated that by utilizing oxime-forming chemistry, numerous large, complex glycoconjugates could be realized, with improved yields over the amide-based strategy, using just a few facile steps. All of the molecules include a minimum of one aminooxy group, with the three linear linkers containing an amino, carboxy or nitrile functional group for further attachment/functionalization, and the two trivalent molecules terminating in three aminooxy groups (Figure 2). All but one molecule possesses an ether group, providing good water-solubility properties. The molecule without the ether, however, is small and polar enough to be completely water-miscible. The aminooxy group(s) in each molecule can be attached via the oxime linkage to an aldehyde/ketone of choice. For linkers 6, 10, and 12, an additional functional group was incorporated at the other end of the chain for attachment to other molecules/surfaces of interest. Linker 6 terminates in a Boc-protected amine, and linker 10 in a carboxylic acid, such that amide-coupling reactions can be used with carboxylic acids and amines, respectively. Linker 12 terminates in a nitrile that can be reduced to an amine or hydrolyzed to yield a carboxylic acid if it is desirable to conduct this transformation after the oxime has been formed. These linear linkers can be used to anchor a molecule to a surface, or as a flexible spacer group to link two molecules of interest together. Trivalent core molecules 15 and 18 can be utilized to create multivalent oxime-linked bioconjugates. These bioconjugates can be probed for biological activities such as protein-carbohydrate binding interactions, as illustrated above. The excellent water-solubility properties, combined with the ease of formation and the hydrolytic stability of the oxime, make the aminooxy-containing linear linkers and trivalent cores described herein useful for a variety of applications.
Figure 2.
Uses of aminooxy-linkers and multivalent cores
Results and Discussion
The synthesis of the three hydrophilic aminooxy linkers 6, 10, and 12, and the two hydrophilic aminooxy trivalent cores 15 and 18, were accomplished in a total of two to five synthetic steps, with yields ranging from 61–100% for all but one reaction, the Michael addition used to create 8, which gave a low yield of 26%. It has been reported in the literature that the low yield for the Michael addition is typical for 8.31 We chose to use the Michael addition reaction because the same chemistry could be applied for the synthesis of both known intermediate compounds, 3 and 8, and also because the reactions could be conducted using greener solvents, namely aqueous NaOH or KOH. All syntheses included a Mitsunobu reaction followed by a hydrazinolysis. The Mitsunobu incorporated the phthalimide group and the N-O linkage. The phthalimide was subsequently removed in hydrazinolysis, yielding the desired aminooxy functionality for all linkers and cores contained herein.
The first linker, 6, has an aminooxy group on one end and a Boc-protected amino group on the other, and contains a single ether group. To begin this synthesis, ethylene glycol, 1, was combined with acrylonitrile, 2, in a biphasic asymmetric Michael addition reaction under the conditions of Mathisen and Albertsson, involving NaOH(aq) as the base, yielding the known hydroxy nitrile compound, 3,32 after flash chromatography (Scheme 1). Compound 3 was next converted to the hydroxy Boc linker, 4, through a two-step one-pot reaction whereby the nitrile was first reduced to the amine by sodium borohydride using nickel chloride hexahydrate as a catalyst, then Boc-protected in situ, yielding 4.33 From compound 4, the phthalimide derivative, 5, was produced using a Mitsunobu reaction, followed by hydrazinolysis to give the target Boc-protected linker, 6.
Scheme 1.
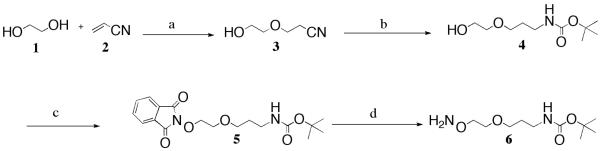
Reagents and conditions: a) NaOH(aq), 45˚C, 85%; b) NiCl2·6 H2O, NaBH4, (Boc)2O, MeOH, 0°C to RT, 72%; c) N-hydroxyphthalimide, PPh3, DIAD, THF, RT, 89%; d) NH2NH2·H2O, EtOH, RT, 93%.
