Abstract
Amyloidosis is a collection of diseases in which different proteins are deposited as insoluble beta-pleated sheets, disrupting organ function. Distribution of these deposits may be diffuse or localized throughout the body, depending on the pathophysiology of the underlying amyloid type. Isolated deposition of amyloid proteins in lung is a very rare entity. They are frequently misdiagnosed as bronchogenic carcinoma, metastatic disease, or focal fungal infections. The treatment of solitary pulmonary amyloidosis is not well-defined. We have treated a 65-year-old female patient with external beam radiation and corticosteroids in palliative intent and she is leading a good quality of life after six months of follow up.
KEY WORDS: Amyloidoma, external beam radiotherapy, lung, radiation
INTRODUCTION
Amyloidosis is a heterogenous group of disease characterized by deposition of misfolded insoluble proteins in extracellular space. Amyloidosis can be either primary or secondary, and deposition of those amyloid proteins can be systemic or localized. Localized amyloid deposition in respiratory tract or lung parenchyma is a rare entity.
CASE REPORT
A 65-year-old female presented with fever for eight months, which was insidious in onset, high-grade intermittent in nature. She also had a long-standing history of intermittent cough and shortness of breath. She smoked 4-6 bidis per day for last 40 years. The physical examination was unremarkable. Her routine blood investigations were within normal limits. CECT (Contrast-enhanced computed tomography) chest revealed an ill-defined 6 × 4 × 7 cm heterogeneously enhancing mass causing abrupt cut off the left lower lobe bronchus with the consequent distal collapse/consolidation of the left lower lobe. The left pulmonary artery is variably encased and attenuated by the lesion. The left inferior pulmonary vein is displaced anterioly by the lesion. Multiple enlarged conglomerate mediastinal lymph nodes with left-sided pleural effusion were also seen [Figure 1]. Bronchoscopy revealed submucosal infiltration in left and right bronchial tree with nodules at secondary carina on left side and anterior segment of right upper lobe. Left lower lobe segment could not be visualized due to stenosis. Endobronchial biopsy revealed multiple fragments lined by respiratory epithelium with focal mucinous metaplasia. The subepithelium showed nodular deposits of eosinophilic, homogenous material with intervening scanty lymphomononuclear infiltrate. These nodular deposits were congophilic and showed apple green birefringence on polarized light. No granuloma or any evidence of malignancy was seen. Features were of amyloidoma [Figures 2 and 3]. FNAC (Fine Needle Aspiration Cytology) from abdominal fat pad came out to be negative. Rest of the systemic amyloidosis work up was negative. Patient was not taken up for resection because of the vascular encasement of the lesion. She was treated with corticosteroids simultaneously along with palliative external beam radiation. Thirty Gy was delivered in ten fractions over two weeks period with photon energy of 6 MV with two fields (AP-PA portals). The steroids were tapered and stopped after one week. After six months of follow up, patient is leading a good quality of life with clinical and radiological complete response.
Figure 1.
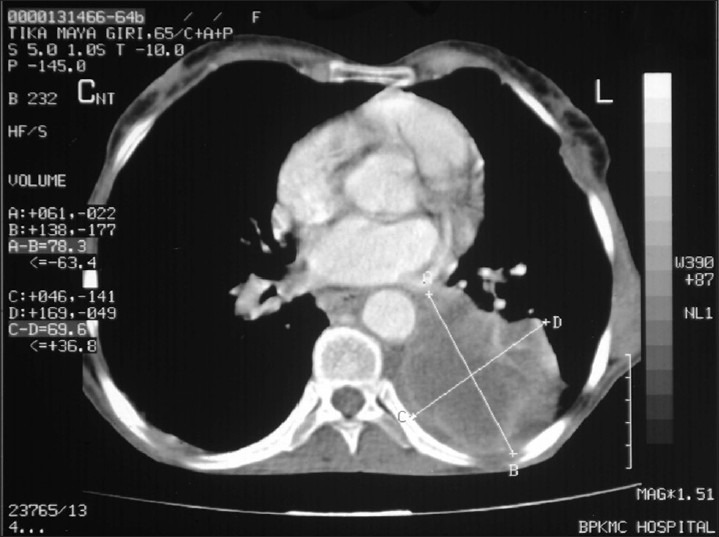
Contrast-enhanced computed tomography chest revealed an ill-defined 6 × 4 × 7 cm heterogeneously enhancing mass causing abrupt cut off the left lower lobe bronchus with the consequent distal collapse/consolidation of the left lower lobe
Figure 2.
