Abstract
Introduction:
Additional cover after neourethra formation to decrease the fistula rate, has been described using the dartos, tunica, denuded skin and corpus spongiosum. The use of corpus spongiosum alone to cover the neourethra is infrequent. The objective of this study was to evaluate the efficacy of spongioplasty alone as an intervening layer in the prevention of urethral fistula following tubularized incised plate urethroplasty (TIPU).
Materials and Methods:
A prospective study was performed including 113 primary hypospadias cases undergoing TIPU with spongioplasty from June 2010 to March 2012. Correction of chordee was carried out by penile degloving alone in 5, mobilization of urethral plate with spongiosum in 22 and combination of both in 45 cases. Intra-operatively, spongiosum was taken to be poorly developed if it was thin and fibrous, moderate if good spongiosal tissue with good vascularization and well-developed if healthy robust spongiosum, which became bulkier than native spongiosum after tubularisation. Spongioplasty was done in a single layer after mobilization of spongiosum, starting just proximal to the native meatus and into the glans distally.
Results:
The mean age of the patients was 11.53 years. The type of hypospadias was distal, mid and proximal in 81, 12 and 20 cases respectively. Spongiosum was poorly developed in 13, moderate in 53 and well-developed in 47 cases. The mean hospital stay was 8-10 days and follow-up ranged from 6 months to 2 years. Urethral fistula was seen in six patients (11.3%) with moderate spongiosum (distal 1, mid 1 and proximal 4), and three (23.03%) with poorly developed spongiosum (one each in distal, mid and proximal) with an overall 7.96% fistula rate. None of the patients with well-developed spongiosum developed a fistula. Poorer spongiosum correlated with a greater number of complications (P = 0.011). Five out of thirteen cases with poor spongiosum (38.46%) had proximal hypospadias, i.e. more proximal was the hypospadias, poorer was the development of the spongiosum (P = 0.05). Meatal stenosis was seen in two patients (1.76%) with proximal hypospadias, one with moderate and the other with poorly developed spongiosum. More proximal was the hypospadias, greater were the number of complications (P = 0.0019).
Conclusion:
TIPU with spongioplasty reconstructs a near normal urethra with low complications. Better developed and thicker spongiosum results in lower incidence of fistula and meatal stenosis. More proximal hypospadias is associated with poorer spongiosum. We recommend spongioplasty to be incorporated as an essential step in all patients undergoing tubularized incised-plate repair for hypospadias.
Keywords: Additional tissue cover, complications, fistula, hypospadias, spongioplasty, urethroplasty
INTRODUCTION
The tubularized incised plate urethroplasty (TIPU) repair is the procedure of choice for distal and mid-shaft hypospadias and is increasingly being used for proximal hypospadias and redo repairs.[1,2,3,4] It has excellent functional and cosmetic outcomes with minimal complications. Post-operatively, urethrocutaneous fistula is the most common complication.[5,6,7] To decrease fistula rate, an additional tissue cover after neourethra reconstruction has been described using transverse island dorsal subcutaneous flap,[8] de-epithelialized skin flaps,[9,10] lateral dartos flap,[11] double dartos flaps,[12,13] double breasted de-epithelialized penile skin flap[14], tunica vaginalis[15,16], and corpus spongiosum.[5,17,18] The use of corpus spongiosum as an intervening layer over the neourethra is less frequent. We evaluated the efficacy of spongioplasty alone after mobilization of corpus spongiosum in prevention of urethral fistula in patients undergoing TIP repair for hypospadias.
MATERIALS AND METHODS
A prospective clinical study was carried out at our institution between June 2010 and March 2012. We included 113 cases of hypospadias undergoing TIPU repair with spongioplasty. All patients presenting with primary hypospadias were included. Re-operative repairs, intersex state and ambiguous genitalia were excluded. The parents (for minors) and patients were informed about the merits and complications of the procedure and a written informed consent was obtained. Approval was sought from the hospital ethical committee. All patients were operated upon by a single surgeon(ALB). Intra-operatively, we divided the corpus spongiosum into three categories depending on the appearance and vascularity.
