Abstract
Pulmonary arterial hypertension (PAH) is a rare but debilitating disease, which if left untreated rapidly progresses to right ventricular failure and eventually death. In the quest to understand the pathogenesis of this disease differences in the profile, expression and action of vasoactive substances released by the endothelium have been identified in patients with PAH. Of these, endothelin-1 (ET-1) is of particular interest since it is known to be an extremely powerful vasoconstrictor and also involved in vascular remodelling. Identification of ET-1 as a target for pharmacological intervention has lead to the discovery of a number of compounds that can block the receptors via which ET-1 mediates its effects. This review sets out the evidence in support of a role for ET-1 in the onset and progression of the disease and reviews the data from the various clinical trials of ET-1 receptor antagonists for the treatment of PAH.
Introduction
The regulation of vascular tone in the pulmonary circulation is a complex and multifactorial process that involves the dispensability of the pulmonary vasculature, the function of the heart, concentration of oxygen in the blood and the capacity of the endothelium to release vasoactive substances. All these mechanisms combine to determine pulmonary vascular resistance and to ensure that the pulmonary circulation is maintained as a low pressure, high blood flow circuit. This prevents the passage of fluid into the interstitial space and allows the right ventricle to operate under optimal conditions. Changes in the pulmonary vascular resistance, which is defined as difference between mean pulmonary artery pressure and left atrial pressure, divided by the cardiac output, can lead to changes in the function of the lungs and eventually the right ventricle. Pulmonary arterial hypertension (PAH) is defined as a pulmonary artery pressure greater or equal to 25 mmHg at rest. 1 The increased pressure in the lung has a knock-on effect on the right ventricle, leading to right ventricular hypertrophy and eventually right heart failure.
Symptoms of the condition include shortness of breath, fatigue, a non-productive cough, angina pectoris, syncope and peripheral oedema. While this is a rare condition affecting 15-50 people per million of the population, its incidence is associated with other morbidities such as HIV (0.5% of patients), systemic sclerosis (7–12% of patients), sickle cell anaemia (2–3.75% of patients) mixed connective tissue disease (10–45% of patients) and systemic lupus erythematosus (1–14% of patients). 2–9 Despite the apparent rareness of the condition, PAH has been classified by the World Health Organistaion (WHO) into 5 distinct categories based on the current understanding of the disease (Table 1). 1
Table 1.
Clinical Classification of Pulmonary Hypertension. (ALK1, activin receptor-like kinase type 1; BMPR, bone morphogenetic protein receptor type 2; HIV, human immunodeficiency virus) (Dana Point, 2008). 1
| Group 1 Pulmonary arterial hypertension (PAH) | |
| 1.1 | Idiopathic PAH |
| 1.2 | Heritable |
| 1.2.1 BMPR2 | |
| 1.2.2 ALK1, endoglin (with or without hereditary hemorrhagic telangiectasia) | |
| 1.2.3 Unknown | |
| 1.3 | Drug- and toxin-induced |
| 1.4 | Associated with |
| 1.4.1 Connective tissue diseases | |
| 1.4.2 HIV infection | |
| 1.4.3 Portal hypertension | |
| 1.4.4 Congenital heart diseases | |
| 1.4.5 Schistosomiasis | |
| 1.4.6 Chronic hemolytic anemia | |
| 1.5 | Persistent pulmonary hypertension of the newborn |
