Abstract
Objective
Activation of the renal renin-angiotensin system in patients with diabetes mellitus appears to contribute to the risk of nephropathy. Recently it has been recognized than an elevation of prorenin in plasma also provides a strong indication of risk of nephropathy. This study was designed to examine renin-angiotensin system control mechanisms in the patient with diabetes mellitus.
Methods
We enrolled 43 subjects with Type 2 diabetes mellitus and, after an acute exposure to captopril (25 mg), randomized them to treatment with either irbesartan (300 mg) or aliskiren (300 mg) for two weeks.
Results
All agents acutely lowered blood pressure and plasma aldosterone, and increased renal plasma flow and glomerular filtration rate. Yet only captopril and Aliskiren acutely increased plasma renin and decreased plasma angiotensin II, while Irbesartan acutely affected neither renin nor angiotensin II. Plasma renin and angiotensin II subsequently did increase upon chronic irbesartan treatment. When given on day 14, irbesartan and aliskiren again induced the above hemodynamic, renal and adrenal effects, yet without significantly changing plasma renin. Irbesartan at that time did not affect plasma angiotensin II, while aliskiren lowered it to almost zero.
Conclusions
The relative resistance of the renal renin response to acute (irbesartan) and chronic (irbesartan and aliskiren) renin-angiotensin system blockade supports the concept of an activated renal renin-angiotensin system in diabetes, particularly at the level of the juxtaglomerular cell, and implies that diabetics require higher doses of renin-angiotensin system blockers to fully suppress the renal renin-angiotensin system.
Keywords: chronic kidney disease, renin-angiotensin system, prorenin, juxtaglomerular cell
Introduction
Diabetes mellitus is the leading cause of nephropathy and end-stage renal disease in the Western world. Abnormalities involving renal perfusion and the renin-angiotensin system (RAS) appear to play an important pathogenic role, but the responsible mechanisms remain obscure [1–4]. A reduction in renal plasma flow (RPF) is common, accompanied by blunting of the renovascular response to angiotensin (Ang II) [1–4]. There is also an accentuated renal vasodilator response to angiotensin-converting enzyme (ACE) inhibition, which in turn corrects the blunting of responsiveness to Ang II. All of these observations suggest activation of the RAS in patients with diabetes mellitus. As plasma renin often is reduced in such patients, the findings suggest that it is the intrarenal RAS that is activated [1,5]. Despite being low, plasma renin is an independent predictor of cardiovascular events in patients with diabetes [6–7]. The rise in plasma renin following RAS blockade is taken to be an indication of the degree of RAS blockade [8]. Importantly, the renin rise following acute exposure to increasing doses of the Ang II type 1 receptor blocker irbesartan in diabetics is blunted and slow as compared to non-diabetic healthy controls, and for a given renin rise a higher irbesartan dose was required in diabetics [1]. Yet, simultaneously, the RPF response to irbesartan was enhanced in diabetics vs. controls at all irbesartan doses tested [1]. Finally, the plasma levels of renin’s precursor, prorenin, are elevated in diabetes, and these elevated levels provide a strong indication of risk of the microvascular complications of this disease, i.e., nephropathy and retinopathy [9–11].
To obtain a better understanding of the dysregulation of the RAS in diabetes, in the present study we carefully compared, in subjects with diabetes, the changes in plasma RAS parameters with the hemodynamic, renal and adrenal responses induced by blockade at the level of renin, ACE and the Ang II type 1 (AT1) receptor, both acutely and chronically. Doses of the 3 types of RAS blockers were chosen in such a manner that identical hemodynamic and renal responses could be expected, to rule out changes in plasma RAS parameters due to systemic or renal hemodynamic differences [12–14]. In addition, subjects were exposed to a high-salt diet to minimize the contribution of the systemic RAS.
Methods
Study protocol
Forty-three subjects with Type 2 diabetes mellitus (29 men, 14 women) averaging 57 years of age were studied on a high-sodium diet (Table 1). Fifty-eight percent were White and 30% were Black. All had hypertension, which was generally well controlled. Most were obese (weight 105±4 kg, body mass index 34.8±1.2 kg/m2). After an outpatient evaluation, which included history, physical examination, screening chemistry, and hematology laboratory tests, all subjects were studied during an 8-day admission to a metabolic ward, the Center for Clinical Investigation of the Brigham and Women’s Hospital. Written, informed consent was obtained from each patient, and the protocol was approved by the Human Subjects IRB Committee of the Institution.
Table 1.