Poorly developed-Thin spongiosal tissue with decreased vascularity; the diameter of the neourethra covered by spongiosum after spongioplasty was less than the proximal healthy urethra [Figure 1].
Figure 1.
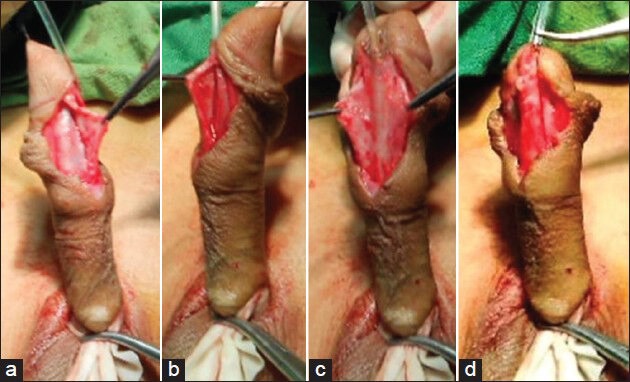
Poorly developed corpus spongiosum (a) Mobilized spongiosum on left side with minimal spongiosum tissue (b) Mobilized spongiosum on right with minimal spongiosum tissue (c) Hypoplastic urethra with mobilized spongiosum on both sides with minimal spongiosum tissue (d) after completion of spongioplasty, proximal normal urethra bulkier than distal neourethra with spongioplasty
Moderately developed-Average thickness and vascularity of spongiosal tissue; the diameter of the neourethra covered by spongiosum after spongioplasty was almost equal to that of the proximal healthy urethra [Figure 2].
Figure 2.
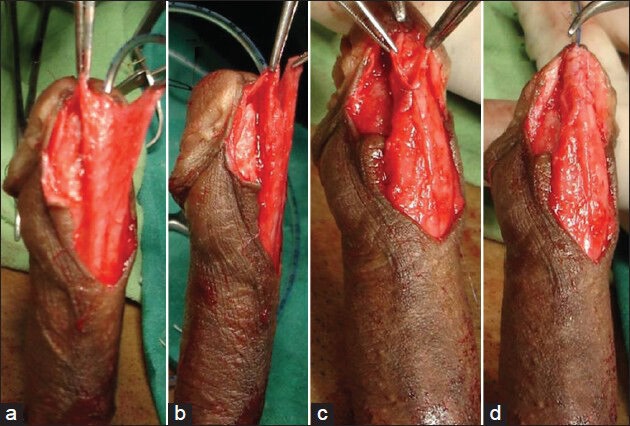
Moderately developed corpus spongiosum (a and b) Both side mobilized spongiosum with moderate spongiosum tissue (c and d) Spongioplasty of mobilized spongiosum
Well-developed-Robust, thick spongiosum with good vascularity; the diameter of the neourethra covered by spongiosum was greater than that of the proximal healthy urethra [Figure 3].
Figure 3.
