Abstract
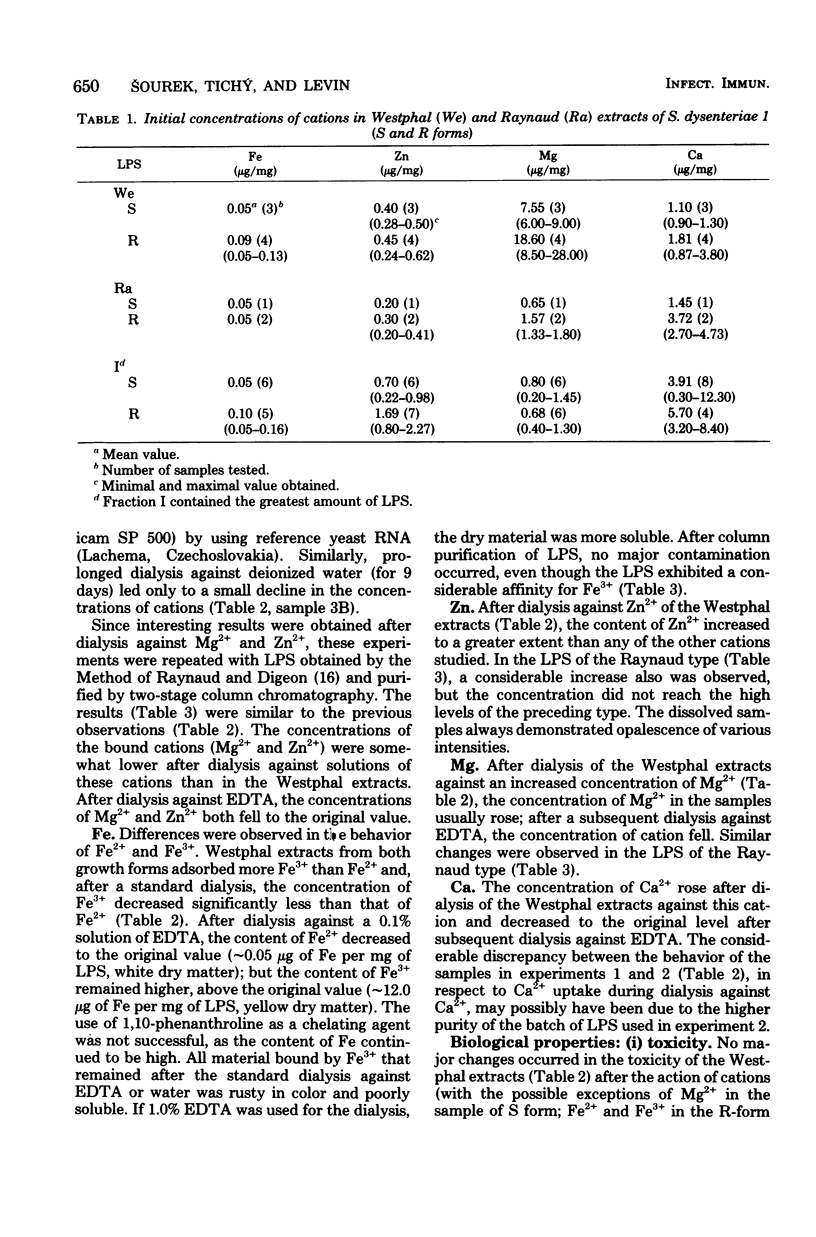
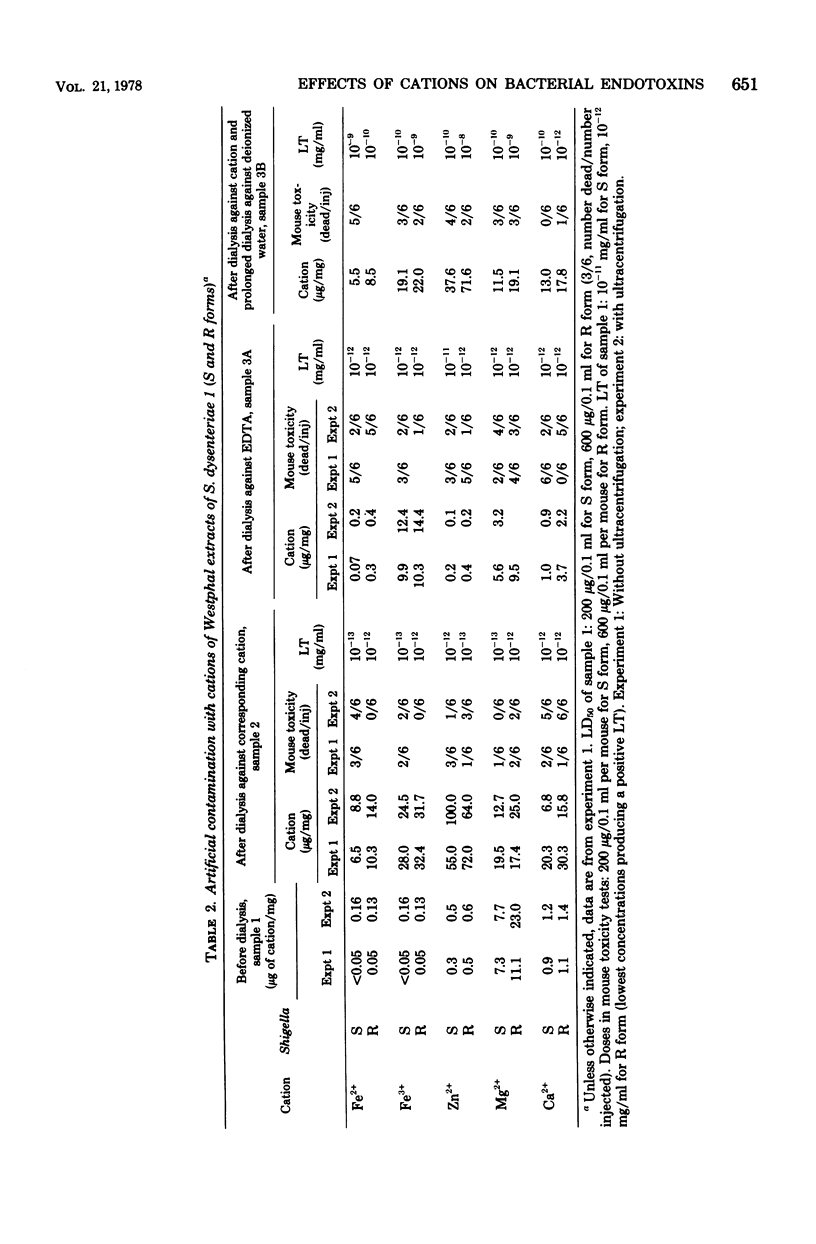
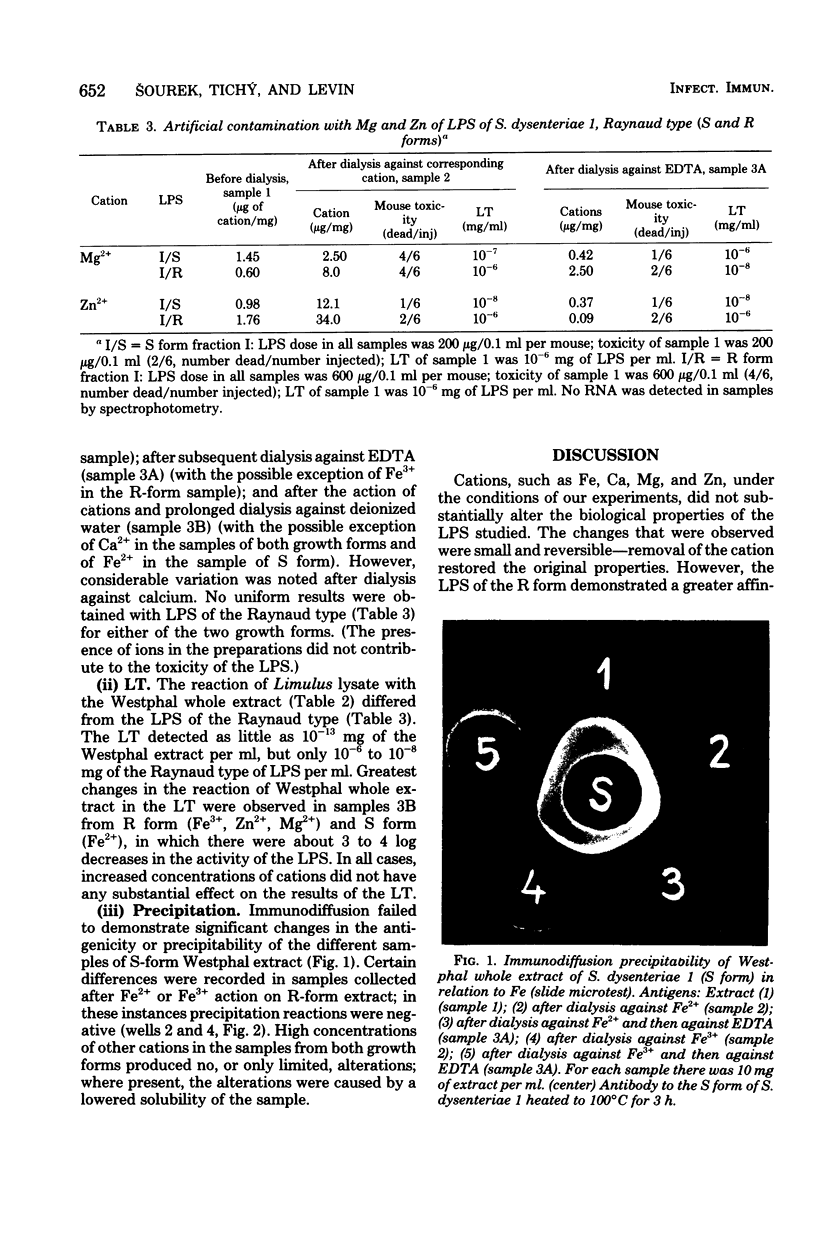
The natural occurrence of cations Fe, Zn, Mg, and Ca in the lipopolysaccharide (LPS) of both the S and R forms of Shigella dysenteriae 1 was studied. LPS preparations were obtained either by phenol-water extraction (according to the method of Westphal et al., Z. Naturforsch. 7b:148-155, 1952) or by extraction of cells with hypertonic sodium chloride-sodium citrate (according to the method of Raynaud and Digeon, C. R. Acad. Sci. (Paris) 229:564-566, 1949), with subsequent chromatographic purification on Sephadex G200 and Sepharose 4B columns. The cation in highest concentration in the Westphal extract was Mg2+ (as much as 30 μg/mg), and the lowest one was Fe (ca. 0.10 μg/mg). In LPS of the Raynaud type, the cation in highest concentration was Ca2+ (as much as 13 μg/mg), and the lowest one was Fe (ca. 0.10 μg/mg). The effects of increasing and decreasing the concentrations of cations (Fe, Zn, Mg, Ca) upon the biological activity of the endotoxins was evaluated by using toxicity in mice and the Limulus test. It appeared that increased concentrations of Fe (chiefly of Fe3+) decreased the toxicity of the R form of LPS, whereas Mg2+ decreased the toxicity of the S form. After prolonged dialysis of LPS preparations against deionized water, there was no consistent relationship between toxicity as determined in white mice and with the Limulus test.