Baseline characteristics. Data are mean±SEM.
| IRBESARTAN (n=21) |
ALISKIREN (n=22) |
|
|---|---|---|
| Age (yrs) | 57.2 ± 1.8 | 57.0 ± 2.5 |
|
Gender # (%) Male Female |
12 (57) 9 (43) |
17 (77) 5 (23) |
|
Race # (%) White Black Other |
12 (57) 6 (29) 3 (14) |
13 (59) 7 (32) 2 (9) |
|
Supine BP (mm Hg) Systolic Diastolic |
140 ± 3 80 ± 2 |
144 ± 3 80 ± 2 |
| Weight (kg) | 101.4 ± 6.0 | 108.2 ± 5.7 |
| Body mass index | 34.8 ± 1.6 | 34.8 ± 1.7 |
| HbA1c (%) | 7.2 ± 0.2 | 7.6 ± 0.3 |
| Fasting serum glucose (mg/dL) | 122 ± 9 | 129 ± 8 |
| Urine sodium (mmol/24 h) | 271 ± 17 | 259 ± 21 |
| Urine potassium (mmol/24 h) | 68 ± 6 | 74 ± 7 |
| Proteinuria # (%) | 7 (33) | 8 (36) |
| Renal plasma flow (mL/min/1.73m2) | 414 ± 19 | 426 ± 13 |
| Glomerular filtration rate (mL/min/1.73m2) | 72 ± 3 | 67 ± 2 |
| Prorenin (ng/L) | 101 ± 20 | 111 ± 25 |
| Renin (ng/L) | 6.4 ± 1.0 | 12 ± 3.8 |
| PRA (ng Ang I/ml per minute) | 0.40 ± 0.08 | 0.72 ± 0.21 |
| Ang I (pmol/L) | 1.9 ± 0.4 | 2.9 ± 0.7 |
| Ang II (pmol/L) | 0.4 ± 0.1 | 0.7 ± 0.2 |
| Aldosterone (ng/L) | 92 ± 26 | 103 ± 20 |
The subjects were placed on a high-sodium, constant isocaloric diet throughout the study and consumed 200 mmol of sodium daily, the first several days as outpatients. Daily dietary potassium (100 mmol) and fluid intake (2500 mL) were constant. Twenty-four-hour urine samples were collected daily; urinary sodium matched sodium intake usually by days 3 to 5. On the first study each subject received the ACE inhibitor captopril at a dose that is at the top of the dose-response relationship (25 mg). On the next day, the subjects were randomized to receive either the AT1 receptor blocker (ARB) irbesartan (300 mg) or the renin inhibitor aliskiren (300 mg). The subjects were then discharged to be readmitted on day 14 for a follow-up study on the same drug. Each had received 14 days of treatment. Exposure to captopril was open, but exposure to aliskiren and irbesartan were randomized and blinded. Phlebotomy limitations prevented subjects from undergoing more than 3 studies each.
Studies began at 7 am. Subjects had been recumbent and fasting overnight and remained recumbent throughout the study. RPF was measured by the clearance of para-aminohippurate(PAH; Clinalfa, Laufelfmgen, Switzerland) and glomerular filtration rate (GFR) by the clearance of inulin (Inutest Polyfructosan, Fresenius Pharma, Linz, Austria) by autoanalyzer methods described previously [15]. After a 60-minute control period to establish basal RPF, the drug was dosed by mouth.
Blood pressure during each infusion was recorded by an automatic recording device (Dinamap, Critikon Inc, Tampa, FL, USA) at 15-minute intervals. Blood pressure fall was analyzed from the lowest single blood pressure reached after each dose compared with the mean of 4 measures taken at the pre-dose baseline on that study day.
Blood samples and biochemical measurements
Blood samples were collected on ice at the start of the PAH infusion, at 90-minute intervals throughout, at the end of each study. Samples were spun immediately, and the plasma was frozen until the time of assay. PAH was measured by an autoanalyzer technique [16]. Renin concentration was measured with a commercial immunoradiometric kit (Renin III, Cisbio, Gif-Sur-Yvette, France) [17]. Total renin concentration was determined simultaneously with the same kit after the induction of a conformational change in the prorenin molecule with aliskiren (10 µmol/L for 48 hours at 4°C), which enabled its recognition by the active site-directed radio labeled antibodies applied in the Cisbio kit [18]. Subtraction of the renin concentration from the total renin concentration yielded the estimated prorenin concentration. Plasma renin activity (PRA) was measured by the trapping of generated Ang I by high-affinity antibodies and by subsequent radioimmunoassay. Plasma Ang I and II were measured specifically by radioimmunoassay after solid-phase extraction and subsequent high-performance liquid chromatography separation [12].