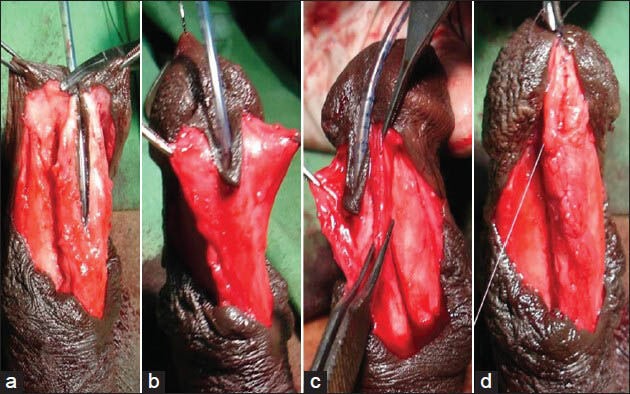
Well-developed corpus spongiosum (a-c) Both side mobilized spongiosum with robust healthy spongiosum tissue (d) Spongioplasty of mobilized spongiosum
Surgical technique
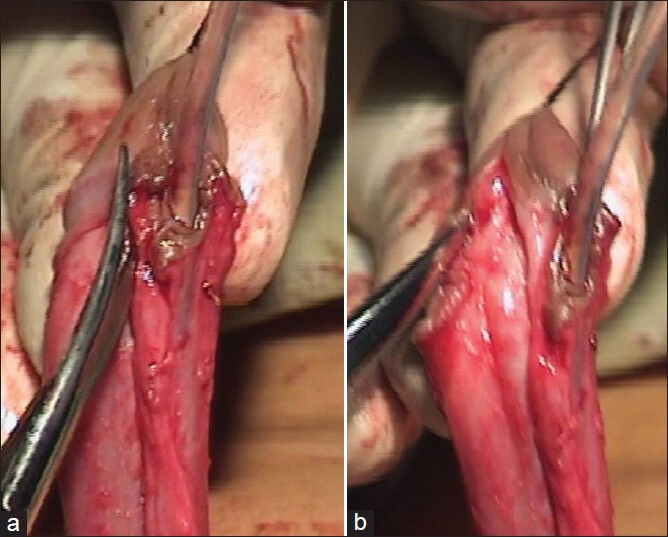
An U-shaped incision is made encircling the meatus up to the corona, preserving the urethral plate and then extended circumferentially around the corona. Penile de-gloving is done when required, up to the root of the penis by creating a plane at the level of Buck's fascia. A Gittes test is carried out to evaluate the chordee after penile de-gloving. Starting well proximal to the hypospadiac meatus, a plane is created between the Buck's fascia and tunica albuginea [Figure 4] and the dissection is carried out on the penile shaft just lateral to the margin of the corpus spongiosum. From lateral to medial, the spongiosum is dissected off the underlying corpora cavernosa. Mobilization is done well beneath the true urethral plate on both sides, lifting off the midline spongiosum and urethral plate [Figure 5] from the corpora cavernosa when required, to correct chordee. Dissection is performed with care, avoiding damage to the corpus spongiosum or corpus cavernosum. The proximal urethra is mobilized according to the severity of ventral curvature up to the bulbar region in case of persistent chordee. Adequacy of curvature correction is confirmed by Gittes test.[17] Since the spongiosal pillars spread beneath the glans wings on each side, care must be taken while mobilizing the glans wings distally with an oblique incision at about 45 degrees, which leaves healthy, thick glans wings yet intact spongiosum when done correctly [Figure 6]. Glanular chordee correction is checked by Gittes test. The urethral plate is then tubularized with a subcuticular 7-0 polydioxanone (PDS) suture after a dorsal incision to create a new urethra. The mobilized spongiosal pillars are then approximated in the midline with 7-0 PDS continuous sutures covering the entire neourethra right up to the glans [Figure 2d]. A 6-to 10 Fr urethral catheter, depending on patient age is left in situ. No additional cover of dartos, tunica vaginalis or de-epithelialized skin was used. Skin is sutured with 7-0 PDS interrupted suture.
Figure 4.
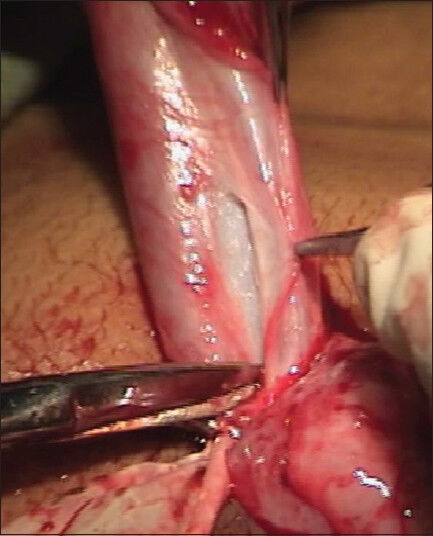
Showing creation of plane proximal to meatus between tunica and Buck's fascia to mobilize the corpus spongiosum
Figure 5.
