Abstract
Pathobionts play a critical role in disease development, but the immune mechanisms against pathobionts remain poorly understood. In this study we have reported a critical role for interleukin-22 (IL-22) in systemic protection against bacterial pathobionts that translocate into the circulation after infection with the pathogen Clostridium difficile. Infection with C. difficile induced IL-22, and infected Il22−/− mice harbored high numbers of pathobionts in extraintestinal organs despite comparable pathogen load and intestinal damage in mutant and wild-type mice. Pathobionts exhibited increased resistant against complement-mediated phagocytosis, and their intravenous administration resulted in high animal mortality. Selective removal of translocated commensals rescued Il22−/− mice and IL-22 administration enhanced the elimination of pathobionts. Mechanistically, IL-22 augmented bacterial phagocytosis by increasing the expression and bacterial binding of complement C3. Our study demonstrates an unexpected role for IL-22 in controlling the elimination of pathobionts that enter the systemic circulation through the regulation of the complement system.
INTRODUCTION
The intestine of humans and animals harbors diverse microbial species that provide numerous benefits to their hosts (Kamada et al., 2013; Round and Mazmanian, 2009). However, the gut microbiota also includes potentially virulent species, called pathobionts, which can cause disease when intestinal homeostasis is disrupted particularly in immunocompromised hosts (Chow and Mazmanian, 2010; Kamada et al., 2013). Indeed, disruption of the healthy microbiota by several mechanisms including antibiotic treatment results in dysbiosis, which can lead to the accumulation of pathobionts and disease (Kamada et al., 2013; Pham and Lawley 2014). However, the mechanisms whereby pathobionts give rise to disease remain poorly understood. Clostridium difficile is a Gram-positive anaerobic bacterium that overgrows during dysbiosis in patients who have received broad-spectrum antibiotics and causes infectious diarrhea and pseudomembranous colitis (Carroll and Bartlett 2011). The severity of C. difficile infection varies from mild diarrhea to septic shock and multiple organ failure, which is associated with high mortality (Eaton and Mazuski, 2013; Sunenshine et al 2006). Moreover, even after treatment with antibiotics such as vancomycin and metronidazole that eradicate C. difficile, 7% of patients will still die from C. difficile infection (Carroll et al., 2011). Although the cause of severe complications and mortality associated with C. difficile infection remains poorly understood, animal studies suggest that commensal bacteria contribute to disease severity. For example after C. difficile infection, mice lacking interleukin-1β (IL-1β), inflammasome components or the innate immune receptor Nod1 exhibit high mortality which is associated with impaired neutrophil recruitment to areas of intestinal damage and increased translocation of commensal bacteria (Hasegawa et al., 2011; Hasegawa et al., 2012). Thus, translocated commensal bacteria may contribute to C. difficile infection-induced mortality, but the mechanisms by which commensal bacteria from the intestine accumulate in extraintestinal tissues and the immune factors that protect the host against their accumulation remain largely unknown.
IL-22, a cytokine produced by RORγt+ innate lymphoid cells, T helper-17 (Th17), Th22 and γδ T cells, has been implicated in the control of commensal bacteria in the intestine (Sonnenberg et al, 2011). Secretion of IL-22 from intestinal immune cells is regulated by IL-23 and IL-1β, with the latter being produced largely via the inflammasome (Sutton et al., 2009). IL-22 enhances the expression of antimicrobial proteins that limit the association of certain intestinal bacteria with the epithelium (Brandl et al., 2007; Zheng et al., 2008). Furthermore, IL-22 regulates the repair of damaged epithelium and maintains homeostasis of the microbiota (Sa et al., 2007, Zheng et al., 2008). Moreover, IL-22 plays an important role in host protection against enteropathogens such as Citrobacter rodentium (Aujla et al., 2008; Basu et al., 2012; Satoh-Takayama et al., 2008; Schulz et al., 2008). Unlike C. difficile infection that induces acute intestinal dysfunction through the production of epithelium-damaging toxins A and B (TcdA and TcdB) (Kuehne et al., 2010), intestinal disease triggered by C. rodentium develops more slowly and pathogen clearance is dependent on acquired immunity (Mundy et al., 2005). Here we report that IL-22-deficient mice exhibit increased mortality following oral infection with C. difficile, and mice were rescued by selective antibiotic treatment that removes commensal bacteria, but not C. difficile. The intestinal burden of C. difficile and the extent of intestinal damage were not altered in infected Il22−/− mice. Instead, IL-22 enhanced the expression of complement factor C3 that contributed to the clearance of specific pathobionts from peripheral organs. Hence, our study underscores a critical role for IL-22 in protecting the host beyond the intestine by facilitating the systemic clearance of intestinal pathobionts.
RESULTS
IL-22-deficient mice are more susceptible to C. difficile infection
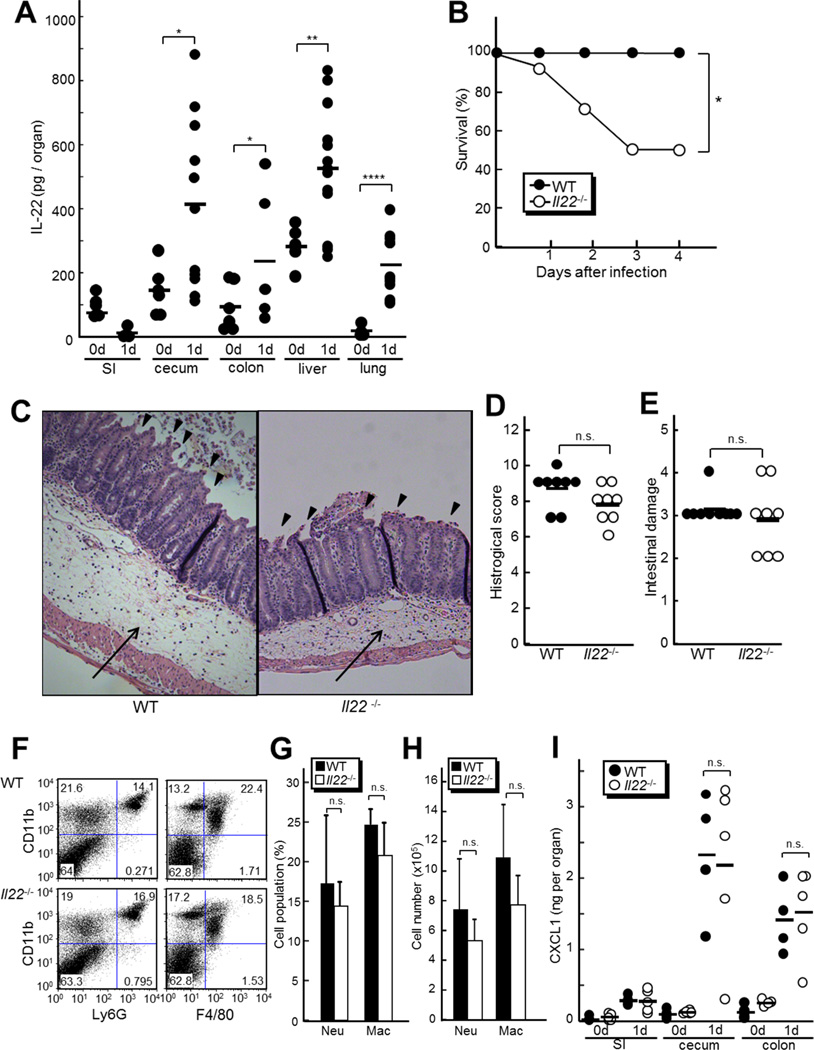
To assess the role of IL-22 in C. difficile-induced colitis, we infected antibiotic-treated mice with the pathogen which recapitulates many of the disease features observed in humans (Chen et al., 2008; Hasegawa et al, 2011). We detected increased production of IL-22 in the intestine, lung, and liver of mice 24 hr after infection with C. difficile (Figure 1A). We also detected marginal induction of IL-17 in cecum after C. difficile infection (Figure S1A). To assess the source of increased IL-22, we infected Rag1−/− mice, which lack T and B cells, and found that increased IL-22 amounts after C. difficile infection was independent of RAG1, whereas no significant IL-17 induction was detected in the absence of RAG1 (Figure S1 A and B). This suggests that the source of increased IL-22 is innate cells but not mature T and B cells including Th17, Th22 and γδ T cells. To investigate the role of IL-22 in host protection during C. difficile infection, we assessed the survival of Il22−/− mice after C. difficile infection, and found a striking increase in mortality of Il22−/− mice when compared to WT mice (Figure 1B). We also found no significant impact of RAG1 deficiency on mouse survival after C. difficile infection(Figure S1C), suggesting the T-cell derived cytokines, including IL-17 in this case, are dispensable for protection against C. difficile infection. To understand the cause of increased mortality in Il22−/− mice associated with C. difficile infection, we analyzed tissue histology and found similar degree of intestinal damage including epithelial disruption, submucosal edema, and infiltration of inflammatory cells including neutrophils in Il22−/− and WT mice 3 days post C. difficile infection(Figure 1C to E). Consistently, flow cytometry analysis showed similar numbers of neutrophils (CD11b+Ly6G+) and macrophages (CD11b+F4/80+) in the intestine of Il22−/− and WT mice 2 days after C. difficile infection(Figure 1F to H). Additionally, Il22−/− and WT mice produced comparable amounts of CXCL1, a chemokine that is important in the recruitment of neutrophils (Figure 1I). We also found comparable weight loss in Il22−/− and WT mice and similar numbers of TUNEL-positive apoptotic cells in the intestinal epithelium after C. difficile infection(Figure S1 D to F). These results indicate that deficiency of IL-22 does not affect the extent of intestinal damage or recruitment of inflammatory cells following C. difficile infection and suggest that IL-22 contributes to protection against C. difficile infection-induced mortality through other host defense mechanisms.