Statistical Analysis
Peak response was taken as the average of the 2 highest consecutive values for PAH/inulin clearance and compared with baseline clearance at the beginning of day 1 of the study. The median value was employed. Linear mixed models were used to model response with an unstructured covariance for the correlation within subjects. An F test is presented for the overall significance of the linear mixed model. For all comparisons, group means have been presented with the SEM as the index of dispersion, and an α-level of 0.05 was used to determine statistical significance. All analyses were performed in SAS version 9.1. All of the hormone measurements were highly skew, but the findings and conclusions were not altered through the use of geometric means and examination of the data by non-parametric tests.
Results
The patients treated with irbesartan or aliskiren were well-matched for age, racial distribution, blood pressure, body habitus, and frequency or severity of obesity (Table 1). They were also well matched for physiologic variables, both hemodynamic and hormonal. They were somewhat less matched for gender and glycemic control, but no difference achieved statistical significance.
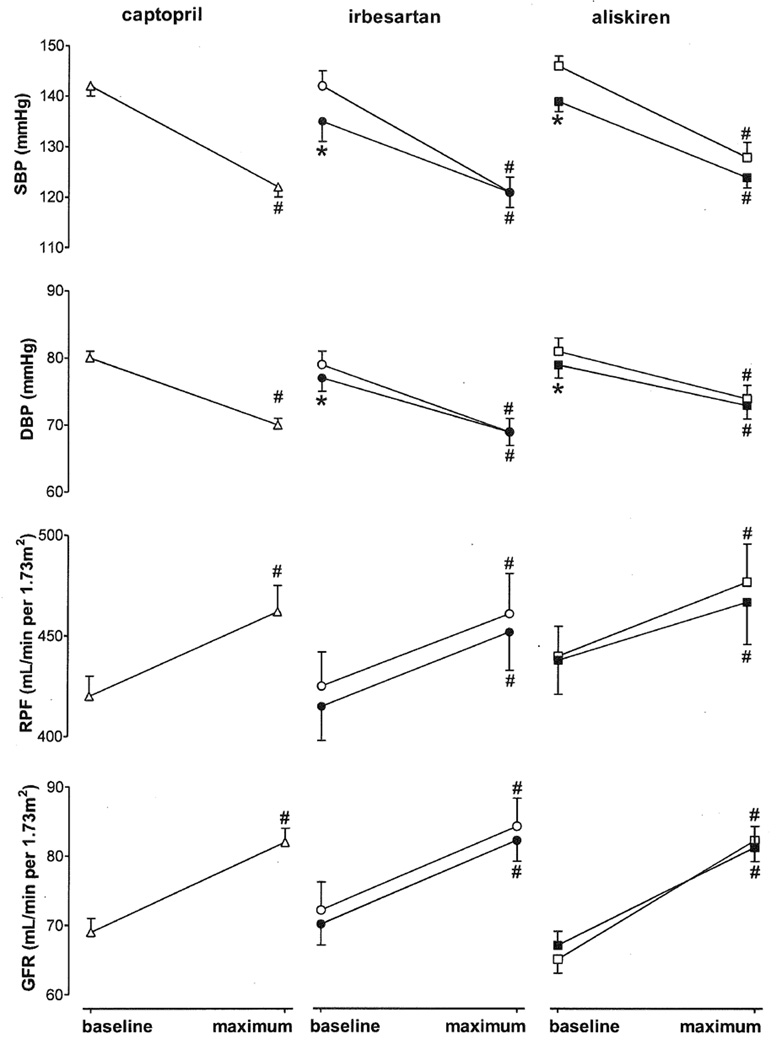
As expected, both systolic and diastolic blood pressure decreased following acute and chronic exposure to the blockers (Figure 1). The acute responses were identical for all 3 blockers, as were the responses to aliskiren and irbesartan on day 14. RPF and GFR also showed an identical response to all 3 blockers, the glomerular response being somewhat larger than the RPF response. Captopril data for the groups that were subsequently treated with either irbesartan or aliskiren were combined since the responses to ACE inhibition were identical in both groups.
Figure 1.
Hemodynamic and renal responses (maximum effect versus baseline) to acute (captopril, irbesartan, or aliskiren; open symbols) and chronic (irbesartan or aliskiren; closed symbols) RAS blockade in diabetic patients. Data are mean±SEM of n=21–43. #P<0.05 vs. t=0, *P<0.05 vs. acute application.