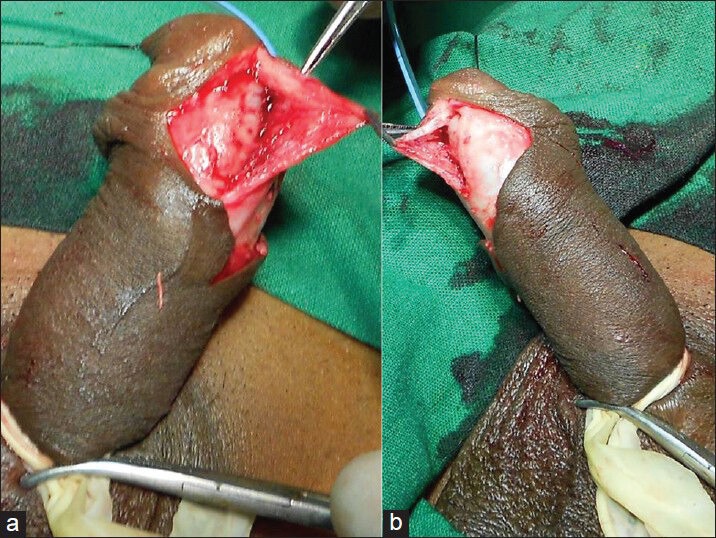
Mobilization of the corpus spongiosum and urethral plate
Figure 6.
Mobilization of the corpus spongiosum and urethral plate into the glans
Cephalosporins with analgesic and anti-inflammatory drugs are given for 7-to 10 days till the catheter is in situ. Patients were evaluated at follow-up visits after 1, 3, 6, 12 months and then yearly. Results were evaluated in terms of patients’, parents’ and surgeon's satisfaction, keeping in mind the stream, cosmesis and lack of complications. If at 3 months follow-up the stream was narrow, we did a meatal/urethral calibration with a 6-10 Fr infant feeding tube.
RESULTS
The age range of the patients was 4 months to 38 years (mean 11.53 years). The hospital stay ranged from 8-10 days and period of follow-up was 6 months to 2 years. The hypospadias was distal, mid and proximal in 81, 12, and 20 cases respectively. Correction of chordee was carried out by penile de-gloving alone in 5, mobilization of urethral plate with spongiosum in 22 and combination of both in 45 cases.
On correlating the type of hypospadias with development of spongiosum; more proximal hypospadias was associated with poorer development of the spongiosum (P = 0.05) [Table 1]. Two patients had meatal stenosis with fistula and seven patients had urethral fistula (Clavien-Dindo grade 3b)[19] alone with an overall complication rate of 7.96%. Fistulas occurred in 2 cases each of distal and mid hypospadias and 5 cases of proximal hypospadias. Both patients who developed meatal stenosis had proximal hypospadias. Complications were higher in proximal hypospadias as compared to distal (P = 0.0019) [Table 1].
Table 1.
Correlation of type of hypospadias, development of spongiosum and complications
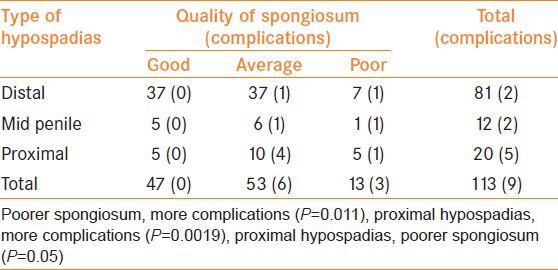
Three (one each in distal, mid and proximal) out of thirteen (23.03%) cases with poorly developed spongiosum and six (distal 1, mid 1 and proximal 4) out of 53 (11.32%) cases with moderately developed spongiosum developed a fistula while none of the cases with well-developed spongiosum developed a fistula. One out of 53 (1.88%) cases with moderately developed spongiosum and 1/13 (7.69%) cases with poorly developed spongiosum developed meatal stenosis. Thus, poorer spongiosum was associated with greater number of complications (P = 0.011) [Table 1].