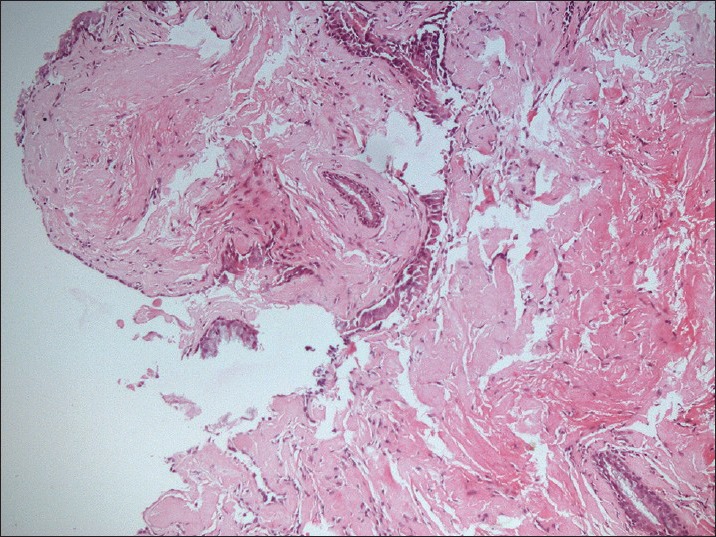
Endobronchial biopsy revealed multiple fragments lined by respiratory epithelium with focal mucinous metaplasia. The subepithelium showed nodular deposits of eosinophilic, homogenous material with intervening scanty lymphomononuclear infiltrate. These nodular deposits were congophilic
Figure 3.
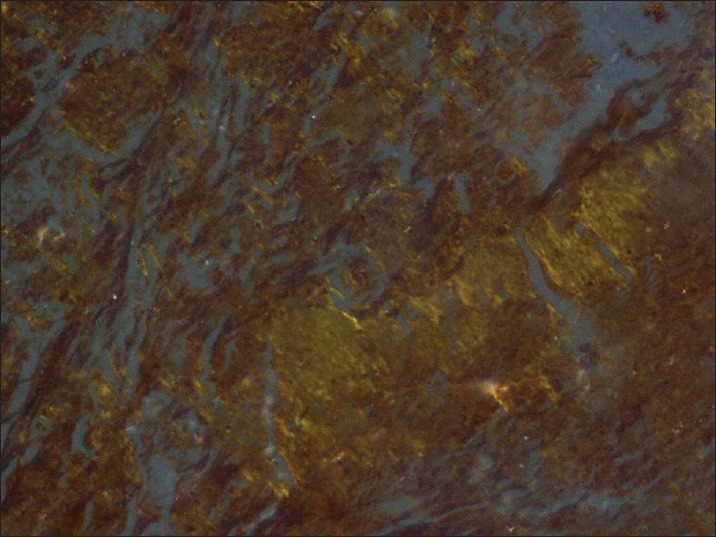
Nodular deposits of eosinophilic, homogenous material with intervening scanty lymphomononuclear infiltrates showing apple green birefringence
DISCUSSION
Amyloidosis is a disease characterized by deposition of abnormal proteins in extracellular tissue. The term amyloid was first used by Rudolf Virchow in 1854.[1] Amyloidosis localized to respiratory tract was first recognized by Lesser in 1877.[2] The locations of the isolated forms of pulmonary amyloidosis are divided into three main anatomical areas; large airway amyloidosis of the larynx and bronchus, parenchymal pulmonary amyloidosis, and mediastinal amyloid.[3] Tracheobronchial amyloidosis is the most common form of localized primary pulmonary amyloidosis.[4] Patients with tracheobronchial amyloidosis present with obstructive manifestations such as cough, dyspnea, and hemoptysis. The second pattern of pulmonary involvement is nodular parenchymal amyloidosis, which is characterized by circumscribed nodule with occasional interstitial and perivascular extension. Clinically, those lesions resemble a neoplasm.[5] The amyloidoma of the lung, when solitary, may be confused with primary bronchogenic carcinoma. When the lesions are multiple, it may be difficult to differentiate these from metastatic cancer of the lung. The slow progression of the lesions, the presence of calcification, and the absence of a primary tumor would suggest the non-malignant nature of the lesion; however, there are no reliable clinical or roentgenographic criteria that can help in making diagnosis of amyloidoma. Diffuse amyloid deposition in the lung parenchyma is usually associated with systemic AL amyloidosis. It is characterized by widespread amyloid deposition involving small vessels and the interstitium.[6]
Solitary pulmonary nodular amyloidosis is an uncommon diagnosis. More common conditions that present similarly should be excluded first, such as neoplastic, infectious, or inflammatory conditions. In an event when clinical findings, radiographic appearances, and pathological conclusions are incongruent with these common diagnoses, amyloid of the lung should be considered.[7]
Due to the variability of amyloid involvement in the pulmonary system, the treatment for respiratory amyloidosis ranges from observation to bronchoscopic or surgical resection based on severity and symptomatology. Management decisions are mostly based on an individual basis. Systemic amyloidosis can be treated with chemotherapy while localized forms are typically treated with local interventions. Infiltrative and systemic disease has been amenable to chemotherapy with oral melphalan and prednisolone.[8] A prospective study done at Mayo Clinic in patients with systemic amyloidosis showed that combination therapy with melphalan, prednisone, and colchicine or melphalan and prednisone resulted in prolonged survival compared to colchicine alone. The median duration of survival after randomization was 8.5 months in the colchicine group, 18 months in the group assigned to melphalan and prednisone, and 17 months in the group assigned to melphalan, prednisolone, and colchicine.[9] Excisional therapy is the standard approach to managing TBA (Tracheobronchial Amyloidosis). In some nodular forms of TBA, neodymium: Yttrium-aluminum-garnet (Nd: YAG) laser treatment removes tissue and eliminates further amyloid deposition in the field, perhaps by laser-induced thermal injury to underlying plasma cells.[10,11]