Similar to the synthesis of 6, the synthesis of linker 10, an ethereal aminooxy-carboxylic acid linker, was undertaken starting with the asymmetric Michael addition of ethylene glycol, 1, with t-butyl acrylate, 7, in 40% (w/v) KOH to give 8 (Scheme 2) in 26% yield. While this is a low yield, it should be noted that this tendency has literature precedence, where this reaction was reported to give a yield of 18% using Na° in THF.31 In our hands, we were able to modestly improve on the reported yield using an aqueous base, either NaOH or KOH. Using NaOH(aq) for the reaction, the yields generally ranged from as low as 10% to as high as 26%. We noted that when the base was prepared fresh, the yields were higher, and that by adding a large excess of ethylene glycol, production of the undesired di-substituted byproduct was minimized. Similar results were obtained when KOH(aq) was used, however, no di-substituted product was detected, making this base the desired choice for this transformation. For either base, the most consistent yields were achieved by allowing the reaction to run for approximately 18–24 hours. Following the Michael addition, a Mitsunobu reaction was employed, followed by deprotection of the t-butyl group, yielding 9, and finally hydrazinolysis, giving the target linker, 10. It should be noted that following the Mitsunobu reaction, only partial purification of the t-butyl-protected phthalimide derivative was possible by flash chromatography. To achieve complete purification, it was necessary to first deprotect the phthalimide intermediate to give carboxylic acid 9, which was then recrystallized from ethyl acetate/hexanes. Compound 9 was finally subjected to hydrazinolysis, with the resultant solution filtered first through Celite, then a PTFE (polytetrafluoroethylene) syringe filter to remove the solid byproduct, 2,3-dihydrophalazine-1,4-dione (DHPD), yielding the oil product 10.34
Scheme 2.
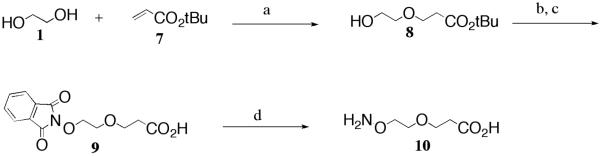
Reagents and conditions: a) 40% (w/v) KOH, RT, 26%; b) N-hydroxyphthalimide, PPh3, DIAD, THF, RT; c) TFA, CH2Cl2, RT, 80% (two-step yield); d) NH2NH2·H2O, EtOH, RT, quantitative.
The synthesis of the next series of compounds, 12 and 15, were achieved using the same two-step reaction Mitsunobu-hydrazinolysis sequence beginning with compound 3, or tri-ethanolamine 13, respectively. The intermediate phthalimines (11 and 14) were purified by first by filtration through Celite, followed by recrystallization. Target aminooxy products, linker 12 and trivalent core 15, were purified via filtration through a combination of Celite and PTFE filters to give the final oil products (Scheme 3).
Scheme 3.
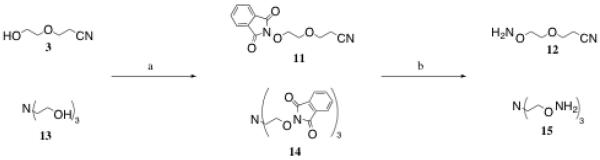
Reagents and conditions: a) N-hydroxyphthalimide, PPh3, DIAD, THF, RT, 73–77%; b) NH2NH2·H2O, EtOH, RT, 83–95%.
For the remaining trivalent core, 18, we desired to create a core that incorporated one of the hydrophilic ether linkers, 9, to bring good water solubility properties to the molecule. In addition, the aminooxy endgroups were extended further away from the core to facilitate oxime linkage formation to a desired aldehyde- or ketone-containing molecule. To accomplish this, commercially available tris(2-aminoethyl)amine, 16, was combined with linker 9 through BOP coupling to yield the tri-phthalimide 17 (Scheme 4). Hydrazinolysis of 17 then afforded oil 18 after filtration through Celite and PTFE filters, followed by FPLC on a BioGel P-10 column.