Figure 1. Il22−/− mice are more susceptible to C. difficile independent of epithelial damage and neutrophil recruitment.
A, WT mice were infected with 108 CFU of Cdiff by gastric gavage after antibiotic treatment as described in Supplemental Experimental Procedures. The amounts of IL-22 in indicated organs from uninfected (0 d, n=6) and mice infected for 24 hr (1 d, n=10) were determined by ELISA. SI, small intestine. Results are representative of three independent experiments. B, Survival of Il22−/− and WT mice infected with Cdiff was monitored for 14 days (n=16 per group). All mice were infected by gastric gavage with 108 CFU of C. difficile. No additional deaths were observed beyond 5 days after infection. C, Representative histology of ceca from Il22−/− and WT mice 3 days after infection. Arrows and arrowheads show submucosal edema and epithelial damage, respectively (200 X magnification). The images are representative of 10 mice per group. D and E, The overall pathology (D) and epithelial damage (E) scores are based on the analysis of ceca on day 3 post-infection. Results are representative of five independent experiments. F to H, Immune cells isolated from ceca and colons of Il22−/− and WT mice (n=5 per group) were analyzed by flow cytometry 2 days post-infection. F, Percentage of cells labeled with anti-CD11b and anti-Ly-6G or anti-CD11b and anti-F4/80 mAbs, respectively, are shown. G and H, Percentage (G) and cell number (H) ± SD of CD11b+Ly-6G+ (neutrophils) or CD11b+F4/80+ (macrophages) are shown. Results are representative of two independent experiments. I, The amounts of CXCL1 in the indicated tissues of Il22−/− and WT mice (n=5 per group) were determined by ELISA 24 hr post-infection. SI, small intestine. Results are representative of three independent experiments. Bars indicate means. *, p < 0.05; **, p < 0.01; ****, p<0.0001; n.s., not significant (p > 0.05)
Reduction of Gammaenterobacteria protects IL-22-deficient mice from lethality after C. difficile infection
The observation that Il22−/− and WT mice exhibit comparable intestinal epithelial damage and inflammatory response after C. difficile infection suggests that epithelial damage per se is unlikely to be the main factor that leads to the death of infected Il22−/− mice. Furthermore, similar numbers of C. difficile were found in the feces of infected Il22−/− and WT mice (Figure S1G), indicating that IL-22 does not protect the host by promoting clearance of C. difficile in the intestine. In addition, C. difficile was not detected in the lung or liver of Il22−/− mice, indicating that the increased mortality of Il22−/− mice was not caused by systemic translocation of C. difficile itself (Figure S1H). Analysis of the gut microbiota by Illumina sequencing of the 16S rRNA gene revealed that IL-22 deficiency did not significantly alter the composition of the fecal microbiota in uninfected and C. difficile-infected mice (Figure S1I to K, Table S1). We also found that IL-22 deficiency did not affect the survival rate of mice infected with streptomycin-resistant strain of Salmonella enterica Typhimurium, whose robust colonization requires elimination of commensals by streptomycin treatment (Figure S1L). We therefore hypothesized that IL-22 may play an important role in the systemic clearance of translocated commensal bacteria following C. difficile infection. To test this, we determined the numbers of commensals in extraintestinal organs of Il22−/− and WT mice before and after C. difficile infection. Notably, increased numbers of commensals were detected in all organs tested, despite similar amounts of fecal bacteria in Il22−/− and WT mice (Figure 2A, Figure S1G). Because no significant differences in bacterial numbers were found in the organs of uninfected Il22−/− and WT mice (Figure 2A), these results indicate that in the absence of IL-22, C. difficile infection results in increased numbers of commensals in extraintestinal tissues. Consistently, the concentrations of serum aspartate transaminase (AST) and alanine transaminase (ALT), which are markers of liver damage, were significantly higher in Il22−/− mice than in WT mice 3 days after C. difficile infection (Figure 2B and C), likely reflecting tissue damage associated with increased burdens of translocated bacteria in the liver of Il22−/− mice after C. difficile infection. Collectively, these results indicate that IL-22 regulates the clearance of translocated intestinal bacteria in extraintestinal organs after C. difficile infection.
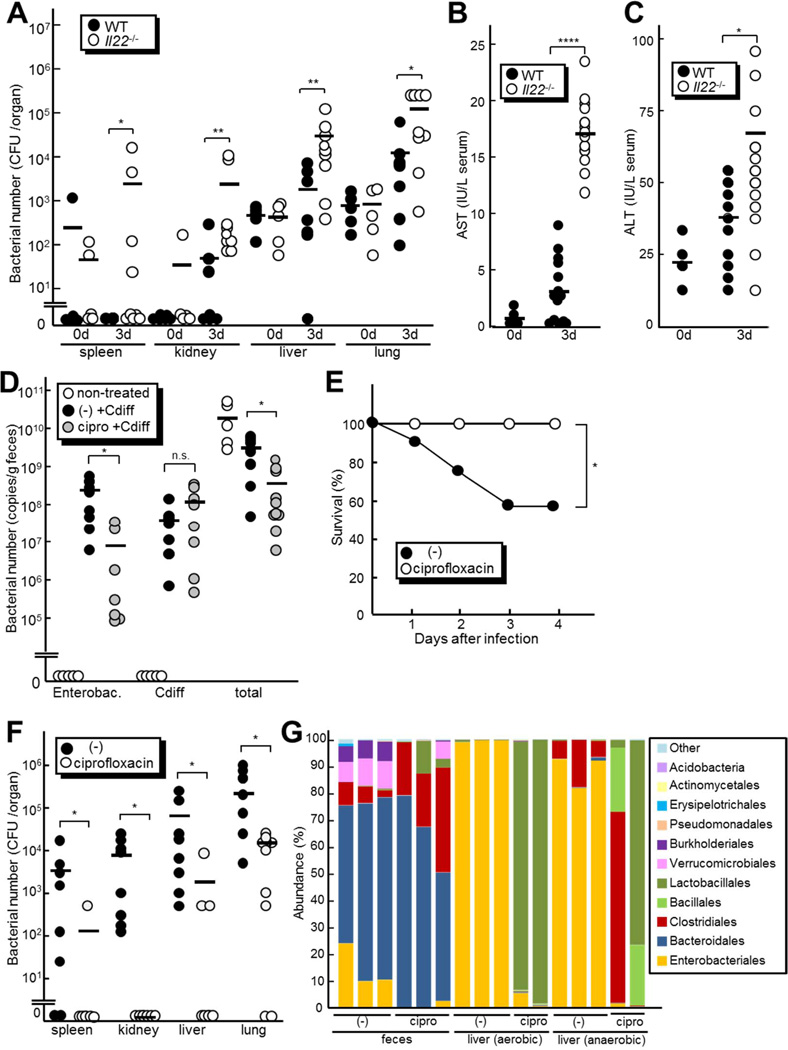
Figure 2. Increased load of commensals in the peripheral organs of infected Il22−/−mice.