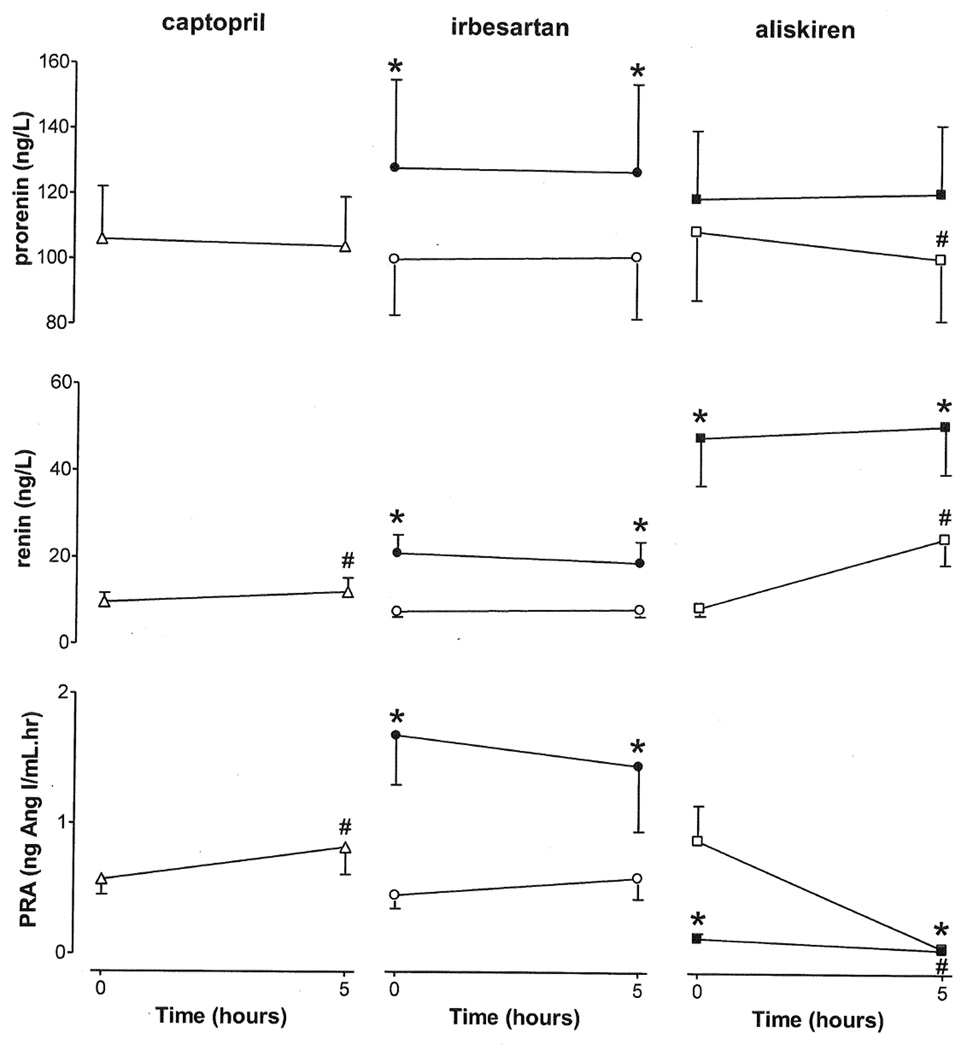
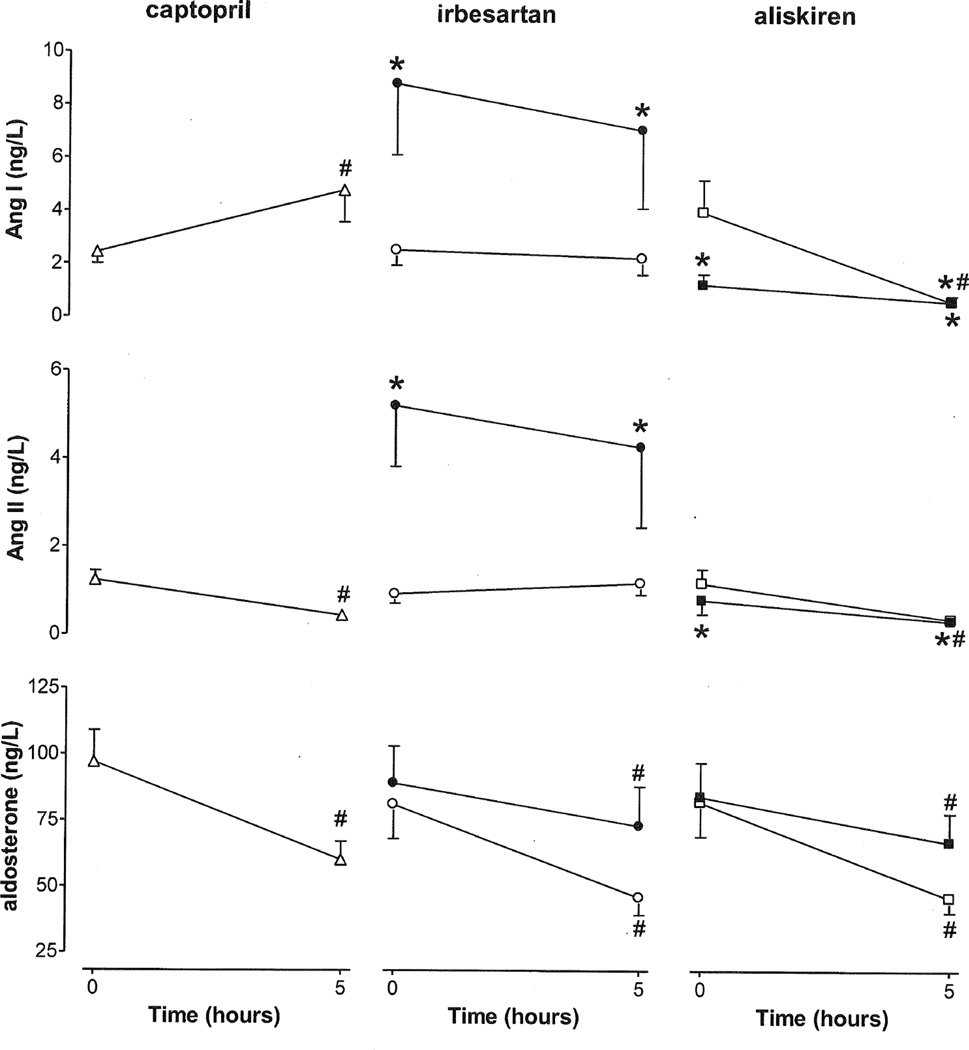
Plasma prorenin increased upon chronic irbesartan treatment only (Figure 2), and decreased upon acute exposure to aliskiren. Plasma renin increased acutely following captopril and aliskiren, but not following acute exposure to irbesartan. Plasma renin increased even further following chronic aliskiren exposure, and also increased following chronic treatment with irbesartan. However, plasma renin did not change on the 14th treatment day after either aliskiren or irbesartan application. PRA followed the renin pattern in the case of captopril and irbesartan, whereas in the case of aliskiren, PRA dropped dramatically, both acutely and on the 14th treatment day. Plasma Ang I rose, and plasma Ang II fell after acute exposure to captopril. Plasma Ang I and II fell acutely in response to aliskiren and fell again to near zero after dosing on day 14 of aliskiren (Figure 3). Conversely, Ang I and II levels did not change acutely in response to irbesartan, and increased significantly after chronic treatment. Application of irbesartan on the 14th treatment day did not change either plasma Ang I or Ang II. Serum aldosterone fell acutely with all 3 drugs. Its fall to irbesartan and aliskiren after two weeks was more modest than that on the first treatment day.
Figure 2.
Responses of prorenin, renin and PRA to acute (captopril, irbesartan, or aliskiren; open symbols) and chronic (irbesartan or aliskiren; closed symbols) RAS blockade in diabetic patients. Data are mean±SEM of n=21–43. #P<0.05 vs. t=0, *P<0.05 vs. acute application.
Figure 3.
Responses of Ang I, Ang II and aldosterone to acute (captopril, irbesartan, or aliskiren; open symbols) and chronic (irbesartan or aliskiren; closed symbols) RAS blockade in diabetic patients. Data are mean±SEM of n=21–43. #P<0.05 vs. t=0, *P<0.05 vs. acute application.
Discussion