| Group 1′ Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary hemangiomatosis (PCH) | |
| Group 2 Pulmonary hypertension owing to left heart disease | |
| 2.1 | Systolic dysfunction |
| 2.2 | Diastolic dysfunction |
| 2.3 | Valvular disease |
| Group 3 Pulmonary hypertension owing to lung diseases and/or hypoxia | |
| 3.1 | Chronic obstructive pulmonary disease |
| 3.2 | Interstitial lung disease |
| 3.3 | Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4 | Sleep-disordered breathing |
| 3.5 | Alveolar hypoventilation disorders |
| 3.6 | Chronic exposure to high altitude |
| 3.7 | Developmental abnormalities |
| Group 4 Chronic thromboembolic pulmonary hypertension (CTEPH) | |
| Group 5 Pulmonary hypertension with unclear multifactorial mechanisms | |
| 5.1 | Hematologic disorders: myeloproliferative disorders, splenectomy |
| 5.2 | Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis: lymphangioleiomyomatosis, neurofibromatosis, vasculitis |
| 5.3 | Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4 | Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis |
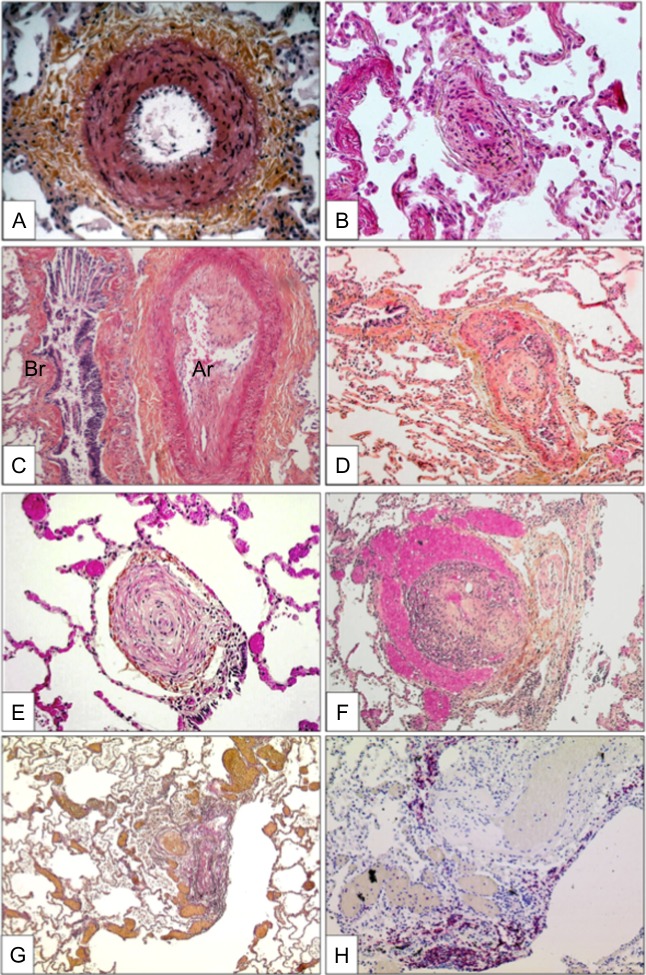
Each of these different categories of PAH have a number of common pathological changes in the pulmonary circulation, which include vasoconstriction of the pulmonary vessels, remodelling of the vessel wall, plexiform lesions characterised by intimal and medial thickening by smooth muscle cells and endothelial cell proliferation, fibrotic changes in the vessel wall, thrombus formation and regions of neovascularisation (Figure 1). 10
Figure 1.
Characteristic histlogical changes seen in the pulmaonray areriesof lungs affected with PAH showing (A) medial hypertrophy, (B) concentric non-laminar intinal fribrosis, (C) eccentric intimal fibrosis, (D) thrombotic lesions, (E) concentric laminar intimal fibrosis, (F) plexiform lesions of small sinusoid-like vessesls, (G)multiple dilation lesions associated with centrally located plexiform lesions and (H) presence of T-lymphocytes (CD-3 positive) cells in a plexifrom lesion). From Montani el al. 11
In the absence of targeted therapies the prognosis of these patients is extremely poor. However with the development of therapies targeted on the pulmonary vasculature the survival of these patients has improved. However this benefit is not seen across all the patient groups, with those who suffer with connective tissue disease or scleroderma fairing much worse than those with an idiopathic cause. 9
PAH is multifactorial disease and a number of different mechanisms have been proposed to contribute to its onset and progression. There are a number of risk factors associated with the disease which relate to the use of drugs such as aminorex, fenfluramine, dexfenfluramine, cocaine, phenylpropanolamine, St. John's Wort, chemotherapeutic agents, serotonin re-uptake inhibitors amphetamines, methamphetamines and L-tryptophan or exposure to chemicals such as toxic rapeseed oil. 11 In addition, mutations in bonemorphogenic protein receptor 2, systemic sclerosis, HIV infection, portal hypertension, congenital heart disease with left-to-right shunts, recent acute pulmonary embolism and sickle cell disease are all conditions for which PAH is a risk factor. 12 Although potential causative agents and other diseases associated with PAH represent an apparently diverse range of clinical conditions, there is general agreement that at the cellular level the disease is mediated by dysfunction of the endothelial cells that line the pulmonary vasculature.