None of the patients developed a stricture urethra and all patients/parents were satisfied with cosmesis. All nine patients with urethral fistula were operated upon 6-12 months later and the fistulae repaired with no further sequelae.
DISCUSSION
The male urethra develops from the urethral folds as they fuse in the midline after the 6th week of fetal life. The endodermally derived distal solid urethral plate must canalize to form the distal most glanular part of the urethra. The urethra forms sequentially after the proximal urethral folds fuse and the mesenchyme within the urethral folds forms the corpus spongiosum. According to the classic theory, embryologically, the last step in urethra formation is the joining of the main endodermal urethral channel with the ectoderm as it invades the glans.[20] The normal and hypospadiac penises differ in the development of glans and urethra, especially their vascularity. In hypospadias, there is failed urethral fold fusion and ectodermal intrusion, histologically characterized by an extremely vascular area with large endothelial lined sinuses around the abortive urethral spongiosum and abnormal glans.[21] The degree of hypoplasia of corpus spongiosum depends upon the severity of hypospadias and proliferation of mesodermal tissue.
We classified the spongiosum according to its development and vascularity as poorly developed, moderately developed and well-developed. No such classification has been reported earlier in literature. This classification is a good parameter to predict the outcome. Further, it may act as a guide to new surgeons attempting hypospadias about the feasibility of spongioplasty in a particular case and whether an additional layer may be required. None of the patients with well-developed spongiosum in distal hypospadias had any complication. Complications in proximal hypospadias with well-developed spongiosum were also very low as compared to poorly developed spongiosum cases. Most importantly, poorer was the spongiosum, greater was the number of complications (P = 0.011). On analysis of our results, we found that more proximal meatus was associated with poorer development of the spongiosum (P = 0.05) and proximal hypospadias was associated with greater incidence of complications (P = 0.0019). However, not all hypospadias with poorly developed spongiosum had complications, suggesting that other factors like development and width of urethral plate also play a role in the outcome of the repair. Since none of the patients of distal hypospadias with well-developed spongiosum had fistula, dorsal dartos cover in such patients may be omitted and the prepuce can be utilized for preputioplasty. However in patients with poorly developed spongiosa, an additional interposing layer like dartos or tunica should be used.
Since its first description by Snodgrass[1] for repair of hypospadias in 1994, TIP has gained widespread acceptance due to its versatility, low complication rate and reliable creation of vertically oriented meatus, with excellent cosmetic outcome. Some authors recommend TIPU as the technique to be considered for primary and re-operative repair of distal and some midshaft hypospadias cases with selected proximal ones.[22,23] The common complications reported after TIPU repair include fistulae, urethral stricture, meatal stenosis, persistent chordee, infections and wound dehiscence[14] with development of urethrocutaneous fistula being the most common. The fistula rate in large series of hypospadias repairs varies from 0% to 33%.[6,14,24] The causes of fistulae are local infection, ischemia, an inadequate procedure, poor tissue healing and distal obstruction due to meatal stenosis/encrustation which promotes fistulae formation.[25]
To decrease the incidence of post-operative fistulae, many modifications in the TIP repair have been tried. However, no single surgical technique can be considered to be a clear winner to prevent the formation of fistulae. One of the most important factors in reducing fistula formation with the TIP technique is the introduction of a protective intermediate layer between the neourethra and the skin. Dorsal,[3] lateral, single or double dartos flaps, ventral based dartos flap, scrotal dartos, de-epithelized local penile skin, preputial flap, paraurethral tissue, spongioplasty, or tunica vaginalis flaps have all been used for the same. Out of all these, the dorsal dartos flap is used by most surgeons, but there is still no consensus over the ideal interposing tissue in TIPU. Because of edema, necrosis of skin, hematoma and torque with mobilization of the dorsal dartos flap, its role as an interposing tissue has been questioned. The corpus spongiosum provides a well-vascularized, spongy protective covering to the normal urethra. It may aid in the natural propulsion of urine and semen. Using this natural, locally available tissue to cover the neourethra in hypospadias seems to make sense. Spongioplasty also decreases the tension on the suture line of urethroplasty in the midline, especially during erections.
