Abstract
Failure of non-surgical primary treatment for localized prostate cancer is a common occurrence, with rates of disease recurrence ranging from 20% to 60%. In a large proportion of patients, disease recurrence is clinically localized and therefore potentially curable. Unfortunately, due to the complex and potentially morbid nature of salvage treatment, radical salvage surgery is uncommonly performed. In an attempt to decrease the morbidity of salvage therapy without sacrificing oncologic efficacy, a number of experienced centers have utilized robotic assistance to perform minimally invasive salvage radical prostatectomy. Herein, we critically evaluate the existing literature on salvage robotic radical prostatectomy with a focus on patient selection, perioperative complications and functional and early oncologic outcomes. These results are compared with contemporary and historical open salvage radical prostatectomy series and supplemented with insights we have gained from our experience with salvage robotic radical prostatectomy. The body of evidence by which conclusions regarding the efficacy and safety of robotic salvage radical prostatectomy can be drawn comprises fewer than 200 patients with limited follow-up. Preliminary results are promising and some outcomes have been favorable when compared with contemporary open salvage prostatectomy series. Advantages of the robotic platform in the performance of salvage radical prostatectomy include decreased blood loss, short length of stay and improved visualization. Greater experience is required to confirm the long-term oncologic efficacy and functional outcomes as well as the generalizability of results achieved at experienced centers.
Keywords: Prostate cancer, prostatectomy, robotics, salvage therapy
INTRODUCTION
Radical prostatectomy and radiation therapy remain the mainstays of primary treatment for clinically localized prostate cancer. Recurrence after primary therapy failure represents a significant and common clinical dilemma, with rates of prostate cancer recurrence reported to range from 15% to 60% depending on the choice of primary treatment and length of follow-up.[1,2,3,4] While the overall utilization of potentially curative local salvage therapies after failure of primary therapy has increased in recent years, this trend has been exclusive of salvage radical prostatectomy (SRP), which is still rarely performed despite evidence of improved safety and oncologic efficacy in modern series.[5,6,7] Furthermore, it has been shown that a high proportion of patients with radiation failure have clinically localized disease recurrence, which may be amenable to cure by local salvage therapy, although the burden of co-morbid conditions in this patient population combined with the difficulty and morbidity of local salvage therapies like SRP limit the applicability of these findings.[5,8]
Patients who are not acceptable surgical candidates or who have limited life expectancy may be more appropriately treated with either salvage cryotherapy or androgen deprivation therapy. However, in appropriately selected patients, it has been shown that SRP can result in excellent cancer-specific survival as well as durable biochemical recurrence (BCR)-free survival.[6] SRP has also been suggested to provide superior cancer-free survival when compared with salvage cryotherapy, and, although salvage cryotherapy results have improved with newer cryoablation systems, further follow-up is required to confirm the oncologic efficacy of salvage cryotherapy.[9,10]
Despite the potential for cure and avoidance of androgen deprivation therapy afforded by SRP, it is rarely performed.[3,5] While the often-elderly and co-morbid status of patients with radio-recurrent disease compounded with concerns for the presence of advanced disease certainly impacts the low rates of SRP, the challenging technical aspects of the procedure, poor functional outcomes and risk of major complications likely contribute as well. In historical series, rates of rectal injury approached 15% and rates of anastomotic stricture have been as high as 32%.[7] While these rates are daunting, improvements in technique and experience have vastly increased the safety of SRP, with rectal injury rates falling to 2-5%.[7,10,11]
The rapid increase in utilization of the robotic platform in the performance of radical prostatectomy in the United States has led to the use of robotic assistance in the salvage setting.[12,13,14,15,16,17,18,19,20] Salvage robotic radical prostatectomy (sRRP) has shown promising early results and appears to be an excellent alternative to open SRP. As for any salvage procedure, prudent patient selection and surgical experience remain crucial for optimal perioperative and oncologic outcomes.
Since 2008, at least six series of sRRP have been published, encompassing approximately 134 patients.[13,14,15,16,17,19,20] In our experience to date, sRRP has been safe and efficacious, with excellent early oncologic results, and has become our preferred method of performing SRP. The precision and dexterity of the robotic instrumentation combined with the superior visualization offered by the three-dimensional magnification of the operative field all facilitate the challenging aspects of the operation-namely, scarring and loss of tissue planes.