Endothelial regulation of the pulmonary circulation
In common with all other surfaces in the body over which the blood flows, a continuous layer of endothelial cells covers the pulmonary circulation. While all these cells share the same phenotypic markers (expression of CD31 and/or von Willebrand factor) they cannot be considered as a homogeneous population of cells. Evidence exists that blood vessels of differing size and anatomical locations respond in specific ways, often determined by their different physiological roles. 13,14 A common feature between endothelial cells is however, the ability to release a range of vasoactive molecules that interact with blood elements and the underlying vascular smooth muscle. These mediators include nitric oxide (NO), prostacyclin and endothelin-1 (ET-1).
These three mediators act to regulate the diameter of the pulmonary vessel by inducing either vasodilatation (NO and prostacyclin) or vasoconstriction (ET-1). In reality a balancing act exists whereby all three mediators may be present to maintain pulmonary vascular tone at an optimal level. Increases or decreases in the amounts of any one agent produced or changes to the receptors/signaling pathway they stimulate may therefore alter the balance towards vasodilation or vasoconstriction. The Hagen-Poiseuille law states that the resistance to flow in a tube is equal to the product of the length of the tube, the viscosity of the fluid, divided by π and the fourth power of the internal radius of the tube. Thus it can be seen that a small change in the radius of the vessel wall will have a significant change to the resistance to flow. 15 Under physiological conditions, such as exercise, this allows for changes in pulmonary vascular resistance due to dilation of pulmonary vessels as well as the recruitment of previously closed capillaries.
In PAH, the profile of endothelium-derived vasoactive factors is changed, with reduced production of vasodilator agents NO and prostacyclin. 16–18 In addition to their action on vessel diameter, these agents also have an inhibitory effect on the regulation of smooth muscle cell proliferation and platelet activation. 19 Both prostacyclin and NO systems have therefore been the target of potential pharmacological interventions for the treatment of the disease. NO-releasing agents were shown to not be long-acting enough and also had the potential to stimulate a reflex tachycardia due to any effect on peripheral vessels. Agents that target the phosphodiesterase-5 enzyme, the predominant isoform of the phosphodiesterase enzymes that are responsible for the breakdown of cyclic guanosine monophosphate (cGMP), the second messenger for NO, have shown some encouraging long-term benefits. 20–22 Agents that can directly activate cGMP have also been a focus of attention and are the subject of clinical trials that are currently in progress. 23,24 Similarly, there are pharmacological agents that stimulate the IP receptors and mimic the effects of prostacyclin that have been used for the treatment of the disease. However, difficulties in the administration of these drugs (they need to be given by inhalation, subcutaneous injection or continuous intravenous infusion), their short half-life and their relative non-specific action at other receptors have limited their use and effectiveness in the treatment of PAH patients.
In contrast to trying to stimulate vasodilation in order to resolve PAH, targeting the vasoconstrictor peptide ET-1 is an alternative or additional strategy that has also been explored. This review will focus on the role of ET-1 in the lung, its biosynthesis, pharmacology, and the evidence for its participation in the pathogenesis of the disease. It will then look at the clinical evidence for efficacy of compounds that block the effects of ET-1 and are currently being used, or are in development, for the treatment of patients with PAH.
Endothelin-1
The endothelins are a family of 3 isopeptides that share a similarity in structure to the sarafotoxins, which are found in the venom of Israeli Mole Viper (Atractaspis engaddensis). Termed ET-1, ET-2 and ET-3 they are all 21 amino acid peptides with a high level of homology and similar structure 25 (Figure 2). The genes for ET-1, ET2, and ET-3 are all located on different chromosomes, with the gene for ET-1 being located on chromosome 6p. While principally found in endothelial cells, a range of other cells types have also been shown to express endothelins including cardiac myocytes, lung epithelium, glomerular kidney cells, mesangial cell, leukocytes and macrophages. 26 ET-1 is the predominant endothelin isoform that is expressed in the cardiovascular system. 27
Figure 2.