Scheme 4.
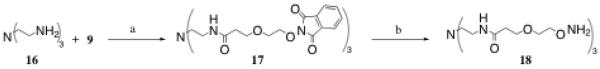
Reagents and conditions: a) BOP, DIPEA, DMF, RT, 86%; b. NH2NH2·H2O, EtOH, RT, 61%.
To demonstrate the ease of creating oxime-linked glycoconjugates from the aminooxy-terminated molecules described above, two examples are presented. In the first, linker 6 was combined with the simple reducing disaccharide, cellobiose 19, in a mild aqueous buffer (pH 4.5 0.1 M NH4OAc), with aniline (0.1 M) added as a catalyst (Scheme 5).1,2 The coupling was accomplished in one day with stirring at room temperature, and yielded 90% of the desired product 20, after flash chromatography. To reveal the amine, compound 20 was next stirred in TFA/CH2Cl2 for two hours at room temperature, then freeze dried, giving an 85% yield of 21. It should be noted that when the oxime is formed, both the E and the Z-isomers are observed in the 1H NMR in D2O (supporting information). These isomers are in equilibrium with the ring-closed form(s) of the sugar, which explains why the integrations for the E/Z isomer peaks are always less than anticipated.2,35 For compound 21, the more stable E isomer was formed in a 7.8/1 ratio compared to the Z isomer. Finally, sugar-linker 21 can be utilized in amide-based coupling reactions with a carboxylic acid-containing molecule of choice.
Scheme 5.
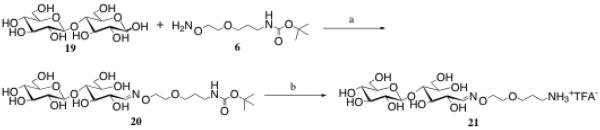
Reagents and conditions: a) 0.1 M NH4OAc, pH = 4.5, 0.1 M aniline, RT, 90%; b) TFA, CH2Cl2, RT, 85%.
Beyond creating monovalent oxime-linked conjugates, we also wanted to show that the oxime linkage could be formed readily in a multivalent sense using a trivalent core. In this example, cellobiose 19 was stirred for 48h at 40°C with trivalent core 15 at pH 4.5 in 0.1 M NH4OAc with 0.1 M aniline added to the reaction as a catalyst (Scheme 6).1,2 The reaction was heated as it had been noted in the literature that mild heating could improve the reaction rate and overall yield of the reaction.2 This is particularly important in a reaction with multiple reactive sites such as this. Purification was carried out by FPLC on a BioGel P-10 column, and resulted in a 61% yield of 22. Finally, by 1H NMR in D2O, it was ascertained that the E/Z isomer ratio was 6.1/1 for 22, favoring the formation of the more stable E isomer.
Scheme 6.
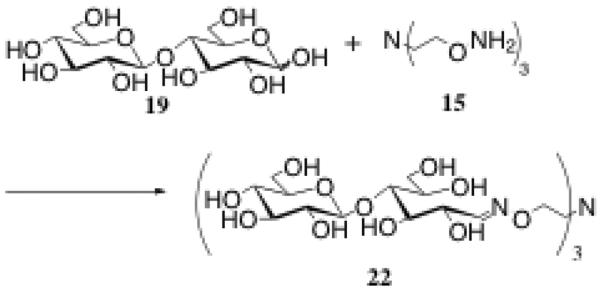
Reagents and conditions: a) 0.1 M NH4OAc, pH = 4.5, 0.1 M aniline, 40°C, 61%.