External beam radiotherapy has been successfully utilized in tracheobronchial amyloidosis in a patient believed not to be a candidate for endoluminal therapy due to the diffuse nature of the airways disease.[12] A total of 24 Gy was delivered in 12 fractions, and colchicine was given as an adjunctive therapy. Improvements were measured by sequential pulmonary function testing, radiographic imaging, bronchoscopic evaluation, and performance status. Kurrus et al. published a case report demonstrating a benefit of external beam radiation (20 Gy in 10 fractions over two weeks) in causing local response in a patient with localized tracheobronchial disease. They treated the patient based on the hypothesis that plasma cells that secrete amyloidogenic protein are radiosensitive.[13] Other hypotheses proffered include radiation injures cells other than plasma cells that may secrete amyloidogenic proteins, and free radicals generated by radiation may modify and enhance the degradation of amyloid protein deposits. Combined endobronchial and radiation therapy have demonstrated a similar beneficial effect.[14]
The index patient has also responded very well both clinically and radiologically with radiotherapy. Hence, radiotherapy can be a viable option for the treatment of solitary pulmonary amyloidoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Saleiro S, Hespanhol V, Magalhães A. Endobronchial amyloidosis. J Bronchol. 2008;15:95–9. [Google Scholar]
- 2.Gillmore JD, Hawkins PN. Amyloidosis and the respiratory tract. Thorax. 1999;54:444–51. doi: 10.1136/thx.54.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachmann HJ, Hawkins PN. Amyloidosis and the lung. Chron Respir Dis. 2006;3:203–14. doi: 10.1177/1479972306070066. [DOI] [PubMed] [Google Scholar]
- 4.Sugihara E, Dambara T, Okamoto M, Sonobe S, Koga H, Inui A, et al. Clinical features of 10 patients with pulmonary amyloidosis. J Bronchol. 2006;13:191–3. [Google Scholar]
- 5.Lee SC, Johnson H. Multiple nodular pulmonary amyloidosis. A case report and comparison with diffuse alveolar-septal pulmonary amyloidosis. Thorax. 1975;30:178–85. doi: 10.1136/thx.30.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai RA, Mahajan VK, Benjamin S, Van Ordstrand HS, Cordasco EM. Pulmonary amyloidoma and hilar adenopathy. Rare manifestations of primary amyloidosis. Chest. 1979;76:170–3. doi: 10.1378/chest.76.2.170. [DOI] [PubMed] [Google Scholar]
- 7.Pollock C, Gray R, Medellin A. Isolated pulmonary nodular amyloidosis: A case report of a rare presentation of amyloidosis in the lung confused with bronchogenic carcinoma. UBCMJ. 2012;3:34–7. [Google Scholar]
- 8.Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. A trial of three regimens for primary amyloidosis: Colchicine alone, melphalan and prednisone, and melphalan, prednisone and colchicine. N Engl J Med. 1997;336:1202–7. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 9.Wechalekar AD, Hawkins PN, Gillmore JD. Perspectives in treatment of AL amyloidosis. Br J Haematol. 2008;140:365–77. doi: 10.1111/j.1365-2141.2007.06936.x. [DOI] [PubMed] [Google Scholar]
- 10.Rubinow A, Celli BR, Cohen AS, Rigden BG, Brody JS. Localized amyloidosis of the lower respiratory tract. Am Rev Resp Dis. 1978;118:603–11. doi: 10.1164/arrd.1978.118.3.603. [DOI] [PubMed] [Google Scholar]
- 11.Ayuso MC, Gilabert R, Bombi JA, Salvador A. CT appearance of localized pulmonary amyloidosis. J Comput Assist Tomogr. 1987;11:197–9. doi: 10.1097/00004728-198701000-00050. [DOI] [PubMed] [Google Scholar]
- 12.Monroe AT, Walia R, Zlotecki RA, Jantz MA. Tracheobronchial amyloidosis: A case report of successful treatment with external bean radiation therapy. Chest. 2004;125:784–9. doi: 10.1378/chest.125.2.784. [DOI] [PubMed] [Google Scholar]
- 13.Kurrus JA, Hayes JK, Hoidal JR, Menendez MM, Elstad MR. Radiation therapy for tracheobronchial amyloidosis. Chest. 1998;114:1489–92. doi: 10.1378/chest.114.5.1489. [DOI] [PubMed] [Google Scholar]
- 14.Kalra S, Utz JP, Edell ES, Foote RL. External-beam radiation therapy in the treatment of diffuse tracheobronchial amyloidosis. Mayo Clin Proc. 2001;76:853–6. doi: 10.1016/S0025-6196(11)63233-3. [DOI] [PubMed] [Google Scholar]