The control of renal perfusion and function in the patient with diabetes mellitus is incompletely understood. Renin release from the kidney appears to be suppressed, although its local intrarenal action has a large effect on perfusion and function [1–4]. Prorenin is elevated in many patients. The 3 types of RAS blockers have large and divergent effects on RAS parameters. This study was undertaken in the hope that the response to blockade at different levels would provide insight into the underlying renal control mechanism in the patient with diabetes mellitus.
Pharmacologic interruption of the RAS is complicated. In the case of ACE inhibitors, the response in part reflects a reduction in Ang II formation, but may well also include elements of bradykinin and nitric oxide pharmacology. In the case of ARBs, the accompanying rise in Ang II may result in Ang II type 2 (AT2) receptor stimulation, in the vessel wall as well as in the kidney [19–21]. Direct renin inhibition is, to date, pharmacologically clean, but we know very little about the local consequences of blockade at this level.
Previous studies have indicated that captopril induces its maximal RPF effect at a dose of 25 mg [13], and that irbesartan induced a maximal response of the renal blood supply at a dose of 300 mg [14]. The maximal RPF response to aliskiren in normal subjects we reported was substantially larger than that to captopril and was achieved with a 600 mg dose [12], while maximal proteinuria reduction in type 2 diabetes patients occurred at 450 mg aliskiren [22]. We anticipated from data in our earlier study [12] that a 300 mg aliskiren dose would come close to inducing a response identical to that of captopril and irbesartan: we did, indeed, achieve an essentially identical acute renal (RPF, GFR) and blood pressure response to the three agents, facilitating comparison of the potentially responsible hormones. In addition, the acute effects of the 3 drugs at the level of the adrenal (resulting in an aldosterone reduction of 40–50%) were also identical.