The use of spongiosal tissue as an intermediate layer between the urethra and skin was described in 2000 by Yerkes[26] and Beaudoin[27] in separate studies. Yerkes reported no fistulae in any of her patients for whom spongioplasty was implemented in a number of urethral repair techniques, whereas Beaudoin described the anatomical characteristics of the spongiosal layer in the hypospadic penis and implemented spongioplasty in patients who underwent urethral tubularization.
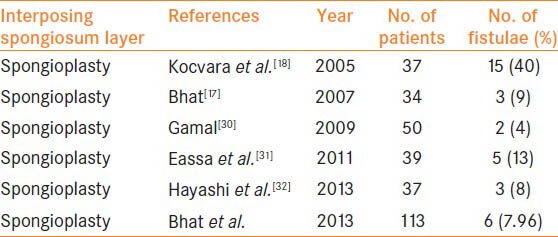
In 2003, Dodat et al.[28] showed no fistula formation in any of the 51 patients who underwent TIPU and spongioplasty. El-Sherbiny et al.[5] reported that covering the neourethra with a flap or spongioplasty minimized the risk of fistula formation. In a series of 500 cases, Sarhan et al.[29] used a dartos flap, spongioplasty, or a combination of these techniques in addition to TIP. They observed a statistically significant decrease in fistula formation in cases in which spongioplasty was implemented compared with cases in which it was not. In the present series we had an acceptable overall fistula rate of 7.96% with no complications in distal hypospadias with well-developed spongiosum where we did not use dorsal/ventral dartos or any other tissue in addition with spongioplasty. These results are comparable with other studies where dorsal dartos has been used with or without spongioplasty. Since the results of well-developed spongioplasty alone in distal hypospadias were very good, additional cover with dorsal dartos can be omitted. Kassaby et al.[17] report that various studies using dartos based flaps, tunica vaginalis and subcutaneous tissue flaps have a fistula rate of 2-33%. When spongioplasty alone was used as an interposing layer, in various series complications ranged from 4% to 40% with 14.2% complication rate in 197 cases whereas in the present series they were seen in 7.96 % cases [Table 2].
Table 2.
Series using spongioplasty alone for hypospadias repair
Our technique of spongioplasty is similar to those mentioned earlier in that we mobilize the urethral plate with the spongiosum completely off the corpora cavernosa starting laterally and moving medially. Furthermore we mobilized the spongiosal pillars into the glans obliquely at an angle of 45° almost up to the tip of the glans. The mobilization of urethral plate with spongiosum have added advantages of correction of torsion and ventral curvature, urethral plate can be tubularized without tension on suture line and many a times without incising the urethral plate.
Complete spongioplasty if done properly as outlined above restores a near normal urethra and provides good support. It is a healthy, vascular cover which probably decreases tension on the midline sutures especially during erections. Better is the spongiosum, lesser are the chances of post-operative fistulae formation.
The strengths of our study are that it is prospective, cases belonged to similar socio-economic status, procedures were performed by a single surgeon in similar circumstances and with adequate follow-up. The weaknesses are that the criteria for development of spongiosum are subjective so there are chances of error in judgment, especially in well and moderately developed spongiosum. To the best of our knowledge, this is the only series in current literature with such a large number of patients undergoing spongioplasty alone with TIP repair.