Conclusion
In conclusion, the syntheses of three water-soluble aminooxy-containing linear linkers and two water-soluble aminooxy-containing trivalent cores were achieved in good yields. These compounds have been employed in the facile synthesis of sugar-linker conjugates and trivalent glycoconjugates such as that illustrated by compounds 20–22 through the formation of the stable oxime linkage. These molecules can be utilized for the creation of larger glycoconjugates such as glycodendrimers, and/or in the study of protein-carbohydrate interactions, which might include a variety of carbohydrate-binding proteins such as lectins. In our lab, we are using these molecules and others derived from them to study the binding to the HIV-1 protein gp120. It is anticipated that these versatile and water-soluble linkers and trivalent cores will realize many other applications for the chemoselective bioconjugation of aldehyde/ketone-containing molecules for the study of biological interactions, or in the development of water-soluble drug conjugates, among others.
Supplementary Material
Acknowledgments
Financial support for this work was gratefully received from NIH-AREA (1R15AI068444-01), Research Corporation Cottrell College Science Award (CC6610), and the CSUS NSM-SURE program for support of H.L. NSF-MRI provided funding for the 500 MHz NMR (CHE MRI-0922676). Special thanks also to Dr. Arpad Somogyi at The University of Arizona for his technical assistance with the MS analysis of Compounds 6, 10 and 12.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data General methods, experimental procedures, and 1H and 13C spectra for target compounds 6, 10, 12, 15, 18, 21 and 22 are contained in the Supporting Information section.
References and Notes
- (1).Dirksen A, Dawson PE. Bioconj. Chem. 2008;19:2543. doi: 10.1021/bc800310p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Thygesen MB, Munch H, Sauer J, Cló E, Jorgensen MR, Hindsgaul O, Jensen KJ. J. Org. Chem. 2010;75:1752. doi: 10.1021/jo902425v. [DOI] [PubMed] [Google Scholar]
- (3).Rashidian M, Mahmoodi MM, Shah R, Dozier JK, Wagner CR, Distefano MD. Bioconj. Chem. 2013;24:333. doi: 10.1021/bc3004167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kalia J, Raines RT. Angew. Chem. Int. Ed. Engl. 2008;47:7523. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tiefenbrunn TK, Dawson PE. Peptide Science. 2009;94:95. [Google Scholar]
- (6).Jones DS, Hammaker JR, Tedder ME. Tett. Lett. 2000;41:1531. [Google Scholar]
- (7).Carrasco MR, Alvarado CI, Dashner ST, Wong AJ, Wong MAJ. Org. Chem. 2010;75:5757. doi: 10.1021/jo101066c. [DOI] [PubMed] [Google Scholar]
- (8).Jin Y, Song L, Su Y, Zhu L, Pang Y, Qiu F, Tong G, Yan D, Zhu B, Zhu X. Biomacromolecules. 2011;12:3460. doi: 10.1021/bm200956u. [DOI] [PubMed] [Google Scholar]
- (9).Sohma Y, Kent SBHJ. Am. Chem. Soc. 2009;131:16313. doi: 10.1021/ja9052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vázquez-Dorbatt V, Tolstyka ZP, Maynard HD. Macromolecules. 2009;42:7650. doi: 10.1021/ma9013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Seo J, Michaelian N, Owens SC, Dashner ST, Wong AJ, Barron AE, Carrasco MR. Org. Lett. 2009;11:5210. doi: 10.1021/ol9021468. [DOI] [PubMed] [Google Scholar]