Azizi et al. have argued that renin release in response to a pharmacologic antagonist provides an index of the effect of the antagonist on Ang II at the level of the renal juxtaglomerular AT1 receptor [8]. In this study our underlying hypothesis was that the renovascular response to blockade would provide a similar index. Surprisingly however, although comparable significant increases in RPF occurred with all 3 RAS blockers, this increase did not correlate at all with the rise in renin. The largest renin rise occurred with aliskiren. A more modest rise occurred following captopril, and no change at all was observed following acute exposure to irbesartan. Renin is the only RAS parameter that should consistently rise during RAS blocker treatment irrespective of the level at which blockade occurs - all other RAS parameters (PRA, Ang I and Ang II) will change in opposite directions. Indeed, PRA and Ang I rose with captopril, decreased with aliskiren and did not change with irbesartan, and Ang II decreased with captopril and aliskiren, while it was unaltered with irbesartan. Prorenin did not increase at all after acute blockade.
We next evaluated the chronic effect of RAS blockade, continuing treatment at the level of either renin or the AT1 receptor. The aliskiren-induced fall in PRA, Ang I and Ang II on first exposure was well sustained for two weeks, thus arguing against the concept of a massive renin rise overcoming the effect of renin inhibition [23–24]. Nevertheless, renin did increase further, reaching levels that were double those after acute exposure. It should be kept in mind that a substantial part of this ‘renin’ increase (up to 40% or more) represents prorenin bound to aliskiren, both acutely and chronically [17]. The underlying mechanism is that aliskiren binds to prorenin, thereby keeping prorenin in an ‘open’ renin-like conformation, allowing prorenin to be recognized as renin in a renin immunoradiometric assay (like the Cisbio assay applied here). This aliskiren-to-prorenin binding simultaneously explains why prorenin decreased following acute exposure to aliskiren and why it did not rise following chronic aliskiren treatment. The latter usually occurs after chronic RAS blockade [5,25], like in the present study after chronic treatment with irbesartan. Such chronic AT1 receptor blocker treatment also increased, as expected, renin, PRA, Ang I and Ang II. The rise in renin was more modest than that observed after aliskiren. However, when taking into consideration the contribution of prorenin to the rise of renin after aliskiren, the renin rise after both drugs would have been comparable. Therefore, at least the long-term change in renin does reflect the equi-effectiveness of the two types of drugs at the level of the renal juxtaglomerular AT1 receptors.
Summarizing, plasma renin rises acutely in diabetics following blockade of Ang II formation with either captopril or aliskiren but not following blockade of AT1 receptors with irbesartan, although all three types of blockade did result in identical acute hemodynamic, renal and adrenal responses. The blunted acute renin response to irbesartan in diabetics has been noted before [1]. A further rise in plasma renin occurred following chronic treatment with either aliskiren or irbesartan, so that at least the chronic changes in renin did parallel the acute responses to RAS blockade, in agreement with the proposal by Azizi et al. [8]. This suggests that in diabetics a higher degree of AT1 receptor blockade needs to be accomplished at the level of the juxtaglomerular cells to affect renin release, which apparently only occurs upon prolonged treatment. This relative resistance points towards a high AT1 receptor receptor occupancy due to exceptionally high Ang II levels at that site, which can only be corrected acutely by drugs that suppress Ang II generation. However, when the RAS had been substantially upregulated following chronic RAS blocker treatment, aliskiren application on day 14 also no longer affected renin release, although it still induced substantial effects on blood pressure, RPF and GFR (comparable to the effects on day 1 of treatment). Its suppressing effects on aldosterone on day 14 were reduced by ~50%. Although the latter confirms that aldosterone release has become more Ang II-independent upon prolonged RAS blockade ‘aldosterone escape’, the lack of effect on renin release suggests that now inhibition of Ang II generation is also insufficient to affect renin release. This is not due to a lack of renin in the kidney, since renin levels in humans may easily rise 100-fold [26]. Clearly therefore, at least in diabetics, renin release is relatively resistant to RAS blockade. Assuming that this reflects a high degree of AT1 receptor occupancy, e.g, as a consequence of an activated renal RAS, it appears that diabetics may require higher doses to fully block all renal effects of Ang II. Indeed, several trials have already indicated that the degree of RAS blockade required to treat diabetic nephropathy (i.e., to slow down or regress proteinuria) are substantially higher than those needed to induce hemodynamic effects [27–29]. Importantly, such high doses are not required for all renal effects of Ang II, since the effects on RPF and GFR occurred acutely and repetitively with all RAS blockers to the same degree independently of changes in circulating renin. Clearly therefore, regional differences in Ang II levels exist in the diabetic kidney. In fact, the existence of such regional differences within the kidney is well-accepted [30–32]. The long-term rise in renin rather than the acute changes in RPF and GFR now may provide the best indication of the degree of blockade at the sites where the highest renal Ang II levels occur. In agreement with this concept, in patients with Type 2 diabetes, the reduction in albuminuria during RAS blocker treatment correlated highly significantly with the rise in immunoreactive renin, i.e., the larger the rise in renin, the larger the reduction in urinary albumin excretion rate [27].