Patient selection
Several groups have sought to define disease characteristics associated with poor outcomes after SRP in order to assist in proper patient selection.[6,19] Patient selection is particularly important in the salvage setting given the potentially substantial impact on quality of life and narrow risk–benefit ratio when compared with prostatectomy in the primary setting. In general, patients selected for sRRP should have a life expectancy of at least 10-15 years and should have clinical stage ≤ T3, post-radiation failure prostate biopsy-proven localized disease with no evidence of metastases on pre-operative imaging. Computerized tomography (CT) scanning can be useful to identify nodal metastases and magnetic resonance imaging (MRI) can be useful to identify the local extent of disease. Other pre-operative clinical characteristics reflective of possible systemic disease are elevated pre-sRRP prostate-specific antigen (PSA) and high pre-operative biopsy Gleason score, short PSA doubling time and short time from primary therapy to recurrence. Pre-operative PSA and biopsy Gleason score have been shown to be predictive of BCR following SRP as well as for the development of metastatic disease.[6]
A thorough discussion should be undertaken with patients giving consideration to sRRP encompassing the substantial risk of impotence and incontinence as well as the increased risk of perioperative complications including the small but increased risk of rectal laceration. We recommend pre-operative mechanical bowel preparation with one bottle of magnesium citrate the day prior to surgery and a clear liquid diet until midnight the night before surgery. We do not recommend the neoadjuvant administration of androgen deprivation therapy as an adjunct to surgery as it has not been shown to be useful.
Operative technique
The surgical technique for sRRP does not significantly depart from that of standard robotic-assisted laparoscopic prostatectomy (RALP). We utilize the six-port transperitoneal approach for both standard RALP and sRRP. The patient is placed in the dorsal lithotomy position and the arms are tucked and padded. After confirming stability on the bed, a Foley catheter is placed on the field and the bladder is emptied. We do not administer pharmaceutical deep vein thrombosis prophylaxis prior to RALP or sRRP. After receiving a single intravenous dose of a first-generation cephalosporin, the patient is positioned in the steep Trendelenburg position after insufflation and the ports are placed in the standard configuration, with a 12 mm supra-umbilical trocar, three robotic trocars, a right lower quadrant assistant 12 mm trocar and, if necessary, an additional 5 mm right-sided assistant trocar.
While others have utilized a posterior approach to first dissect free the seminal vesicles and the plane between the prostate and the rectum before taking down the bladder, we generally perform an anterior approach for both standard RALP and sRRP.[20] Many surgeons-especially those with less experience-may find it easier to identify the posterior plane with the posterior approach, although in our experience the bladder neck (anterior) approach is preferable. For any given patient, either approach is acceptable and it is to the discretion of the individual surgeon based upon training and experience as to which approach is utilized. The initial steps of sRRP are identical to a standard RALP-the peritoneum is incised lateral to each medial umbilical ligament and the space of Retzius is developed after division of the urachus and medial umbilical ligaments. The periprostatic fatty tissue is dissected free and the superficial dorsal venous branches are cauterized and divided, exposing the endopelvic fascia. The effects of prior radiation therapy are often readily apparent at the level of the endopelvic fascia, which is frequently thickened, fibrotic and adherent to the underlying tissues [Figure 1]. The endopelvic fascia is carefully incised with judicious cautery as required to cut through the thick tissue [Figure 2]. During salvage procedures, we generally omit ligation of the deep dorsal venous complex at this point to allow for improved mobility during dissection of the prostatic apex. Furthermore, it has been our experience that dorsal venous complex bleeding is minimal in the salvage setting due to radiation effects.
Figure 1.