Full text
PDF



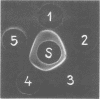
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. The role of multivalent cations in the organization and structure of bacterial cell walls. Biochem Biophys Res Commun. 1966 Mar 22;22(6):664–671. doi: 10.1016/0006-291x(66)90198-7. [DOI] [PubMed] [Google Scholar]
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Sandberg A. L., Rosentreich D. L. Comparison of the pyrogenicity, Limulus activity mitogenicity and complement reactivity of several bacterial endotoxins and related compounds. J Immunol. 1976 Oct;117(4):1238–1242. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Greer G. G., Epps N. A., Vail W. J. Interaction of lipopolysaccharides with mitochondria. II. Effects of magnesium ions on toxicity of a rough lipopolysaccharide. J Infect Dis. 1973 Dec;128(6):724–729. doi: 10.1093/infdis/128.6.724. [DOI] [PubMed] [Google Scholar]
- Gärtner H., Grigo J. Die Verteilung eines Shigella dysenteriae Exotoxins im Versuchstier. Arch Hyg Bakteriol. 1969 Jun;153(3):270–277. [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Olins A. L., Warner R. C. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967 Nov 10;242(21):4994–5001. [PubMed] [Google Scholar]
- Prigal S. J., Herp A., Gerstein J. The detoxification of lipopolysaccharides derived from bacterial endotoxins by ferric chloride. J Reticuloendothel Soc. 1973 Sep;14(3):250–257. [PubMed] [Google Scholar]