Given the elevated prorenin levels in diabetes, particularly when nephropathy is present, one possibility is that this increased renal Ang II production, at the site of the juxtaglomerular cell and elsewhere in the kidney, involves prorenin, e.g. via its activation through binding to the recently discovered prorenin receptor [18,33]. If so, the high plasma prorenin levels might be taken as a reflection of the activation of the renal RAS, and patients with high prorenin levels would then require higher RAS blocker doses. To answer this question, additional studies are required that involve patient treatment on the basis of their prorenin levels. If prorenin truly contributes to renal Ang II levels, renin inhibitors, being capable of blocking prorenin-dependent Ang I generation [18], seem the most appropriate drugs to block the renal RAS. The ongoing ALTITUDE trial, aimed at determining whether aliskiren on top of conventional RAS blockade reduces cardiovascular and renal morbidity and mortality, will address this issue [34].
Acknowledgements
All collection, analysis and presentation of data were performed by the scientific investigators at Brigham and Women’s Hospital, University Hospital Lausanne, and Erasmus MC. The Trial (NCT00660309) was registered on Clinicaltrials.gov by the Sponsor, Novartis Pharmaceuticals. The project described was supported by Grant Number 1 UL 1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Grants and Other Support/Disclosures: Drs. Moukarbel, Nussberger, Barkoudah, Danser, and Hollenberg received research funding from Novartis Pharmaceuticals, who also supplied Aliskiren.
Footnotes
Conflicts of Interest: There are no potential conflicts of interest.
References
- 1.Price DA, Porter LE, Gordon M, Fisher ND, De'Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 2.Lansang MC, Price DA, Laffel LM, Osei SY, Fisher ND, Erani D, Hollenberg NK. Renal vascular responses to captopril and to candesartan in patients with type 1 diabetes mellitus. Kidney Int. 2001;59:1432–1438. doi: 10.1046/j.1523-1755.2001.0590041432.x. [DOI] [PubMed] [Google Scholar]
- 3.De'Oliveira JM, Price DA, Fisher ND, Allan DR, McKnight JA, Williams GH, Hollenberg NK. Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int. 1997;52:771–777. doi: 10.1038/ki.1997.394. [DOI] [PubMed] [Google Scholar]
- 4.Hollenberg NK, Price DA, Fisher ND, Lansang MC, Perkins B, Gordon MS, Williams GH, Laffel LM. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003;63:172–178. doi: 10.1046/j.1523-1755.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 5.Danser AHJ, Derkx FHM, Schalekamp MADH, Hense HW, Riegger GAJ, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16:853–862. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr066. in press. [DOI] [PubMed] [Google Scholar]
- 7.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 8.Azizi M, Bissery A, Lamarre-Cliche M, Ménard J. Integrating drug pharmacokinetics for phenotyping individual renin response to angiotensin II blockade in humans. Hypertension. 2004;43:785–790. doi: 10.1161/01.HYP.0000125698.00128.64. [DOI] [PubMed] [Google Scholar]
- 9.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of micro vascular complications. N Engl J Med. 1985;312:1412–1417. doi: 10.1056/NEJM198505303122202. [DOI] [PubMed] [Google Scholar]
- 10.Deinum J, Ronn B, Mathiesen E, Derkx FHM, Hop WC, Schalekamp MADH. Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999;42:1006–1010. doi: 10.1007/s001250051260. [DOI] [PubMed] [Google Scholar]
- 11.Danser AHJ, van den Dorpel MA, Deinum J, Derkx FHM, Franken AAM, Peperkamp E, de Jong PTVM, Schalekamp MADH. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68:160–167. doi: 10.1210/jcem-68-1-160. [DOI] [PubMed] [Google Scholar]
- 12.Fisher NDL, Danser AHJ, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117:3199–3205. doi: 10.1161/CIRCULATIONAHA.108.767202. [DOI] [PubMed] [Google Scholar]
- 13.Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–392. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- 14.Price DA, Fisher ND, Lansang MC, Stevanovic R, Williams GH, Hollenberg NK. Renal perfusion in blacks: alterations caused by insuppressibility of intrarenal renin with salt. Hypertension. 2002;40:186–189. doi: 10.1161/01.hyp.0000024349.85680.87. [DOI] [PubMed] [Google Scholar]