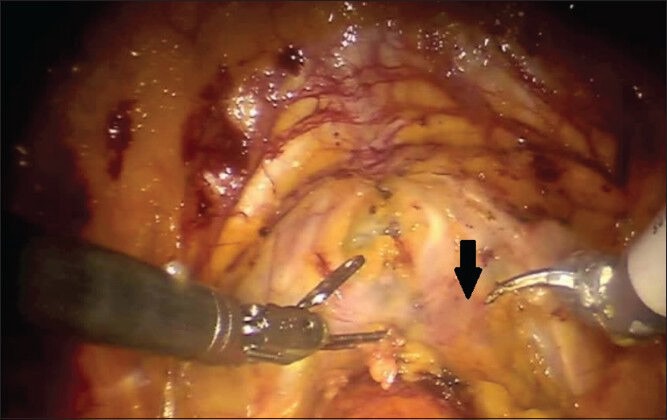
Fibrotic and thickened endopelvic fascia in a patient undergoing salvage robotic radical prostatectomy after external beam radiation therapy
Figure 2.
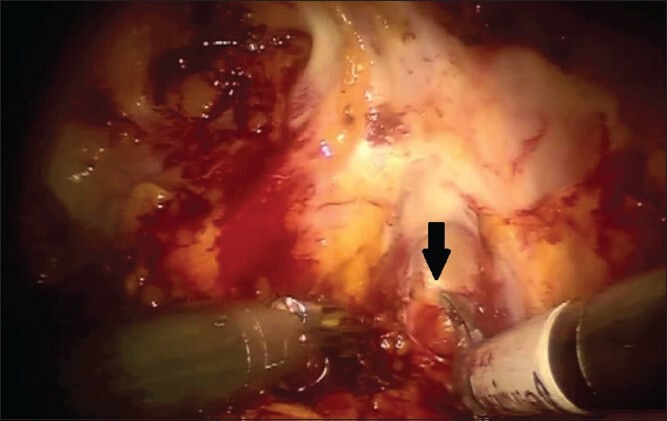
The thickened right endopelvic fascia is incised
The bladder neck is then identified and incised as per a standard RALP and the bilateral vasa deferentia are divided and the seminal vesicles dissected free. Complete excision of the seminal vesicles is prudent during sRRP as 30–35% of patients will have seminal vesicle invasion.[6,19] The posterior plane is then developed. Although the inter-fascial plane above the Denonvillier's fascia can be quite adherent to the prostate, Denonvillier's fascia is relatively inviolate and the plane posterior to Denonvillier's fascia is generally well preserved. Furthermore, developing this plane anterior to the perirectal fat allows for wide excision of the prostate in case of extracapsular extension [Figure 3]. The improved visualization of this plane with the robotic platform greatly facilitates this dissection, although caution is still required. Because of the high incidence of locally advanced disease in this population, we generally perform a wide excision of the lateral prostatic fascia and neurovascular bundle to optimize oncologic outcomes.
Figure 3.
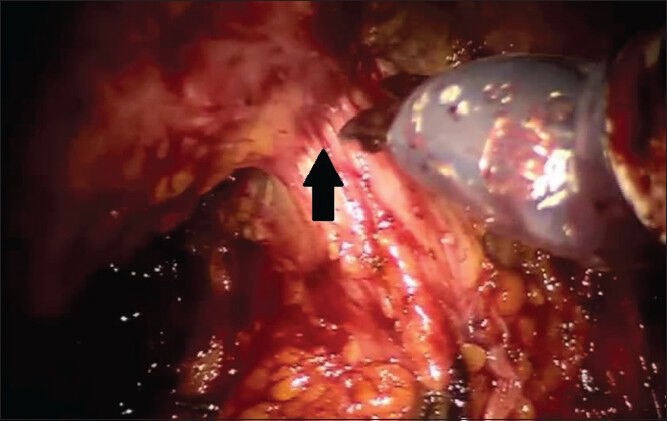
Dissection of the posterior plane deep to Denonvillier's fascia is almost completed. The rectum is tented up as the last remaining attachments between posterior prostatic apex and rectum are divided
The apex of the prostate is often quite immobile in the post-radiation setting and, in the case of primary brachytherapy, the posterior prostatic apex is often particularly fibrotic and adherent [Figure 4]. Therefore, the lateral margins of the prostatic are completely freed in order to allow full mobilization of the prostate prior to division of the dorsal venous complex. The prostate is retracted posteriorly and cranially with the fourth arm as the dorsal venous complex is divided and then laterally as needed to improve visualization of the urethra and posterior apex. The urethra is sharply divided, the catheter is removed and any remaining apical tissue is dissected free. The improved visualization provided by robotic approach is remarkably useful at this point as periapical fibrosis may obscure the boundaries of the prostate. Additional margins can be taken as needed. Further hemostatic sutures can be placed in the dorsal venous complex at this time if required.