CONCLUSIONS
A more proximal hypospadias is associated with poorer spongiosum and this is likely to increase the chances of complications. Better developed and thicker spongiosum results in lower incidence of fistula formation after TIPU. Spongioplasty reconstructs a near normal urethra as per anatomical principles of surgery with least complications. Finally, it adds an extra layer of locally available healthy vascular tissue avoiding dissection for local flaps. We recommend spongioplasty be incorporated as an essential step in all patients undergoing TIP repair for hypospadias.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Snodgrass W. Tubularized, incised plate urethroplasty for distal hypospadias. J Urol. 1994;151:464–5. doi: 10.1016/s0022-5347(17)34991-1. [DOI] [PubMed] [Google Scholar]
- 2.Snodgrass WT, Lorenzo A. Tubularized incised-plate urethroplasty for proximal hypospadias. BJU Int. 2002;89:90–3. [PubMed] [Google Scholar]
- 3.Snodgrass WT, Lorenzo A. Tubularized incised-plate urethroplasty for hypospadias reoperation. BJU Int. 2002;89:98–100. doi: 10.1046/j.1464-4096.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MT, Snodgrass WT. Tubularized incised plate hypospadias reoperation. J Urol. 2004;171:2404–6. doi: 10.1097/01.ju.0000125018.90605.a5. [DOI] [PubMed] [Google Scholar]
- 5.El-Sherbiny MT, Hafez AT, Dawaba MS, Shorrab AA, Bazeed MA. Comprehensive analysis of tubularized incised-plate urethroplasty in primary and re-operative hypospadias. BJU Int. 2004;93:1057–61. doi: 10.1111/j.1464-410X.2004.04781.x. [DOI] [PubMed] [Google Scholar]
- 6.Borer JG, Bauer SB, Peters CA, Diamond DA, Atala A, Cilento BG, Jr, et al. Tubularized incised plate urethroplasty: Expanded use in primary and repeat surgery for hypospadias. J Urol. 2001;165:581–5. doi: 10.1097/00005392-200102000-00075. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor KM, Kiely EA. Lessons learned using Snodgrass hypospadias repair. Ir J Med Sci. 2006;175:37–9. doi: 10.1007/BF03168998. [DOI] [PubMed] [Google Scholar]
- 8.Ross JH, Kay R. Use of a de-epithelialized local skin flap in hypospadias repairs accomplished by tubularization of the incised urethral plate. Urology. 1997;50:110–2. doi: 10.1016/S0090-4295(97)00212-4. [DOI] [PubMed] [Google Scholar]
- 9.Sugarman ID, Trevett J, Malone PS. Tubularization of the incised urethral plate (Snodgrass procedure) for primary hypospadias surgery. BJU Int. 1999;83:88–90. doi: 10.1046/j.1464-410x.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 10.Jayanthi VR. The modified Snodgrass hypospadias repair: Reducing the risk of fistula and meatal stenosis. J Urol. 2003;170:1603–5. doi: 10.1097/01.ju.0000085260.52825.73. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hunayan AA, Kehinde EO, Elsalam MA, Al-Mukhtar RS. Tubularized incised plate urethroplasty: Modification and outcome. Int Urol Nephrol. 2003;35:47–52. doi: 10.1023/a:1025995811691. [DOI] [PubMed] [Google Scholar]
- 12.Kamal BA. Double dartos flaps in tubularized incised plate hypospadias repair. Urology. 2005;66:1095–8. doi: 10.1016/j.urology.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Bakan V, Yildiz A. Dorsal double-layer dartos flap for preventing fistulae formation in the Snodgrass technique. Urol Int. 2007;78:241–4. doi: 10.1159/000099345. [DOI] [PubMed] [Google Scholar]
- 14.el-Kassaby AW, Al-Kandari AM, Elzayat T, Shokeir AA. Modified tubularized incised plate urethroplasty for hypospadias repair: A long-term results of 764 patients. Urology. 2008;71:611–5. doi: 10.1016/j.urology.2007.11.121. [DOI] [PubMed] [Google Scholar]