A, The number of total cultivable bacteria in spleen, kidney, liver, lung and feces from Il22−/− (n=9) and WT mice (n=8) infected with 108 CFU of C. difficile. Bacterial loads were determined on day 0 and 3 post-infection by plating on non-selective BHI. B and C, The concentrations of AST (B) and ALT (C) in sera from Il22−/− (n=12) and WT mice (n=10) 3 days after C. difficile infection. Results are representative of two independent experiments. D to G Il22−/− mice were treated with 417 mg/kg ciprofloxacin in the drinking water or left alone (−) after C. difficile infection. D, The numbers of total bacteria, enterobacteria and C. difficile in feces of ciprofloxacin-treated and non-treated control mice (n=7 per group) on day 2 post-infection were determined by quantitative RT-PCR. E, Survival of ciprofloxacin-treated (n=8) and non-treated mice (n=7) infected with C. difficile was monitored for 14 days. No additional deaths were observed beyond 5 days after infection. F, The number of total cultivable bacteria in spleen, kidney, liver and lung from ciprofloxacin-treated (n=9) and non-treated mice (n=8) on day 3 infection with C. difficile. Results are representative of two independent experiments. G, Taxonomic composition of fecal bacteria and cultivable bacterial populations in the liver of ciprofloxacin-treated and non-treated mice day 3 after C. difficile infection (n=3 or 2 per group). Bars indicate means. *, p < 0.05; **, p < 0.01; ****, p<0.0001; n.s., not significant (p > 0.05).
To determine the contribution of translocated commensals to the pathogenesis of C. difficile infection, we investigated next the survival rate of C. difficile-infected Il22−/− mice in the absence or presence of ciprofloxacin, an antibiotic that effectively kills many commensals, but not C. difficile in vitro (Figure S1M). Quantitative PCR analysis using bacterial group-specific primers showed that ciprofloxacin treatment reduced the numbers of total bacteria and enterobacteria ~ 8 and 30 fold, respectively, but it did not affect the numbers of C. difficile in feces (Figure 2D). Histological analysis showed that C. difficile infection induced similar degree of epithelial damage and submucosal edema in ciprofloxacin-treated and control mice (Figure S1N), which is consistent with comparable numbers of C. difficile in antibiotic-treated and untreated mice. Notably, ciprofloxacin treatment completely rescued IL22−/− mice from C. difficile infection-induced lethality (Figure 2E). Importantly, ciprofloxacin treatment dramatically reduced the number of bacteria translocated in extraintestinal organs after C. difficile infection (Figure 2F). Furthermore, Illumina sequencing revealed that ciprofloxacin treatment strongly reduced the number of Enterobacteriales species in the liver and feces (Figure 2G). Taken together, these results suggest that translocated commensal bacteria after C. difficile infection contributes to the death of Il22−/− mice, which can be rescued by limiting the extent of commensal bacteria translocation with ciprofloxacin treatment.
Translocation of specific species of pathobionts after C. difficile infection contributes to lethality in IL-22-deficient mice
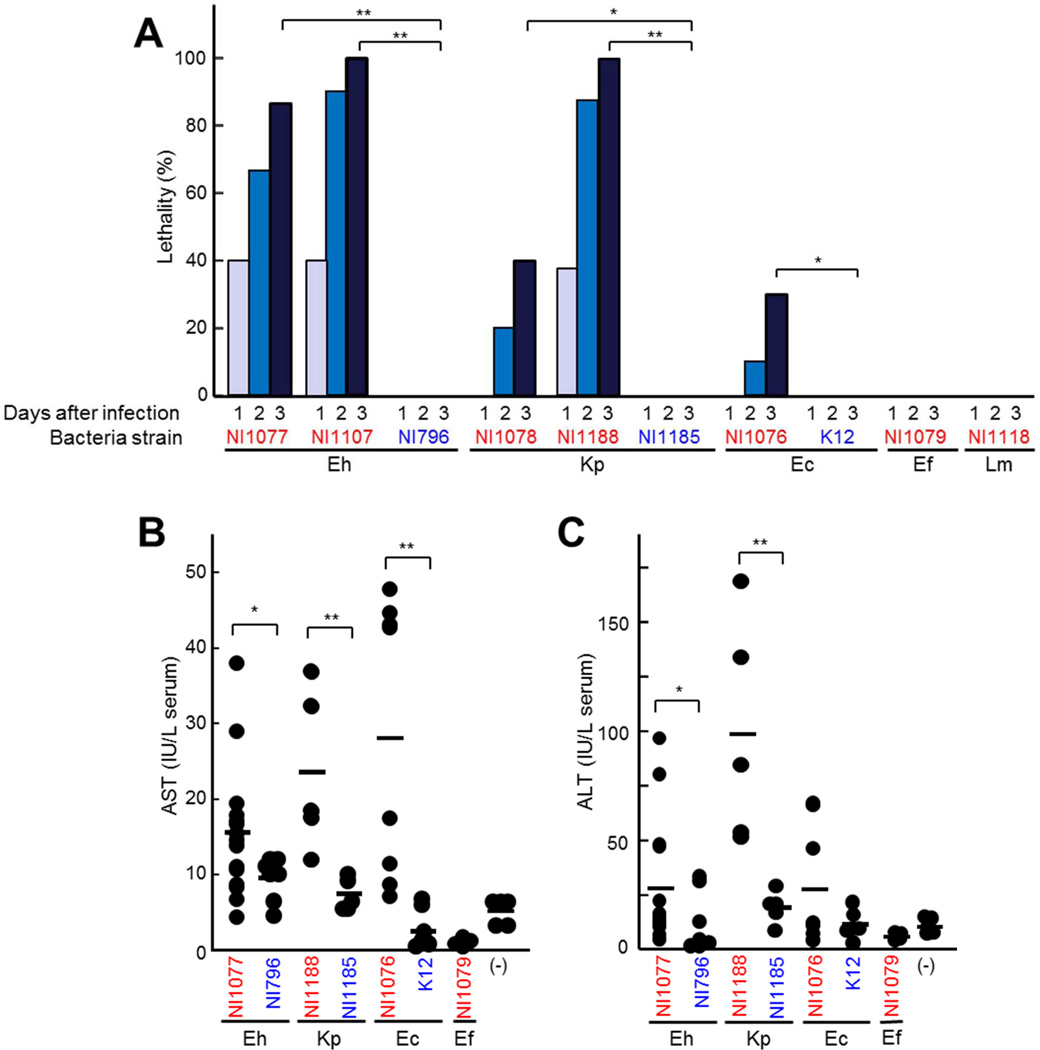
To investigate the role of individual bacterial species in C. difficile-induced disease, we further characterized the composition of the bacteria present in the liver of C. difficile-infected Il22−/− mice and isolated dominant bacteria from the organs of C. difficile-infected mice to assess their pathogenicity. The most abundant bacteria in the liver after C. difficile infection were Enterobacteriaceae species including several belonging to the Enterobacter cloacae complex, Klebsiella pneumoniae and Escherichia coli (Figure 2G). These bacteria represented ~ 89 % of anaerobically cultivable bacterial species detected as operational taxonomic units (OTUs) in Figure 2G. In addition, Lactobacillales species including Enterococcus faecalis and Lactobacillus murinus were identified as the second dominant (~ 1% of total bacteria) group in the liver after C. difficile infection (Figure 2G). To evaluate the ability of these dominant bacterial species to cause lethality, we infected WT mice with individual bacterial clones by intravenous administration to mimic bacterial translocation after C. difficile infection. WT mice infected with enterobacterial species isolated from the tissues of C. difficile-infected Il22−/− mice (NI1077, NI1107, NI1078, and NI1188) died by day 3 after bacterial administration (Figure 3A), whereas all mice infected with two Lactobacillales species (NI1079 and NI1118) also isolated from the liver survived (Figure 3A). Importantly, i.v. administration of the same number of 16S rRNA phylotype-matched enterobacterial strains (NI796 and NI1185) isolated from the mouse feces did not result in lethality (Figure 3A). These results suggest that pathogenicity of enterobacterial species is strain-dependent. Consistently, administration of pathobiont strains, but not that of control phylotype-matched strains of Enterobacteriaceae induced liver damage as assessed by ALT and AST concentrations in serum (Figure 3B and C). Collectively, our data suggests that particular strains of enterobacterial pathobionts that accumulate and translocate into extraintestinal organs during C. difficile infection displayed remarkable lethality once in the circulation.