Figure 4.
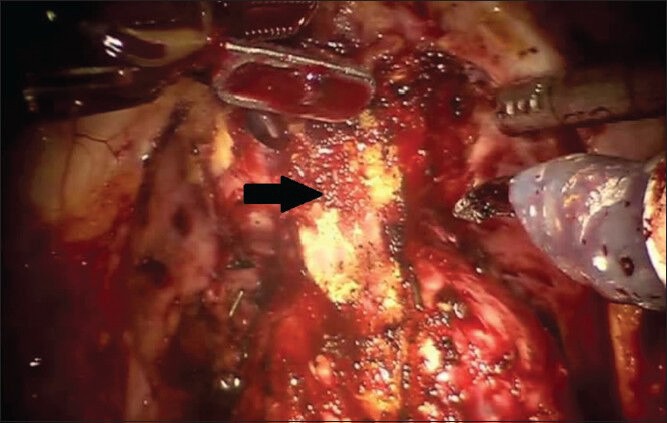
The dorsal venous complex has been divided. Note the marked periapical fibrosis
A standard bilateral pelvic lymphadenectomy is generally performed and, although we have become more aggressive with node dissections in patients with advanced disease in recent years, the improved staging and possibility of trivial survival benefit with lymphadenectomy must be weighed against the increased morbidity of a surgical node dissection. This is particularly relevant in patients with favorable disease characteristics. The vesicourethral anastomosis is then performed in the standard fashion and a surgical drain is placed through the left lateral robotic trocar site only if a lymphadenectomy was performed.
Post-operative care
Post-operative management after sRRP follows our standard RALP pathway, which has been previously published and includes early ambulation, a full liquid diet on the first post-operative morning, intravenous ketorolac to reduce the utilization of narcotics and an aggressive bowel regimen including milk of magnesia and bisacodyl suppositories.[21] Surgical drains are generally removed on post-operative day one, unless the output is greater than 200 cc over 24 hours. It has been our experience that salvage status should not prolong length of hospital stay, and greater than 94% of patients undergoing sRRP have been discharged on the first post-operative day.[19] Post-operative cystography is rarely performed and Foley catheters are left in place for 10-14 days.
Complications
Complication rates in published sRRP series are generally comparable to or lower than those of contemporary open SRP series. Major complications are uncommon and a distinct advantage of performing SRP with robotic assistance is decreased blood loss. Median estimated blood loss has ranged from 75 cc to 175 cc in published series, with no blood transfusions or conversions from laparoscopic to open in all 134 patients.[13,14,15,16,17,19,20] Another distinct advantage of the robotic approach is the low rate of anastomotic stricture, ranging from 0% to 17% in the six sRRP series.[14,15,16,17,19,20] Even in contemporary open SRP series, anastomotic stricture rates remain troublesome, ranging from 11% to 30%.[7,10,11] A small percentage (0–33%) of patients develop anastomotic leaks following sRRP requiring prolonged catheterization.[14,15,16,17,19,20] Four patients with thrombotic complications (deep vein thrombosis or pulmonary emboli) have been reported.[17,19,20] Two enterotomies during lysis of adhesions have also been reported.[16,20] Of the 134 patients undergoing sRRP in published series, only two (1.5%) rectal injuries have been described-a rate lower than most contemporary open SRP series, which have ranged from 2% to 4%.[7,10,11,14,15,16,17,19,20] If a rectal laceration occurs, we recommend primary, multilayer closure, tissue interposition and strong consideration for fecal diversion given the increased propensity for rectourethral fistula in the salvage setting.