- 15.Babu R, Hariharasudhan S. Tunica vaginalis flap is superior to inner preputial dartos flap as a waterproofing layer for primary TIP repair in midshaft hypospadias. J Pediatr Urol. 2013;9:804–7. doi: 10.1016/j.jpurol.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee US, Mandal MK, Basu S, Das R, Majhi T. Comparative study of dartos fascia and tunica vaginalis pedicle wrap for the tubularized incised plate in primary hypospadias repair. BJU Int. 2004;94:1102–4. doi: 10.1111/j.1464-410X.2004.05111.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhat A. Extended urethral mobilization in incised plate urethroplasty for severe hypospadias: A variation in technique to improve chordee correction. J Urol. 2007;178:1031–5. doi: 10.1016/j.juro.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 18.Kocvara R, Dvorácek J, Díte Z, Sedlácek J, Molcan J. Comprehensive long-term analysis of hypospadias repair using vascularized flaps and tubularized incized plates-Report on 588 cases. Cas Lek Cesk. 2005;144:7–11. [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinman FJ. Penis and male urethra. In: Hinman FJ, editor. UroSurgical Anatomy. Ch. 16. Philadelphia: W. B. Saunders Co; 1993. p. 418. [Google Scholar]
- 21.Baskin LS, Erol A, Li YW, Cunha GR. Anatomical studies of hypospadias. J Urol. 1998;160:1108–15. doi: 10.1097/00005392-199809020-00039. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno K, Hayashi Y, Kojima Y, Tozawa K, Sasaki S, Kohri K. Tubularized incised plate urethroplasty for proximal hypospadias. Int J Urol. 2002;9:88–90. doi: 10.1046/j.1442-2042.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 23.Retik AB, Borer JG. Primary and reoperative hypospadias repair with the Snodgrass technique. World J Urol. 1998;16:186–91. doi: 10.1007/s003450050050. [DOI] [PubMed] [Google Scholar]
- 24.Cheng EY, Vemulapalli SN, Kropp BP, Pope JC, 4th, Furness PD, 3rd, Kaplan WE, et al. Snodgrass hypospadias repair with vascularized dartos flap: The perfect repair for virgin cases of hypospadias? J Urol. 2002;168:1723–6. doi: 10.1097/01.ju.0000026940.33540.31. [DOI] [PubMed] [Google Scholar]
- 25.Bhat A, Mandal AK. Acute postoperative complications of hypospadias repair. Indian J Urol. 2008;24:241–8. doi: 10.4103/0970-1591.40622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerkes EB, Adams MC, Miller DA, Pope JC, 4th, Rink RC, Brock JW., 3rd Y-to-I wrap: Use of the distal spongiosum for hypospadias repair. J Urol. 2000;163:1536–8. [PubMed] [Google Scholar]
- 27.Beaudoin S, Delaage PH, Bargy F. Anatomical basis of surgical repair of hypospadias by spongioplasty. Surg Radiol Anat. 2000;22:139–41. doi: 10.1007/s00276-000-0139-7. [DOI] [PubMed] [Google Scholar]
- 28.Dodat H, Landry JL, Szwarc C, Culem S, Murat FJ, Dubois R. Spongioplasty and separation of the corpora cavernosa for hypospadias repair. BJU Int. 2003;91:528–31. doi: 10.1046/j.1464-410x.2003.04110.x. [DOI] [PubMed] [Google Scholar]
- 29.Sarhan OM, El-Hefnawy AS, Hafez AT, Elsherbiny MT, Dawaba ME, Ghali AM. Factors affecting outcome of tubularized incised plate (TIP) urethroplasty: Single-center experience with 500 cases. J Pediatr Urol. 2009;5:378–82. doi: 10.1016/j.jpurol.2009.02.204. [DOI] [PubMed] [Google Scholar]
- 30.Gamal W, Zaki M, Rashid A, Mostafa M, Abouzeid A. Tubularized Incised Plate (TIP) repair augmented by spongioplasty for distal and midpenile hypospadias. Uro Today Int J. 2009;2:3. [Google Scholar]
- 31.Eassa W, Jednak R, Capolicchio JP, Brzezinski A, El-Sherbiny M. Risk factors for re-operation following tubularized incised plate urethroplasty: A comprehensive analysis. Urology. 2011;77:716–20. doi: 10.1016/j.urology.2010.07.467. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi Y, Mizuno K, Moritoki Y, Nakane A, Kato T, Kurokawa S, et al. Can spongioplasty prevent fistula formation and correct penile curvature in TIP urethroplasty for hypospadias? Urology. 2013;81:1330–5. doi: 10.1016/j.urology.2013.01.005. [DOI] [PubMed] [Google Scholar]


