Abstract
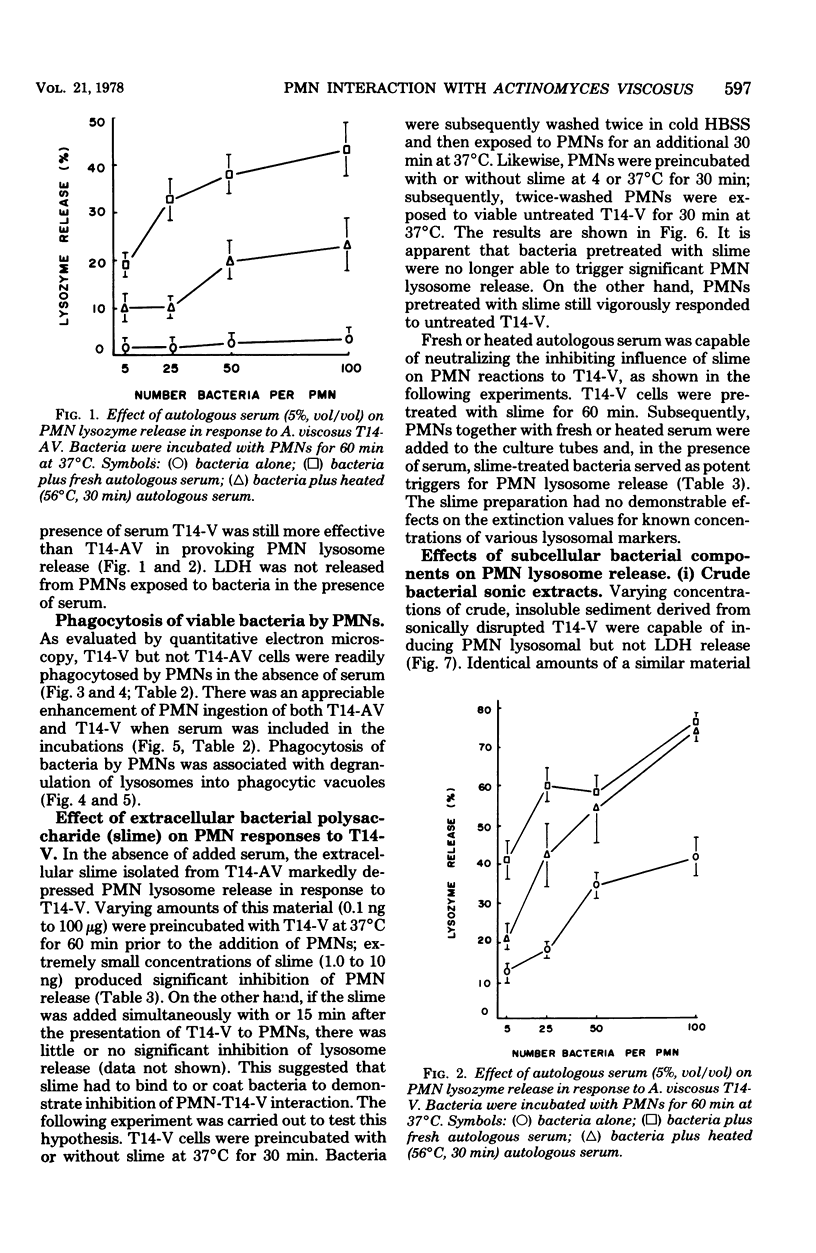
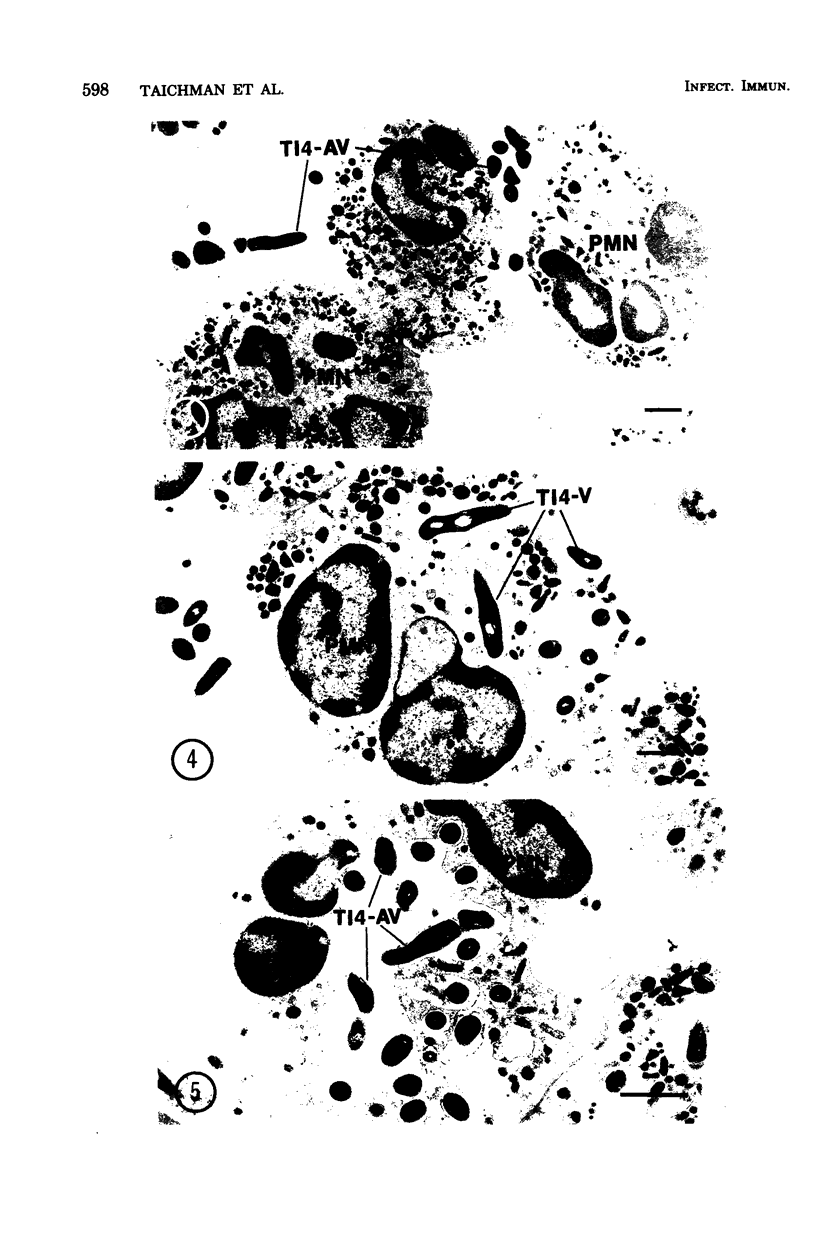
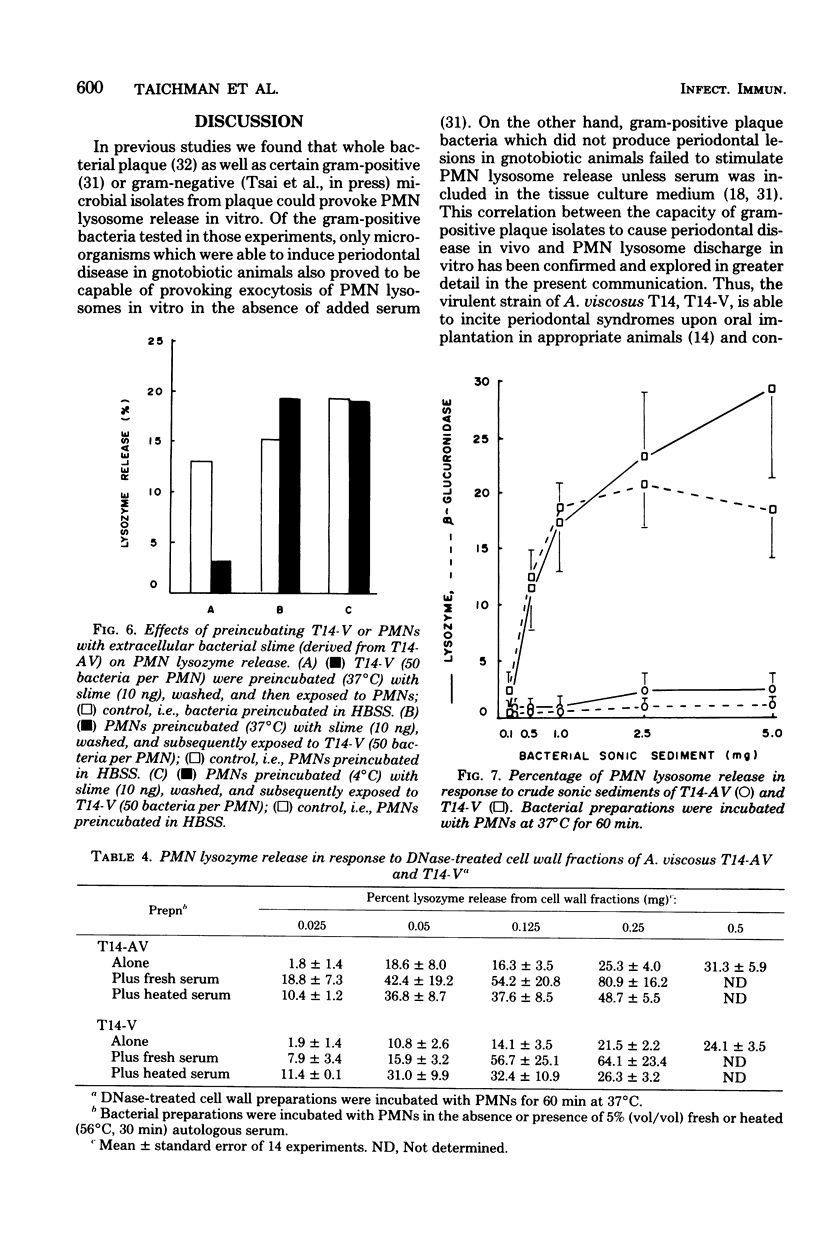
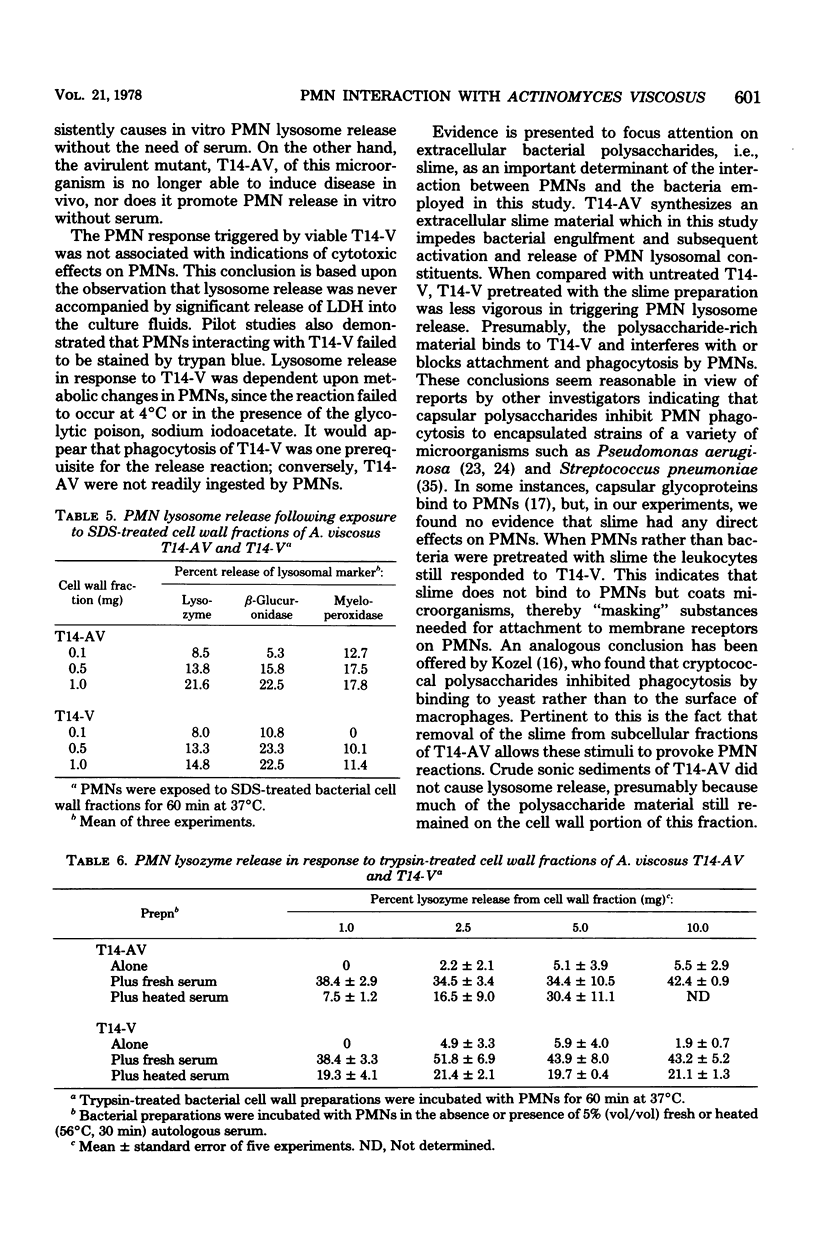
Both virulent (V) and avirulent (AV) strains of Actinomyces viscosus T14 are capable of colonizing the oral cavity of gnotobiotic rats, but only T14-V causes destructive periodontal disease. The basis for this difference in in vivo pathogenicity has not been adequately defined. In the present study we compared the capacities of T14-AV and T14-V to provoke in vitro extracellular release of lysosomal constituents from human polymorphonuclear leukocytes (PMNs). In serum-free cultures, viable T14-V but not T14-AV stimulated discharge of PMN lysosomes. The release response was correlated with PMN phagocytic activity; thus, PMNs readily ingested T14-V but not T14-AV. To explain these differences in PMN-bacteria interactions, subcellular fractions of T14-AV or T14-V were incubated with PMNs. A crude, insoluble sonic extract derived from T14-V caused PMN lysosome release, but a similar fraction from T14-AV was inactive. However, following extensive washing and treatment with deoxyribonuclease