Oncologic outcomes
The assessment of the oncologic efficacy of sRRP is limited at this point with a median follow-up ranging from 4 to 36 months in published series.[14,15,16,17,19,20] Despite this, preliminary results are encouraging. Of the 134 sRRP patients described in the literature, 34 (25%) had positive margins, comparable to contemporary open SRP margin rates, which have ranged from 11% to 33%.[6,7,13,14,15,16,17,19,20,22] Positive margins have been shown to be associated with BCR following radical prostatectomy in the primary and salvage settings.[22,23]
Of the 117 patients in published series undergoing sRRP who received a bilateral pelvic lymphadenectomy, seven (5.2%) were detected to have positive nodes.[14,15,16,17,19,20] In our experience, six patients (18%) developed BCR with 16 months of median follow-up.[19] In another large series of 55 patients undergoing sRRP, 10 (18%) developed BCR with a median follow-up of 36 months.[20] These results are similar to the 25% BCR rate at a median follow-up of 16 months reported by a large multi-institutional series of more than 400 patients receiving open SRP.[6]
Functional outcomes
Functional outcomes following SRP are generally worse than radical prostatectomy performed in the primary setting. While follow-up is limited in many sRRP series, continence rates (0-1 pad per day) have ranged from 33% to 80%, with the two largest series reporting rates of 39-45%.[14,15,16,17,19,20] Reported continence rates will likely improve as sRRP series mature and follow-up increases.
Pre-operative and post-operative erectile function in the post-radiation setting is universally poor. The majority of patients have impaired pre-operative erectile function-only 21-23% of patients were considered potent pre-operatively in the two largest sRRP series.[19,20] High rates of post-operative erectile dysfunction have been a consistent finding in other sRRP series and, although limited follow-up may underestimate improvements in erectile function, potency rates in contemporary, mature open SRP series are invariably poor.[6,7,11,14,15,16,17]
CONCLUSIONS
Performance of radical prostatectomy in the salvage setting, whether open or robotic, is a technically formidable procedure. While this is in part related to the radiation changes to the tissue and subsequent increased risk of serious complication, it is also related to the increased incidence of locally advanced disease. Therefore, patient selection is crucial and sRRP is not recommended for the novice robotic surgeon. Utilization of robotic assistance is particularly well suited for the performance of radical prostatectomy in the post-radiation setting, and is almost universally our modality of choice in this patient population. Improved visualization and precise tissue handling greatly facilitate performance of the procedure. The literature in support of sRRP to date has demonstrated excellent safety with low rates of perioperative complications. Some outcomes may compare favorably to open SRP series, including low blood loss, short length of stay and decreased anastomotic stricture rates. Furthermore, early oncologic results are encouraging, although improvements in functional outcomes are still required. As follow-up increases and experience is gained, it is hoped that wider and safer delivery of a potentially curative therapeutic option to a difficult patient population may be facilitated by the robotic platform.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zelefsky MJ, Kuban DA, Levy LB, Potters L, Beyer DC, Blasko JC, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327–33. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Coen JJ, Dallow KC, Shipley WU. The treatment of prostate cancer by conventional radiation therapy: An analysis of long-term outcome. Int J Radiat Oncol Biol Phys. 1995;32:287–92. doi: 10.1016/0360-3016(95)00123-G. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–14. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Cary KC, Paciorek A, Fuldeore MJ, Carroll PR, Cooperberg MR. Temporal trends and predictors of salvage cancer treatment after failure following radical prostatectomy or radiation therapy: An analysis from the CaPSURE registry. Cancer. 2014;120:507–12. doi: 10.1002/cncr.28446. [DOI] [PubMed] [Google Scholar]