Figure 3. Enterobacteria isolated from liver of mice infected with C. difficile exhibit enhanced lethality after intravenous administration.
A to D, Enterobacteriaceae E. hormaechei (Eh) NI1077, NI1109, NI796; K. pneumoniae (Kp) NI1078, NI1188, NI1185; E. coli NI1076, K12; Lactobacillales E. faecalis (Ef) NI1079 and L. murinus (Lm) NI1118 were administered (2×108 CFU) to WT mice i.v.. A, Survival of infected mice (n=10) were monitored for 14 days. No additional deaths were observed beyond 5 days after infection. Results are representative of three independent experiments. B and C, The concentrations of AST (B) and ALT (C) in the serum 3 days after infection. Strains isolated from the liver after C. difficile infection are indicated in red, whereas control strains (isolates from the murine intestine and K-12) are indicated in blue. Bars indicate means. *, p < 0.05; **, p < 0.01. Results are representative of three independent experiments.
Enterobacterial pathobionts isolated from C. difficile-infected Il22−/− mice are resistant to complement-mediated phagocytosis
The differential ability of individual commensals to induce tissue damage and lethality in vivo could be due to differences in cytotoxicity. However, both pathobiont and non-pathobiont commensal strains showed similar cytotoxicity and cytokine induction in vitro (Figure S2 A to C). Therefore, we hypothesized that pathobiont strains possess a unique ability to escape elimination from host tissues. To test this, we determined the survival of individual commensals after i.v. administration into WT mice. Comparison of bacterial numbers in the blood, liver and lung of infected mice showed that pathobionts had higher survival rates than control commensal bacterial strains (Figure 4A and Figure S3A). Moreover, the survival rates of individual commensals were proportional to their ability to induce host lethality and organ damage as revealed by release of AST and ALT into the circulation after inoculation in mice (Figure 3). These results suggest that pathobionts cause detrimental tissue damage by evading host protective immunity.
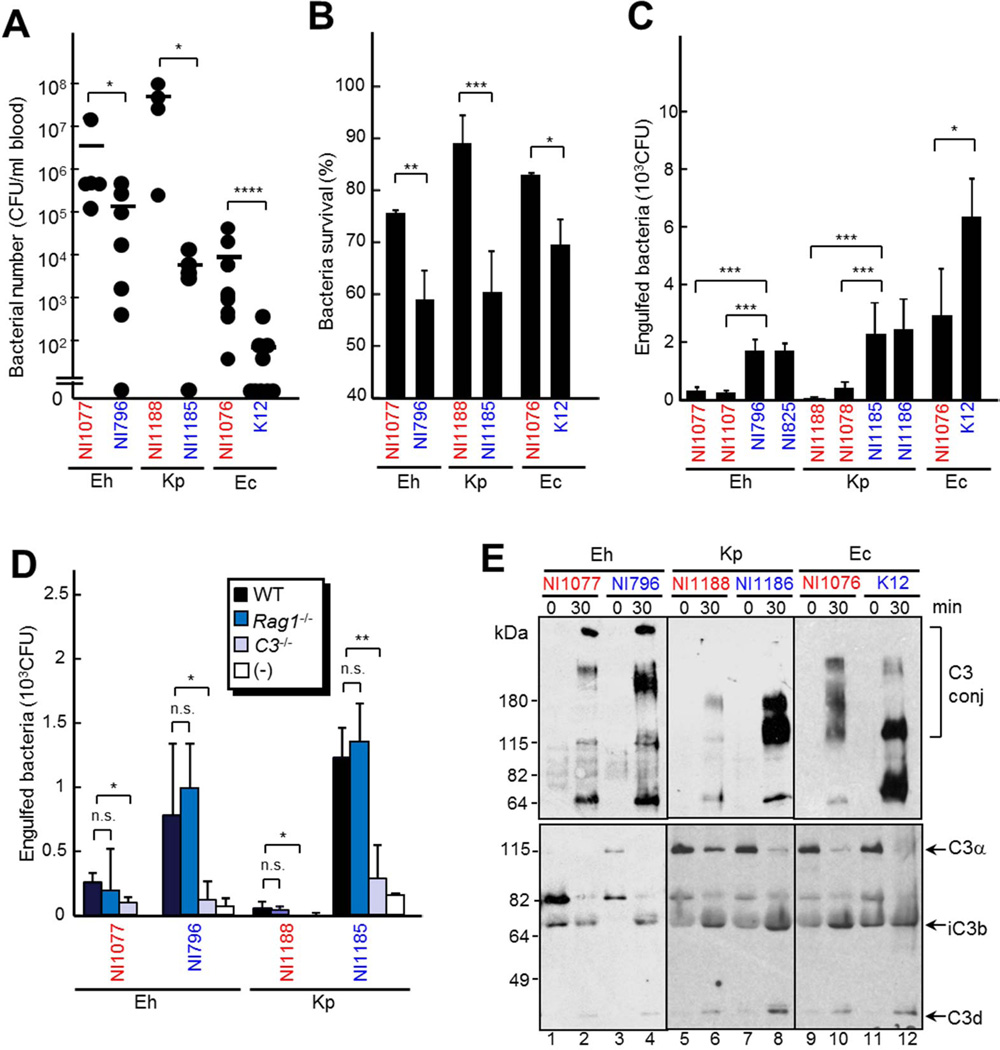
Figure 4. Increased survival of enterobacteria in tissues is associated with impaired phagocytosis by neutrophils.
A, WT mice were infected with 1 × 108 CFU of E. hormaechei (Eh) NI1077, NI796; K. pneumoniae (Kp) NI1078, NI1188 and E. coli NI1076, K12 i.v.. The number of bacteria in the blood from mice (n=7 per group) on day 2 after infection was determined by plating on BHI media. B, Neutrophils were incubated with 5×103 CFU of the indicated strains in 2.5% fresh mouse serum for 2 hr. Surviving bacteria were counted by plating on BHI. C, Bone marrow macrophages were cultured with the indicated strains at MOI 1:1 in the presence of 5% fresh mouse serum. 20 min after infection cells, the cells were treated with gentamycin. Internalized living bacteria were counted by plating on BHI. D, Bone marrow macrophages were cultured with the indicated strains at MOI 1:1 in 5% fresh mouse serum from WT, Rag1−/− or C3−/− mice. 20 min after infection, the cells were treated with gentamycin. Internalized bacteria were counted by plating on BHI. E, C3 processing and deposition on indicated strains. 5 × 108 CFU bacteria were incubated in mouse serum for 30 min and C3 subfragments deposited on bacteria (upper panel) and total reaction mixture (lower panel) were detected by anti-C3 antibody. C3 conj, C3 conjugated with bacteria. Bars indicate means ± SD. The strains that were isolated from liver after C. difficile infection are indicated in red, whereas control intestinal strains are indicated in blue. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p<0.0001; n.s., not significant (p > 0.05). Results are representative of four independent experiments.
Previous studies have shown a critical role for neutrophils in the elimination of translocated commensals at intestinal sites in the C. difficile infection mouse model (Hasegawa et al., 2010; Hasegawa et al., 2012). Therefore, we hypothesized that the enterobacterial pathobionts isolated from Il22−/− mice after C. difficile infection have the ability to avoid elimination by neutrophils or other phagocytic cells. Indeed, isolated pathobionts were more resistant to both killing by neutrophils (Figure 4B) and phagocytosis by macrophages (Figure 4C) than control phylotype-matched commensal strains. To determine more comprehensively the susceptibility to phagocytosis of translocated pathobionts that survived in organs such as the liver after C. difficile infection, we examined the sensitivity to phagocytosis of 100 randomly selected bacterial clones isolated from the livers and feces of Il22−/− mice 3 days after C. difficile infection. The proportion of phagocytosis-resistant commensal clones was significantly higher in the livers of C. difficile-infected Il22−/− mice than in the feces of the same mice (Figure S3B). These results suggest that some translocated commensals are able to evade phagocytosis leading to their enhanced survival in the mouse organs after C. difficile infection.