- 15.Nasjletti A, Colina-Chourio J, McGiff JC. Disappearance of bradykinin in the renal circulation of dogs. Effects of kininase inhibition. Circ Res. 1975;37:59–65. doi: 10.1161/01.res.37.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Fisher ND, Allan D, Kifor I, Gaboury CL, Williams GH, Moore TJ, Hollenberg NK. Responses to converting enzyme and renin inhibition. Role of angiotensin II in humans. Hypertension. 1994;23:44–51. doi: 10.1161/01.hyp.23.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Krop M, Garrelds IM, de Bruin RJA, van Gool JMG, Fisher NDL, Hollenberg NK, Danser AHJ. Aliskiren accumulates in renin secretory granules and binds plasma prorenin. Hypertension. 2008;52:1076–1083. doi: 10.1161/HYPERTENSIONAHA.108.123042. [DOI] [PubMed] [Google Scholar]
- 18.Batenburg WW, de Bruin RJ, van Gool JM, Muller DN, Bader M, Nguyen G, Danser AH. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1151–1157. doi: 10.1161/ATVBAHA.108.164210. [DOI] [PubMed] [Google Scholar]
- 19.Schuijt MP, Basdew M, van Veghel R, de Vries R, Saxena PR, Schoemaker RG, Danser AHJ. AT2 receptor-mediated vasodilation in the heart: effect of myocardial infarction. Am J Physiol. 2001;281:H2590–H2596. doi: 10.1152/ajpheart.2001.281.6.H2590. [DOI] [PubMed] [Google Scholar]
- 20.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Esch JHM, Schuijt MP, Sayed J, Choudry Y, Walther T, Danser AHJ. AT2 receptor-mediated vasodilation in the mouse heart depends on AT1A receptor activation. Br J Pharmacol. 2006;148:452–458. doi: 10.1038/sj.bjp.0706762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, Danser AH, Boomsma F, Frandsen E, Parving HH. Optimal antiproteinuric dose of aliskiren in type 2 diabetes mellitus: a randomised crossover trial. Diabetologia. 2010;53:1576–1580. doi: 10.1007/s00125-010-1789-6. [DOI] [PubMed] [Google Scholar]
- 23.Sealey JE, Laragh JH. Aliskiren, the first renin inhibitor for treating hypertension: reactive renin secretion may limit its effectiveness. Am J Hypertens. 2007;20:587–597. doi: 10.1016/j.amjhyper.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Danser AHJ, Charney A, Feldman DL, Nussberger J, Fisher N, Hollenberg N. The renin rise with aliskiren: it's simply stoichiometry. Hypertension. 2008;51:e27–e28. doi: 10.1161/HYPERTENSIONAHA.108.109967. [DOI] [PubMed] [Google Scholar]
- 25.Schalekamp MADH, Derkx FHM, Deinum J, Danser AHJ. Newly developed renin and prorenin assays and the clinical evaluation of renin inhibitors. J Hypertens. 2008;26:928–937. doi: 10.1097/HJH.0b013e3282f6a671. [DOI] [PubMed] [Google Scholar]
- 26.Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AHJ. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J. 2009;30:805–812. doi: 10.1093/eurheartj/ehp012. [DOI] [PubMed] [Google Scholar]
- 27.Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, Danser AH, Boomsma F, Frandsen E, Parving HH. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care. 2009;32:1873–1879. doi: 10.2337/dc09-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 29.Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care. 2003;26:2268–2274. doi: 10.2337/diacare.26.8.2268. [DOI] [PubMed] [Google Scholar]
- 30.Schalekamp MADH, Danser AHJ. Angiotensin II production and distribution in the kidney: I. A kinetic model. Kidney Int. 2006;69:1543–1552. doi: 10.1038/sj.ki.5000303. [DOI] [PubMed] [Google Scholar]
- 31.Schalekamp MADH, Danser AHJ. Angiotensin II production and distribution in the kidney: II. Model-based analysis of experimental data. Kidney Int. 2006;69:1553–1557. doi: 10.1038/sj.ki.5000305. [DOI] [PubMed] [Google Scholar]
- 32.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJA, Burcklé C, Müller DN, Bader M, Nguyen G, Danser AHJ. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 34.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, Armbrecht J, Pfeffer MA. Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant. 2009;24:1663–1671. doi: 10.1093/ndt/gfn721. [DOI] [PubMed] [Google Scholar]