or sodium dodecyl sulfate, cell wall fractions of T14-AV stimulated lysosome release. These procedures apparently removed an extracellular polysaccharide slime which is synthesized by T14-AV but not by T14-V. There was a significant reduction in the capacities of viable T14-V or cell wall fractions of T14-V or T14-AV to provoke PMN lysosome release when these agents were preincubated with a slime material isolated from T14-AV. This inhibitory influence of slime was overcome by the addition of fresh or heated (56°C, 30 min) serum to the PMN-bacteria cultures. The data suggest a relationship between the abilities of the avirulent and virulent strains of A. viscosus T14 to act as periodontal pathogens in vivo and to serve as stimuli for PMN lysosome release in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. Studies in immunochemistry. 11. The action of dilute alkali on the N-acetylhexosamines and the specific blood-group mucoids. Biochem J. 1952 Jun;51(3):379–389. doi: 10.1042/bj0510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attström R. Studies on neutrophil polymorphonuclear leukocytes at the dento-gingival junction in gingival health and disease. J Periodontal Res Suppl. 1971;8:1–15. [PubMed] [Google Scholar]
- Attström R. The roles of gingival epithelium and phagocytosing leukocytes in gingival defence. J Clin Periodontol. 1975 Feb;2(1):25–32. doi: 10.1111/j.1600-051x.1975.tb01723.x. [DOI] [PubMed] [Google Scholar]
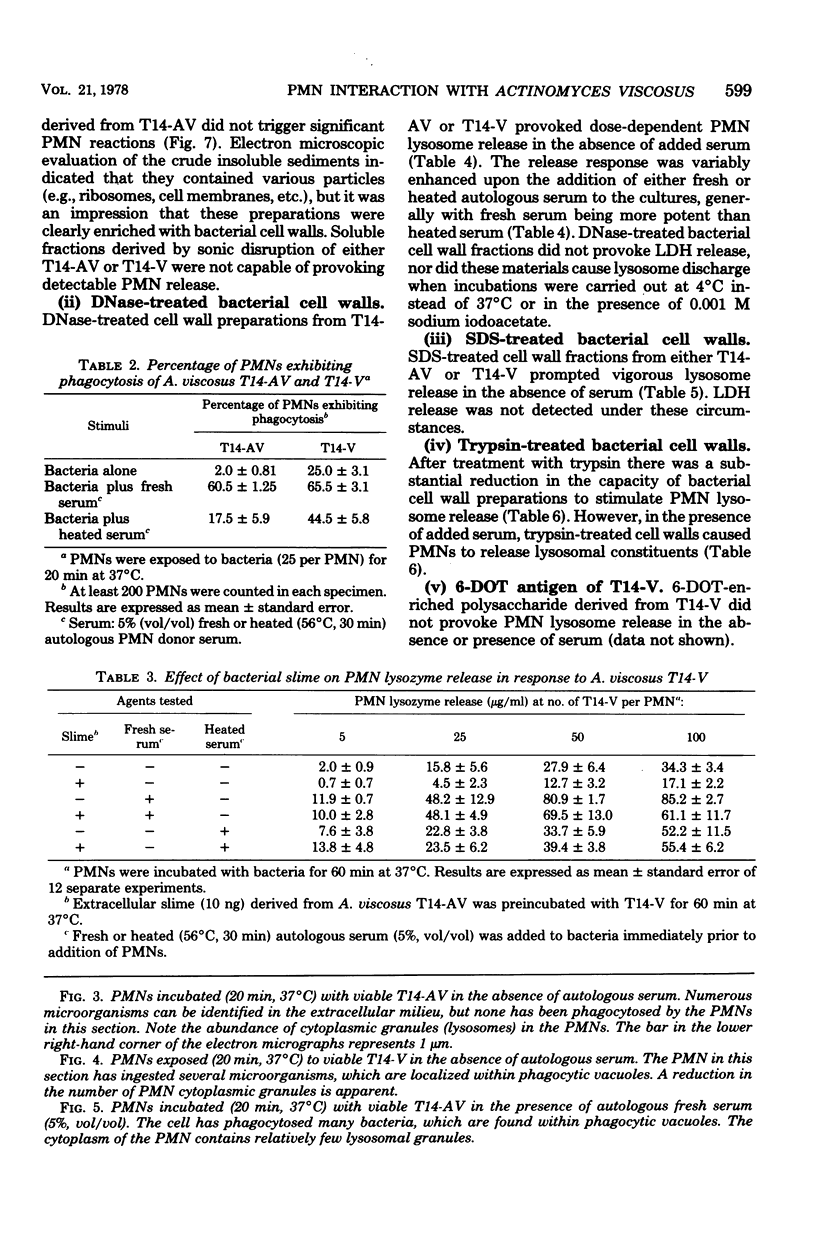
- Baehni P., Listgarten M. A., Taichman N. S., McArthur W. P. Electron microscopic study of the interaction of oral microorganisms with polymorphonuclear leukocytes. Arch Oral Biol. 1977;22(12):685–692. doi: 10.1016/0003-9969(77)90098-x. [DOI] [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D., Epps D. V., Clagett J. In vivo and in vitro studies on possible pathogenic mechanisms of Actinomyces viscosus. Infect Immun. 1976 Aug;14(2):548–554. doi: 10.1128/iai.14.2.548-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D., Schroeder H. E., Page R. C. Morphological features and functional properties of human fibroblasts exposed to Actinomyces viscosus substances. Infect Immun. 1978 Jan;19(1):287–295. doi: 10.1128/iai.19.1.287-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant P. R. Plaque-neutrophil interaction in monoinfected rats as visualized by transmission electron microscopy. J Periodontol. 