Phagocytosis of bacteria is facilitated by opsonization with immunoglobulins (Igs) and complement factors (Noris and Remuzzi 2013). Therefore, we tested the ability of the sera from Rag1−/− and C3−/− mice, which are deficient in Ig production and all complement pathways, respectively (Noris and Remuzzi 2013), to facilitate phagocytosis of pathobionts and control commensals. The sera from C3−/− mice promoted poorly the phagocytosis of all tested bacteria by macrophages, although pathobiont strains were more resistant to C3-mediated phagocytosis (Figure 4D). In contrast, the sera from Rag1−/− and WT mice showed a similar ability to facilitate phagocytosis of both groups of bacteria (Figure 4D). These results suggest that C3, but not antibodies, is important for phagocytosis of commensal bacteria. To test whether pathobionts are less susceptible to complement-mediated phagocytosis by either inhibiting C3 activation or deposition on their surface, the bacteria were incubated in fresh murine serum as a complement source for 30 min. After incubation comparable production of C3 subfragments were detected by immunoblotting, suggesting that pathobiont and control bacterial strains elicit similar complement activation (Figure 4E, lower panel). To assess the amounts of C3 subfragments deposited on bacteria, unbound C3 was removed by extensive washing and bacterial extracts were immunoblotted with anti-C3 antibody. Notably, C3 deposition were lower in pathobionts than in phylotype-matched commensals (Figure. 4E, upper panel). These results indicate that pathobionts exhibit reduced ability to induce C3 deposition, but similar ability to induce C3 processing. Similar amounts of bacteria-bound C3 were detected when bacteria were incubated with serum from Rag1−/− and WT mice (Figure S3 C and D), indicating that C3 deposition by commensals is dependent on the lectin or alternative pathway, but not the classical Ig-mediated pathway. To further test which complement pathway is involved in C3 deposition on commensal bacteria, we tested the ability of commensals to bind MBL proteins, which are responsible for C3 deposition in the lectin pathway (Noris and Remuzzi 2013). We found that the tested commensals did not bind MBL proteins (Figure S3 E and F), suggesting that C3 deposition on intestinal bacteria is mediated by the alternative pathway.
Recombinant IL-22 improves the survival of C. difficile-infected mice by enhancing systemic clearance of intestinal pathobionts
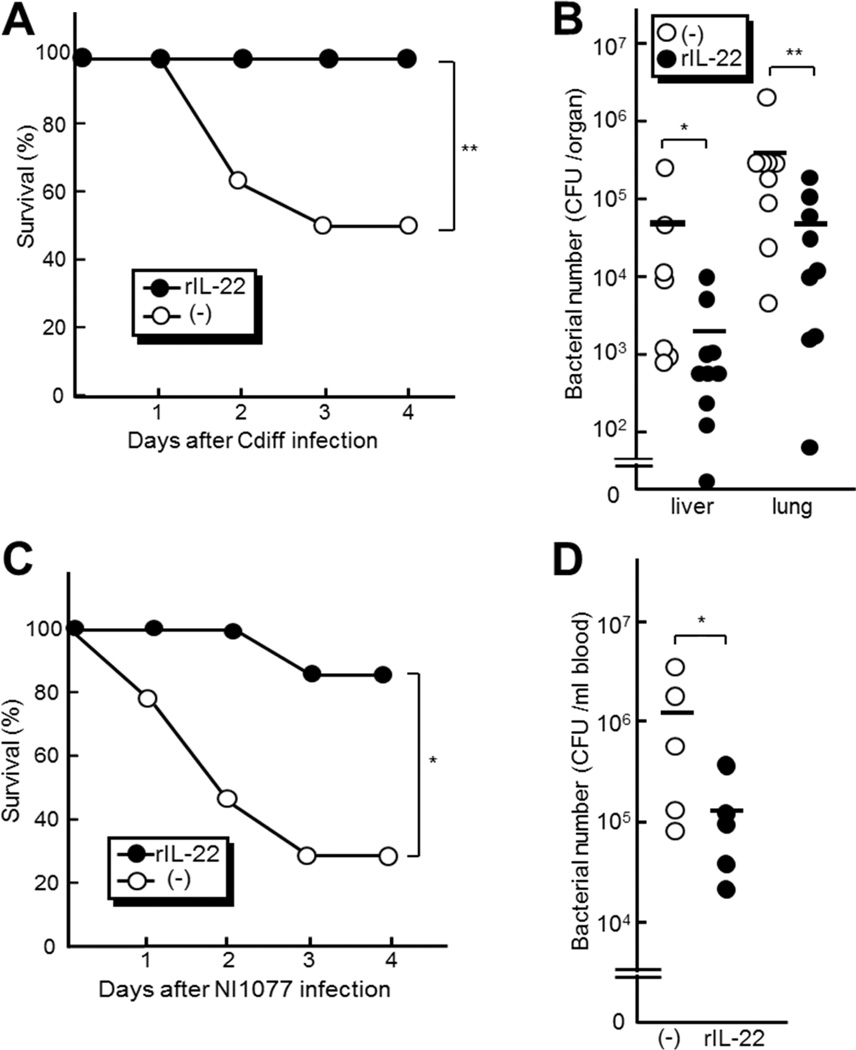
Il22−/− mice showed defective clearance of translocated pathobionts in peripheral organs after C. difficile infection (Figure 4A). We therefore determined whether systemic administration of recombinant IL-22 protects Il22−/− mice from C. difficile infection-induced mortality. Intraperitoneal administration of IL-22 rescued mice from mortality after C. difficile infection (Figure 5A), which was associated with reduced numbers of translocated bacteria in the liver and lung (Figure 5B). To further investigate the ability of IL-22 to confer systemic protection against pathobionts, we used NI1077, one of the dominant pathobionts that translocate into extraintestinal organs after C. difficile infection. We found that intravenous administration of pathobiont NI1077 induced IL-1β production, but no or minimal induction of IL-22 in the liver, lung or cecum (Figure S4 A to E). In contrast, high amounts of IL-22 were detected after C. difficile infection (Figure 1A). Thus, robust induction of IL-22 requires intestinal infection in the C. difficile infection model. To assess the role of IL-22 in protection against exposures to pathobionts, we intravenously injected NI1077 to Il22−/− and WT mice, and found comparable mortality in Il22−/− and WT mice (Figure S4F). These results suggest that IL-22 protects the host only when robustly induced after C. difficile intestinal infection. To assess the protective ability of IL-22, we pre-treated mice with recombinant IL-22 and then intravenously injected NI1077 to Pycard−/− mice that are deficient in C. difficile infection-induced production of IL-22 in the intestine (Figure S4 G and H). Pycard −/− mice were used to avoid the induction of IL-22 because Pycard−/− mice exhibit impaired production of IL-1p which is required for IL-22 production in the liver and lung after i.v. administration of NI1077 (Figure S4 A to C). Notably, administration of recombinant IL-22 improved the survival of NI1077-infected Pycard−/− mice which was associated with reduced numbers of NI1077 in the blood (Figure 5C and D). These results suggest that IL-22 protects the host by inducing systemic immune responses that promote the clearance of harmful pathobionts, resulting in improved host survival after C. difficile infection.
Figure 5. Intraperitoneal administration of recombinant IL-22 protects the host from systemic infection with pathobionts.
A and B Il22−/− mice were treated with recombinant IL-22 (1µg per mouse) or mock by i.p. injection 12 hr after infection with 108 CFU of C. difficile A, The survival of infected mice was monitored for 7 days. No additional deaths were observed beyond 4 days after infection. B, The number of total cultivable bacteria in liver and lung from Il22−/− (n=10 per group). The bacterial number in the tissues on day 3 post-infection was determined by plating on BHI media. Results are representative of three independent experiments. C and D Pycard−/− mice were pretreated with recombinant IL-22 (1µg per mouse) (n=6) or mock (n=10) by i.p. injection one day prior to i.v. infection with 2 × 108 CFU of the pathobiont NI1077. C, The survival of NI1077-infected mice was monitored for 7 days. No further deaths were observed beyond 4 days after infection. D, The bacterial number in blood on day 1 post-infection was determined by plating on BHI media (n=5 per group).*, p < 0.05; **, p < 0.01. Results are representative of three independent experiments.