- RAYNAUD M., DIGEON M. Sur une nouvelle toxine du bacille typhique extraite des formes rough. C R Hebd Seances Acad Sci. 1949 Sep 12;229(11):564–566. [PubMed] [Google Scholar]
- ROSEN F. S., SKARNES R. C., LANDY M., SHEAR M. J. Inactivation of endotoxin by a humoral component. III. Role of divalent cation and a dialyzable component. J Exp Med. 1958 Nov 1;108(5):701–711. doi: 10.1084/jem.108.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourek J., Tichý The influence of some cations on bacterial endotoxins: copper. Zentralbl Bakteriol Orig A. 1975;231(1-3):259–268. [PubMed] [Google Scholar]
- Sourek J., Trnka T., Digeon M., Raynaud M. Some immunochemical and chemical aspects of S and R Shigella dysenteriae 1 endotoxins. J Hyg Epidemiol Microbiol Immunol. 1975;19(3):356–374. [PubMed] [Google Scholar]
- Tomasulo P. A., Levin J., Murphy P. A., Winkelstein J. A. Biological activities of tritiated endotoxins: correlation of the Limulus lysate assay with rabbit pyrogen and complement-activation assays for endotoxin. J Lab Clin Med. 1977 Feb;89(2):308–315. [PubMed] [Google Scholar]
- VANHEYNINGEN W. E., ARSECULERATNE S. N. EXOTOXINS. Annu Rev Microbiol. 1964;18:195–216. doi: 10.1146/annurev.mi.18.100164.001211. [DOI] [PubMed] [Google Scholar]
- Wang W. S., Korczynski M. S., Lundgren D. G. Cell envelope of an iron-oxidizing bacterium: studies of lipopolysaccharide and peptidoglycan. J Bacteriol. 1970 Oct;104(1):556–565. doi: 10.1128/jb.104.1.556-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]