1976 Mar;47(3):132–138. doi: 10.1902/jop.1976.47.3.132. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Mashimo P. A., Krygier G., Ellison S. A. Antibody-mediated effects on the periodontium. J Periodontol. 1974 May;45(5):330–337. doi: 10.1902/jop.1974.45.5.330. [DOI] [PubMed] [Google Scholar]
- Ginsburg I., Sela M. N. The role of leukocytes and their hydrolases in the persistence, degradation, and transport of bacterial constituents in tissues: relation to chronic inflammatory processes in staphylococcal, streptococcal, and mycobacterial infections and in chronic periodontal disease. CRC Crit Rev Microbiol. 1976 Mar;4(3):249–322. doi: 10.3109/10408417609106944. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn M., Sensakovic J. W., Bartell P. F. In vivo distribution of Pseudomonas aeruginosa slime glycolipoprotein: association with leukocytes. Infect Immun. 1977 Jan;15(1):109–114. doi: 10.1128/iai.15.1.109-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur W. P., Baehni P., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. III. Modulation of rabbit polymorphonuclear leukocyte hydrolase release response to Actinomyces viscosus and Streptococcus mutans by immunoglobulins and complement. Infect Immun. 1976 Dec;14(6):1315–1321. doi: 10.1128/iai.14.6.1315-1321.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Pathogenesis of the chronic inflammatory lesion induced by group A streptococcal cell walls. Lab Invest. 1974 May;30(5):568–581. [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- Rosan B., Hammond B. F. Extracellular polysaccharides of Actinomyces viscosus. Infect Immun. 1974 Aug;10(2):304–308. doi: 10.1128/iai.10.2.304-308.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Biological activity of fragments derived from the extracellular slime glycolipoprotein of Pseudomonas aeruginosa. Infect Immun. 1975 Oct;12(4):808–812. doi: 10.1128/iai.12.4.808-812.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillitoe E. J., Lehner T. Immunoglobulins and complement in crevicular fluid, serum and saliva in man. Arch Oral Biol. 1972 Feb;17(2):241–247. doi: 10.1016/0003-9969(72)90206-3. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Freedman H. L., Uriuhara T. Inflammation and tissue injury. I. The response to intradermal injections of human dentogingival plaque in normal and leukopenic rabbits. Arch Oral Biol. 1966 Dec;11(12):1385–1392. doi: 10.1016/0003-9969(66)90028-8. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., McArthur W. P. Interaction of inflammatory cells and oral bacteria: release of lysosomal hydrolases from rabbit polymorphonuclear leukocytes exposed to gram-positive plaque bacteria. Arch Oral Biol. 1976;21(4):257–263. doi: 10.1016/0003-9969(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Tsai C. C., Baehni P. C., Stoller N., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun. 1977 Jun;16(3):1013–1023. doi: 10.1128/iai.16.3.1013-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Nilsson U. R., McArthur W. P., Taichman N. S. Activation of the complement system by some gram-positive oral bacteria. Arch Oral Biol. 1977;22(5):309–312. doi: 10.1016/0003-9969(77)90027-9. [DOI] [PubMed] [Google Scholar]
- Wallinder I. B., Neujahr H. Y. CELL WALL AND PEPTIDOGLYCAN FROM Lactobacillus fermenti. J Bacteriol. 1971 Mar;105(3):918–926. doi: 10.1128/jb.105.3.918-926.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]