IL-22 enhances elimination of commensal bacteria during C. difficile infection through the induction of complement factor C3 expression and deposition
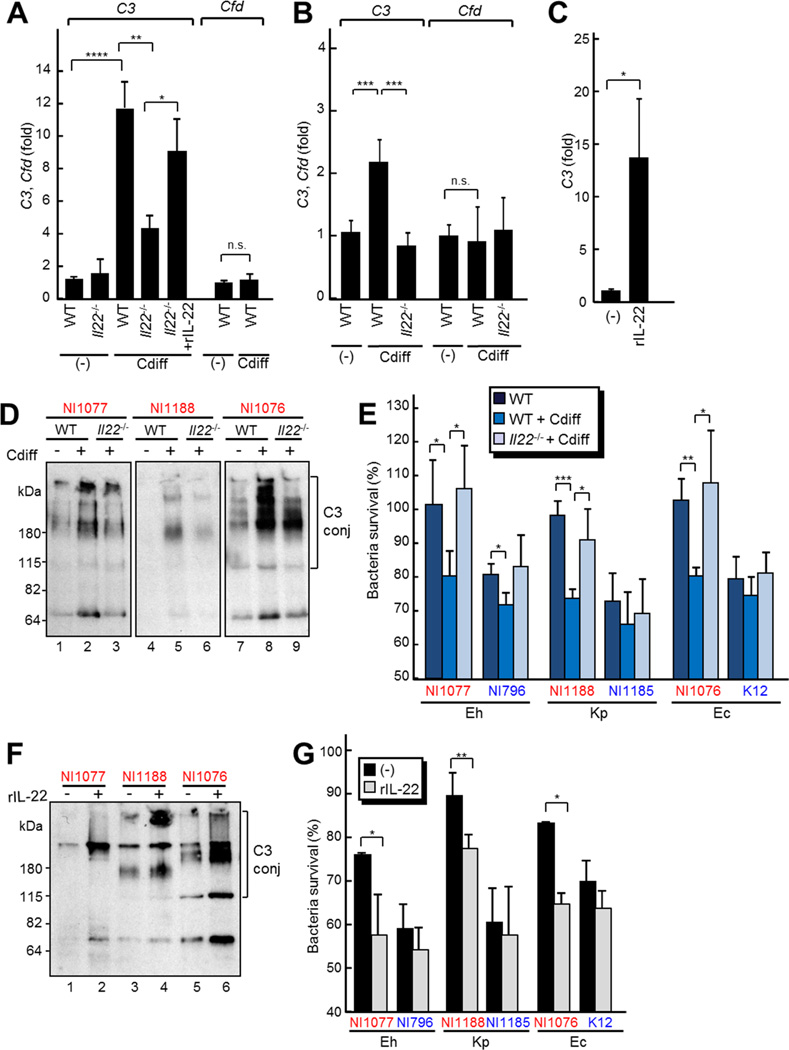
To determine the mechanisms by which IL-22 enhances the host protective response against translocated pathobionts after C. difficile infection, we first tested the expression of β-defensins, Defb4 and Defb14, whose human orthologues are induced by IL-22 stimulation in keratinocytes (Wolk et al., 2004). We found increased Defb4 mRNA expression in the cecum after C. difficile infection, but undectable Defb14 mRNA expression in WT and Il22−/− mice (Figure S5A). Neutrophils isolated from Il22−/− and WT mice showed a similar bactericidal activity (Figure S5B), indicating that IL-22 does not affect the bactericidal activity of neutrophils. To further study the immune responses elicited by IL-22, we reviewed the GEO dataset (accession GDS3226 and GSE44091) of gene expression in IL-22-treated or TcdA and TcdB toxins-treated murine intestines (Zheng et al., 2008; D‧Auria et al., 2013). In addition to genes known to be induced by IL-22, such as S100 proteins and acute phase proteins (Liang et al., 2010; Sonnenberg et al., 2012), analysis of the datasets revealed increased expression of complement factor C3 in IL-22- and TcdA/TcdB toxins-treated mice (Figure S5 C and D). Therefore, we determined if IL-22 regulates the induction of C3 expression in response to C. difficile infection. Quantitative reverse transcriptase (RT)-PCR analyses showed that the mRNA expression of C3, but not Cfd (Factor D, a component of alternative complement pathway) were increased in the liver and intestine 2 days after C. difficile infection (Figure 6A and B). We also confirmed the induction of other known IL-22 regulated genes after C. difficile infection, including those encoding S100 proteins and acute phase proteins (Figure S5E to H). Notably, the induction of C3 was significantly reduced in Il22−/− mice, compared with that observed in control WT mice (Figure 6A and B). We also found that the induction of C3 was independent on RAG1 (Figure S5I). Furthermore, injection of recombinant IL-22 induced C3 mRNA expression in WT mice (Figure 6C) and increased the expression of C3 in the liver of Il22−/− mice after C. difficile infection (Figure 6A). We additionally performed immunoblotting analysis to verify that C3 protein in serum were also increased in C. difficile-infected WT and Il22−/− mice were defective in the induction of C3 (Figure S5J). Comparison of C3 expression in organs revealed the liver as the potential major source of increased C3 after IL-22 treatment (Figure S5K). Indeed, stimulation of the human hepatocyte cell line HepG2 with IL-22 increased C3 expression, which was inhibited by incubation with the STAT3-specific inhibitor WP1066 (Figure S5L). In addition, the amounts of C3 deposition on pathobionts increased when the bacteria were incubated with sera from infected WT mice, compared with sera from uninfected WT mice (Figure 6D, compare lanes 1 and 2; 4 and 5; 7 and 8). Moreover, C3 deposition by pathobionts was reduced when the bacteria were incubated with sera from infected Il22−/− mice compared with that of infected WT mice (Figure 6D, compare lanes 2 and 3; 5 and 6; 8 and 9). Likewise, the bactericidal activity of neutrophils was enhanced by incubation with sera from C. difficile-infected WT mice, but not Il22−/− mice (Figure 6E). Furthermore, the sera from mice treated with IL-22 enhanced both C3 deposition on pathobionts (Figure 6F) and the bactericidal activity of neutrophils (Figure 6G). These results indicate that IL-22 promotes C3 expression and deposition on bacteria, thereby enhancing the ability of neutrophils to kill pathobionts.
Figure 6. IL-22 controls C. difficile infection-induced complement factor C3 expression and bacterial deposition.
A and B Il22−/− and WT mice were infected with 108 CFU of C. difficile. The expression of C3 and Cfd mRNAs in liver (A) and cecum (B) of Il22−/− (n=12) and WT mice (n=19) on day 0 (−) and day 2 post-infection were determined by quantitative RT-PCR. C, WT mice (n=5 per group) were treated with or without recombinant IL-22 (1µg per mouse) by i.p. injection. C3 expression in liver were determined by quantitative RT-PCR. D, C3 deposition on the indicated bacterial strains were detected as described in Fig. 4 after incubation with sera from Il22−/− and WT mice infected (+) or uninfected (−) with C. difficile E, Neutrophils were incubated with indicated strains in mouse sera from Il22−/− and WT mice infected or uninfected with C. difficile. The surviving bacteria were counted by plating on BHI. F and G, WT mice were treated with or without recombinant IL-22 (1ug per mouse) by i.p. injection. F, C3 deposition on the indicated bacterial strains was detected after incubation with sera from WT mice treated with recombinant IL-22. C3 conj, C3 conjugated with bacteria. G, Neutrophils were incubated with the indicated strains with mouse sera from WT mice treated with or without recombinant IL-22. The survival of bacteria was assessed by plating on BHI. Bars indicate means ± SD. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p<0.0001; n.s., not significant (p > 0.05). Results are representative of three independent experiments.