- 6.Chade DC, Shariat SF, Cronin AM, Savage CJ, Karnes RJ, Blute ML, et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur Urol. 2011;60:205–10. doi: 10.1016/j.eururo.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich A, Richter S, Thuer D, Pfister D. Prognostic parameters, complications, and oncologic and functional outcome of salvage radical prostatectomy for locally recurrent prostate cancer after 21 st-century radiotherapy. Eur Urol. 2010;57:437–43. doi: 10.1016/j.eururo.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Zagars GK, Pollack A, Von Eschenbach AC. Prostate cancer and radiation therapy-the message conveyed by serum prostate-specific antigen. Int J Radiat Oncol Biol Phys. 1995;33:23–35. doi: 10.1016/0360-3016(95)00154-Q. [DOI] [PubMed] [Google Scholar]
- 9.Pisters LL, Leibovici D, Blute M, Zincke H, Sebo TJ, Slezak JM, et al. Locally recurrent prostate cancer after initial radiation therapy: A comparison of salvage radical prostatectomy versus cryotherapy. J Urol. 2009;182:517–25. doi: 10.1016/j.juro.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Ward JF, Sebo TJ, Blute ML, Zincke H. Salvage surgery for radiorecurrent prostate cancer: Contemporary outcomes. J Urol. 2005;173:1156–60. doi: 10.1097/01.ju.0000155534.54711.60. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Scardino PT, Bianco FJ, Jr, DiBlasio CJ, Fearn PA, Eastham JA. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004;172:2239–43. doi: 10.1097/01.ju.0000140960.63108.39. [DOI] [PubMed] [Google Scholar]
- 12.Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 13.Jamal K, Challacombe B, Elhage O, Popert R, Kirby R, Dasgupta P. Successful salvage robotic-assisted radical prostatectomy after external beam radiotherapy failure. Urology. 2008;72:1356–8. doi: 10.1016/j.urology.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kaouk JH, Hafron J, Goel R, Haber GP, Jones JS. Robotic salvage retropubic prostatectomy after radiation/brachytherapy: Initial results. BJU Int. 2008;102:93–6. doi: 10.1111/j.1464-410X.2008.07570.x. [DOI] [PubMed] [Google Scholar]
- 15.Boris RS, Bhandari A, Krane LS, Eun D, Kaul S, Peabody JO. Salvage robotic-assisted radical prostatectomy: Initial results and early report of outcomes. BJU Int. 2009;103:952–6. doi: 10.1111/j.1464-410X.2008.08245.x. [DOI] [PubMed] [Google Scholar]
- 16.Eandi JA, Link BA, Nelson RA, Josephson DY, Lau C, Kawachi MH, et al. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J Urol. 2010;183:133–7. doi: 10.1016/j.juro.2009.08.134. [DOI] [PubMed] [Google Scholar]
- 17.Chauhan S, Patel MB, Coelho R, Liss M, Rocco B, Sivaraman AK, et al. Preliminary analysis of the feasibility and safety of salvage robot-assisted radical prostatectomy after radiation failure: Multi-institutional perioperative and short-term functional outcomes. J Endourol. 2011;25:1013–9. doi: 10.1089/end.2010.0564. [DOI] [PubMed] [Google Scholar]
- 18.Rocco B, Cozzi G, Spinelli MG, Grasso A, Varisco D, Coelho RF, et al. Current status of salvage robot-assisted laparoscopic prostatectomy for radiorecurrent prostate cancer. Curr Urol Rep. 2012;13:195–201. doi: 10.1007/s11934-012-0245-1. [DOI] [PubMed] [Google Scholar]
- 19.Kaffenberger SD, Keegan KA, Bansal NK, Morgan TM, Tang DH, Barocas DA, et al. Salvage robotic assisted laparoscopic radical prostatectomy: A single institution, 5-year experience. J Urol. 2013;189:507–13. doi: 10.1016/j.juro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuh B, Ruel N, Muldrew S, Mejia R, Novara G, Kawachi M, et al. Complications and outcomes of salvage robot-assisted radical prostatectomy: A single-institution experience. BJU Int. 2014;113:769–76. doi: 10.1111/bju.12595. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MR, Baumgartner RG, Anderson LW, Smith JA, Jr, Chang SS, Herrell SD, et al. The evidence-based pathway for peri-operative management of open and robotically assisted laparoscopic radical prostatectomy. BJU Int. 2007;99:1103–8. doi: 10.1111/j.1464-410X.2007.06777.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson KM, Penson DF, Cai J, Groshen S, Stein JP, Lieskovsky G, et al. Salvage radical prostatectomy: Quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol. 2006;176:2025–31. doi: 10.1016/j.juro.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 23.Grossfeld GD, Chang JJ, Broering JM, Miller DP, Yu J, Flanders SC, et al. Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: Data from the CaPSURE database. J Urol. 2000;163:1171–7. [PubMed] [Google Scholar]