- (12).Renaudet O, Dumy P. Tett. Lett. 2001;42:7575. [Google Scholar]
- (13).Richard A, Barras A, Ben Younes A, Monfilliette-Dupont N, Melnyk P. Bioconj. Chem. 2008;19:1491. doi: 10.1021/bc700444t. [DOI] [PubMed] [Google Scholar]
- (14).Thygesen MB, Sauer J, Jensen KJ. Chem. Eur. J. 2009;15:1649. doi: 10.1002/chem.200801521. [DOI] [PubMed] [Google Scholar]
- (15).Jones DS, Cockerill KA, Gamino CA, Hammaker JR, Hayag MS, Iverson GM, Linnik MD, McNeeley PA, Tedder ME, Ton-Nu H-T, Victoria EJ. Bioconj. Chem. 2001;12:1012. doi: 10.1021/bc015512x. [DOI] [PubMed] [Google Scholar]
- (16).Grover GN, Lam J, Nguyen TH, Segura T, Maynard HD. Biomacromolecules. 2012;13:3013. doi: 10.1021/bm301346e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Matsushita T, Nagashima I, Fumoto M, Ohta T, Yamada K, Shimizu H, Hinou H, Naruchi K, Ito T, Kondo H, Nishimura S-I. J. Am. Chem. Soc. 2010;132:16651. doi: 10.1021/ja106955j. [DOI] [PubMed] [Google Scholar]
- (18).Stukel JM, Li RC, Maynard HD, Caplan MR. Biomacromolecules. 2010;11:160. doi: 10.1021/bm9010276. [DOI] [PubMed] [Google Scholar]
- (19).Rodriguez EC, Winans KA, King DS, Bertozzi CR. J. Am. Chem. Soc. 1997;119:9905. [Google Scholar]
- (20).Carrasco MR, Nguyen MJ, Burnell DR, MacLaren MD, Hengel SM. Tett. Lett. 2002;43:5727. [Google Scholar]
- (21).Pandey D, Katti SB, Haq W, Tripathi CKM. Bioorg. Med. Chem. 2004;12:3807. doi: 10.1016/j.bmc.2004.05.011. [DOI] [PubMed] [Google Scholar]
- (22).Szabó I, Manea M, Orbán E, Csámpai A, Bősze S, Szabó R, Tejeda M, Gaál D, Kapuvári B, Przybylski M, Hudecz F, Mező G. Bioconj. Chem. 2009;20:656. doi: 10.1021/bc800542u. [DOI] [PubMed] [Google Scholar]
- (23).Lee SC, Parthasarathy R, Botwin K, Kunneman D, Rowold E, Lange G, Klover J, Abegg A, Zobel J, Beck T, Miller T, Hood W, Monahan J, McKearn JP, Jansson R, Voliva CF. Biomed. Microdevices. 2004;6:191. doi: 10.1023/B:BMMD.0000042048.18186.ff. [DOI] [PubMed] [Google Scholar]
- (24).Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. Nat. Methods. 2009;6:207. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Crisalli P, Hernández AR, Kool ET. Bioconj. Chem. 2012;23:1969. doi: 10.1021/bc300344b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Oyelaran O, Gildersleeve JC. Curr. Opin. Chem. Biol. 2009;13:406. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cló E, Blixt O, Jensen KJ. Eur. J. Org. Chem. 2010:540. [Google Scholar]
- (28).Dettin M, Muncan N, Bugatti A, Grezzo F, Danesin R, Rusnati M. Bioconj. Chem. 2011;22:1753. doi: 10.1021/bc200254u. [DOI] [PubMed] [Google Scholar]
- (29).Zamboanga (McReynolds) K. United States patent pending. 2013
- (30).Clayton R, Hardman J, LaBranche CC, McReynolds KD. Bioconjugate Chem. 2011;22:2186. doi: 10.1021/bc200331v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chen Q, Gabathuler R. Synth. Commun. 2004;34:2425. [Google Scholar]
- (32).Mathisen T, Albertsson A-C. Macromolecules. 1989;22:3838. [Google Scholar]
- (33).Caddick S, Judd DB, Lewis A. K. de K., Reich MT, Williams MRV. Tetrahedron. 2003;59:5417. [Google Scholar]
- (34).Khan MN. J. Org. Chem. 1995;60:4536. [Google Scholar]
- (35).Peri F, Dumy P, Mutter M. Tetrahedron. 1998;54:12269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.