To determine the role of C3 in IL-22-mediated protective responses, we determined the effect of C3 depletion by cobra venom factor (CVF) on the ability of serum to augment the pathobiont killing by neutrophils. Immunoblotting analysis showed that C3 protein in the serum was increased after IL-22 treatment (Fig. S5M and N). Injection of CVF depleted C3 in naive and infected mice after treatment with IL-22 (Fig. S5N). Notably, C3 depletion by CVF was confirmed by the disappearance of the C3 peak in the chromatographic profile and of the C3 a chain in Coomassie Blue staining (Fig. S5 M and N, lower panel), indicating that C3 is highly abundant in the serum, consistent with published serum protein database (Nanjappa V et al., 2014). Because IL-22 potentially can induce multiple serum proteins, we separated serum proteins by UnoQ column chromatography and determined if the C3-containing fraction would specifically augment pathobiont killing by neutrophils. Immunoblotting analysis showed that C3 was separated from most of other serum proteins and detected in Fraction 3 (Fig. S5M and N). The serum from IL-22-treated mice showed increased protein amounts in Fraction 3 as well as in other fractions, whereas CVF-treated mice with and without IL-22 co-injection showed reduced protein amounts only in Fraction 3 (Figure S5M), consistent with the finding by Coomassie blue staining. Using UnoQ column fractionation, we found that the presence of C3 (fraction 3) correlated with increased pathobiont killing activity by neutrophils, which was enhanced by IL-22 and blocked by C3 depletion with CVF (Figure S5O). Therefore, C3 but not other abundant serum proteins, appears to be particularly important for pathobiont killing activity by neutrophils. To determine if C3 is important for host mortality and bacterial elimination after C. difficile infection and i.v. injection of the pathobiont NI1077, we treated mice with CVF to deplete C3 before C. difficile infection or pathobiont i.v. administration. We found that CVF-treated mice were more susceptible to both C. difficile infection (Figure S5P) and i.v. administration of the pathobiont (Figure S5R). Importantly, IL-22 -injection failed to rescue CVF-treated mice from mortality induced by i.v. administration of the pathobiont (Figure S5R). Consistently, we found increased bacterial load in the liver and lung of C. difficile-infected mice that had been pre-treated with CVF (Figure S5Q), and in the blood of pathobiont-infected mice when compared to mice that were pre-treated with CVF as well as mice without CVF treatment (Figure S5S). Collectively, these results suggest that C3 plays an important role in IL-22-mediated pathobiont elimination during C. difficile infection and protects the host from pathobiont-induced lethality.
DISCUSSION
In this study, we have demonstrated a protective role for IL-22 in host defense against C. difficile infection. Il22−/− mice displayed higher mortality after C. difficile infection than WT mice despite comparable degree of intestinal damage and pathogen burden. In Il22−/− mice after C. difficile infection, we found increased translocation of enterobacterial pathobionts to extraintestinal organs including the liver, which was associated with increased degree of liver injury. These pathobionts exhibited high pathogenicity when present in the blood, which was associated their resistance to complement-mediated phagocytosis and killing by phagocytes. Importantly, IL-22 was required for induction of C3 after C. difficile infection and IL-22 administration protected the mice from pathobiont accumulation in extraintestinal organs and pathobiont-associated mortality. Therefore, our study highlights an important role for IL-22 in limiting pathobiont-induced systemic disease and identifies a mechanism by which pathobionts can evade host immunity.
IL-22 has been shown to be critical for host defense against other pathogens (Aujla et al., 2008; Basu et al., 2012; Satoh-Takayama et al., 2008; Schulz et al., 2008). In addition, IL-22 has been implicated in the clearance of Alcaligenes species in lymphoid tissues (Sonnenberg et al., 2012) and affects the composition of the microbiota in mice infected with S. enterica Typhimurium (Behnsen et al., 2014). However, we found no significant differences between Il22−/− and WT mice in the microbiota composition before or after C. difficile infection, suggesting that IL-22 does not regulate the composition of microbiota in all infection models. In other infection models, IL-22 protects the host against pathogens via the induction of antimicrobial peptides such as RegIII proteins and enhances the repair of the intestinal epithelium, at least in part, by preventing epithelial cell death (Brandl et al., 2007; Zheng et al., 2008). Deficiency in IL-22 also results in increased translocation of intestinal bacteria into the liver after C. rodentium infection. Although IL-22 protects the host by regulating the elimination of C. rodentium (Basu et al., 2012), IL-22 did not affect the number of C. difficile in the intestine. This suggests that IL-22 can also promote host survival through mechanisms distinct from pathogen elimination. Our studies highlight the importance of systemic protection against pathobionts after translocation into peripheral organs as a result of intestinal epithelial barrier defects induced by C. difficile infection. In the C. difficile infection model, IL-22 plays a critical role in complement-mediated elimination of pathobionts that have disseminated systemically, at least in part, by upregulating C3 expression. Therefore, IL-22 protects the host not only by promoting intestinal barrier function, but also by promoting protective systemic immune responses. In the C. difficile infection model, inflammasome-mediated IL-1β production is also critical for both local (intestinal) and systemic immune responses by facilitating neutrophil recruitment and promoting elimination of commensals after C. difficile infection (Hasegawa et al. 2011; Hasegawa et al. 2012). IL-1β is also important for IL-22 production by innate lymphoid cells (Sutton et al., 2009). It is possible that the host has additional protective mechanisms against infection which may link local to systemic immune responses against pathogens and pathobionts. Moreover, C. difficile infection induced several proteins including S100 proteins and acute phase proteins in an IL-22 dependent manner. S100 proteins are known to regulate C3 expression (Schonthaler et al., 2013). Therefore, S100 may also contribute to increased C3 expression during C. difficile infection. C3 activation by bacteria results in production of C3a and the downstream factor C5a (Noris and Remuzzi 2013). C3a and C5a are known to be involved in the recruitment of IL-1β-producing inflammatory cells, inflammasome activation and IL-22 induction (Liu et al., 2011; Laudisi et al., 2013; Noris and Remuzzi 2013). Therefore, innate responses mediated by complement fragments, IL-1β and IL-22 may exert a positive feedback loop as a part of the complex host defense mechanism that confers both intestinal and systemic protection against pathobiont-induced morbidity. Complement fragments and IL-22 are also known to be involved in liver repair (Strey et al., 2003; Radaeva et al., 2004). Therefore, signaling mediated by complement, L-1β and L-22 may also play an important role during the recovery phase after C. difficile infection. Additional studies are needed to fully understand the mechanisms by which the host coordinates local and systemic immunity.
After C. difficile infection a subset of patients with pseudomembranous colitis develops sepsis and multiple organ failure, which is associated with high mortality (Eaton and Mazuski, 2013; Sunenshine et al., 2006). The elimination of commensals by antibiotics improves host survival after C. difficile infection in the mouse model, suggesting a potential therapeutic application. C. difficile possesses toxins that disrupt epithelial barrier function (Kuehne et al., 2010), which subsequently lead to translocation of commensals and immunostimulatory molecules through the damaged intestinal epithelium. Neutrophils that are recruited into the damaged intestine can eliminate many of the translocated commensals after C. difficile infection (Hasegawa et al., 2011; Hasegawa et al., 2012). However, we show here that certain pathobionts are resistant to host defense responses including phagocytosis, and eventually translocate into peripheral organs to cause organ damage. Therefore, host mechanisms to boost systemic protection against translocated pathobionts, such as those mediated through IL-22, are important for survival against C. difficile infection and could be exploited in the treatment against C. difficile infection. Our study showed γ-enterobacterial commensals as one of the dominant pathobionts that are responsible for complications of C. difficile infection in the mouse model. Importantly, individual strains within the same γ-enterobacterial species display differential sensitivity to complement-mediated phagocytosis and cause varying degrees of lethality to the host. One of the complement-resistance mechanisms exhibited by bacteria is capsular polysaccharide (CPS) on the bacterial surface that can shield bacteria from recognition by complement factors (Rautemaa and Meri, 1999). In addition, the outer structures of lipopoly(oligo)saccharides (LPS or LOS) are crucial for protecting bacteria against host immune responses, including complement activity (Roberts, 1996). The complement-resistant structural determinants of CPS and LPS are highly diverse among enterobacteria (Rautemaa and Meri, 1999). Therefore, the differential sensitivity of individual strains within the same bacterial species to complement may be ultimately dependent on their surface structures. Our study highlights the importance of complement resistance in the pathogenesis of commensal-mediated intestinal disease. Therefore, understanding the mechanisms by which pathobionts acquire complement resistance will be critical for treating systemic complications caused by gut commensal bacteria. The presence of commensals is important to suppress C. difficile expansion in the intestine, and elimination of competitive commensals by antibiotics results in overgrowth of C. difficile and C. difficile infection-related disease (Carroll et al., 2011). Currently, elimination of C. difficile with vancomycin and/or metronidazole is the first-line treatment for C. difficile-infected patients (Rupnik et al., 2009). However, some patients suffer relapsing disease that is refractory to standard antibiotic treatment. Transplantation of fecal microbiota from healthy donors has been shown to be effective for the prevention of disease recurrence (Koenigsknecht and Young, 2013; Lo Vecchio and Cohen, 2014). Because individual strains within the same species possess differential resistance against host elimination and can give rise to varying degrees of disease severity, it will be important to assess in detail the composition of the donor microbiota in fecal transplants to avoid transplanting potential pathobionts to patients already sickened by infection and dysbiosis.
EXPERIMENTAL PROCEDURES
Bacterial strains
C. difficile VPI10463 and E. coli K-12 were cultured as described (Hasegawa et al., 2012). E. hormaechei NI1077, NI1109 and NI796; K. pneumoniae NI1078, NI1188 and NI1185; E. coli NI1076; E. faecalis NI1079 and L. murinus NI1118 were isolated from the intestine of C. difficile infected or non-infected mice and cultured at 37 °C in Brain Heart Infusion (BHI) medium (BD, Franklin Lakes, NJ) under an aerobic environment, and their 16S rRNA gene phylotypes were determined as described (Hasegawa et al., 2012).
Mice
WT, C3−/− and Rag1−/− C57BL/6 (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Il22−/− mice in B6 background were a gift from Dr. Wenjun Ouyang (Genentech, South San Francisco, CA). All mice were housed, bred and maintained under specific pathogen-free (SPF) conditions as described (Hasegawa et al., 2012). Mice in different cages or derived from different sources were co-housed for two weeks to normalize the microbiota composition before experiments. The mouse studies were approved by the University of Michigan Committee on Use and Care of Animals. [Where were Pycardi−/− mice obtained?]
C. difficile infection and analyses
Eight-week old mice were infected with 108 CFU of C. difficile VPI10463 after antibiotic treatment as described (Hasegawa et. al, 2011). Post-infection analyses were performed as described in Supplemental Experimental Procedures.
Microbiota composition analysis
Bacterial DNAs were extracted from mouse intestines as described (Hasegawa et al., 2010). The V4 region of the 16S rRNA gene (252 bp) was sequenced using an Illumina MiSeq sequencer and ≈22K reads were analyzed by Mothur (Schloss et al., 2009; Kozich et al., 2013). OTUs were classified into taxonomic phylotypes at >97 % identity using Mothur (Schloss et al., 2009).
Preparation of intestinal immune cells and flow cytometric analysis
Intestinal lamina propria cells were prepared from mice 2 days post-infection, and immunostaining and flow cytometric analysis were performed as previously described (Hasegawa et al., 2011). Briefly, isolated lamina propria cells were stained with FITC-labeled CD11b monoclonal antibody (mAb) (M1/70), APC-labeled mAb F4/80 (BM8), and PE-labeled Ly-6G mAb (1A8). Isotype-matched antibodies (BD) were used as controls. Dead cells were excluded with 7-AAD staining. Neutrophils were defined as CD11b+Ly-6G+ cells, and macrophages were defined as CD11b+F4/80+ cells.
Systemic infection assay
Mice were injected with 2 × 108 CFU of isolated commensals intravenously. Bacterial number in the blood on day 3 was determined by serial plating of diluted blood and colonies counted on BHI plates after 24hr-incubation under aerobic conditions.
Phagocytosis and bacterial killing assay
Bone marrow-derived macrophages were cultured with isolated bacteria (MOI 1:1) for 20 minutes with or without 5 % fresh mouse serum and then treated with 50 µg/ml gentamycin for 1hr after five times wash with phosphate-based saline (PBS). The cells were lysed with 0.1 % Nonidet P-40 in PBS and plated on BHI. Under these conditions, 91 ± 3 % of bacteria survived in macrophages for two hours and 97.2 ± 0.7 % of bacteria were killed within 24 hr. For bacterial killing assay, mouse peritoneal neutrophils were collected from the intraperitoneal cavity 4 hr after thioglycollate medium i.p. injection. 5 × 105 neutrophils were incubated with 5 × 103 bacteria with 2.5 % (v/v) fresh mouse serum for 2 hr. Bacterial survival was assessed by plating on BHI.
Western blotting for C3 deposition
5 × 108 bacteria were incubated in 50 % (v/v) mouse serum for 30 min at 37 °C. Unbound C3 was removed from bacterial pellets by washing with ice-cold PBS. Samples and control 1/200 total reaction mixtures were boiled in Laemmli’s buffer, separated by 10 % SDS-PAGE (1 × 108 bacteria per lane). For C3 deposition, C3 protein was examined by immunoblotting using anti-mouse C3d antibody (R&D). For binding assay of MBL proteins, MBLs were examined by immunoblotting using anti-mouse MBL1 and MBL2 antibodies (gifts from Steffen Thiel, Aarhus University, Aarhus, Denmark). For equal loading, aliquots from each sample were plated and the same number of CFUs were lysed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software version 6.0 (GraphPad Software, La Jolla, CA). Differences between two groups were evaluated using unpaired two-tailed Student’s t-test. False discovery rate q-values were calculated by Mothur (Schloss et al., 2009) for comparison of OTU abundances. For multiple group comparisons, statistical analysis was performed using one-way ANOVA and then the Dunnett’s or Sidak’s test as a posthoc test. The survival rate of infected mice was analyzed using the log-rank test. Differences at p<0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Wenjun Ouyang (Genentech) for Il22−/− mice, Steffen Thiel (Aarhus University) for anti-MBL antibodies, Mohamed ElSayed (University of Michigan) for HepG2 and Melody Zeng, Jessica Werner and Grace Chen (University of Michigan) for careful review and stimulating discussion of the manuscript. The work was supported by National Institutes of Health grants R01 DE018503 (to N Inohara), R01 DK091191 (to G Nuñez) and the Crohn’s and Colitis Foundation of America and Michigan Gastrointestinal Peptide Research Center NIDDK 5P30DK034933 (to N Kamada).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicting financial interests.
REFERENCES
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M. The Cytokine IL-22 Promotes Pathogen Colonization by Suppressing Related Commensal Bacteria. Immunity. 2014;40:262–273. doi: 10.1016/j.immuni.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: Implications for Epidemiology and Diagnosis. Annu. Rev. Microbiol. 2011;65:501–521. doi: 10.1146/annurev-micro-090110-102824. [DOI] [PubMed] [Google Scholar]
- Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria KM, Kolling GL, Donato GM, Warren CA, Gray MC, Hewlett EL, Papin JA. In vivo physiological and transcriptional profiling reveals host responses to Clostridium difficile toxin A and toxin B. Infect Immun. 2013;81:3814–3824. doi: 10.1128/IAI.00869-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SR, Mazuski JE. Overview of Severe Clostridium difficile Infection. Critical Care Clinics. 2013;29:827–839. doi: 10.1016/j.ccc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Osaka T, Tawaratsumida K, Yamazaki T, Tada H, Chen GY, Tsuneda S, Núñez G, Inohara N. Transitions in oral and intestinal microflora composition and innate immune receptor-dependent stimulation during mouse development. Infect. Immun. 2010;78:639–650. doi: 10.1128/IAI.01043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Núñez G, Inohara N. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol. 2011;186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kamada N, Jiao Y, Liu MZ, Núñez G, Inohara N. Protective Role of Commensals against Clostridium difficile Infection via an IL-1β-Mediated Positive-Feedback Loop. J Immunol. 2012;189:3085–3091. doi: 10.4049/jimmunol.1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht MJ, Young VB. Faecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection: current promise and future needs. Curr Opin Gastroenterol. 2013;29:628–632. doi: 10.1097/MOG.0b013e328365d326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- Laudisi F, Spreafico R, Evrard M, Hughes TR, Mandriani B, Kandasamy M, Morgan BP, Sivasankar B, Mortellaro A. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1β release. J. Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J. Transl. Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, AFouser L. IL-22 induces an acute-phase response. J. Immunol. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- Lo Vecchio A, Cohen MB. Fecal microbiota transplantation for Clostridium difficile infection: benefits and barriers. Curr Opin Gastroenterol. 2014;30:47–53. doi: 10.1097/MOG.0000000000000023. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Review Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Nanjappa V, Thomas JK, Marimuthu A, Muthusamy B, Radhakrishnan A, Sharma R, Ahmad Khan A, Balakrishnan L, Sahasrabuddhe NA, Kumar S, et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2014;42:D959–D965. doi: 10.1093/nar/gkt1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Remuzzi G. Overview of complement activation and regulation. Semin. Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Lawley TD. Emerging insights on intestinal dysbiosis during bacterial infections. Curr Opin Microbiol. 2014;17C:67–74. doi: 10.1016/j.mib.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- Rautemaa R, Meri S. Complement-resistance mechanisms of bacteria. Microbes Infect. 1999;10:785–794. doi: 10.1016/s1286-4579(99)80081-1. [DOI] [PubMed] [Google Scholar]
- Roberts IS. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J. Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Applied and Environmental Microbiology. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SM, Köhler G, Schütze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73:187–197. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.