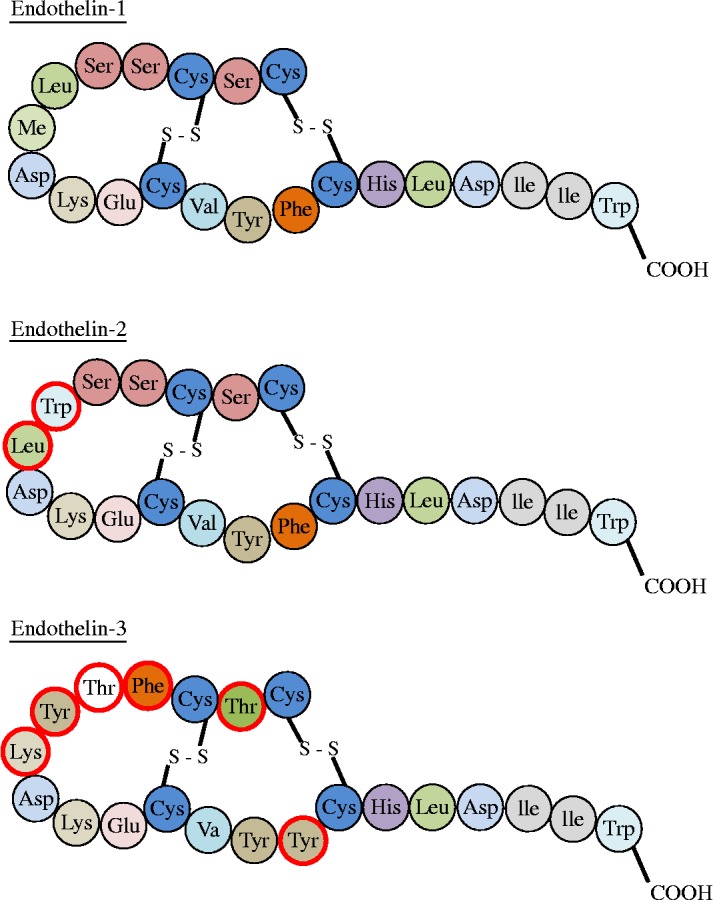
Amino-acid structure of isoforms of endothelin. Chnages in specfic aminio acids in the peptide sequence compared to ET-1 circled in red.
Biosynthesis
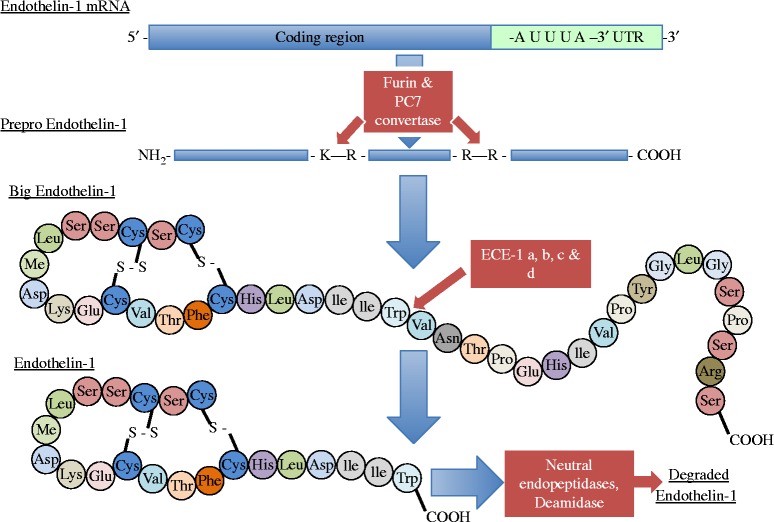
ET-1 is not stored in endothelial cells. Its release is dependent upon transcription of the gene, with the rate of transcription being responsive to stimulants and inhibitors to allow rapid changes in the amounts released. Transcription of the ET-1 gene is regulated by a number of factors including c-fos, c-jun, acute phase reactant regulatory elements and nuclear factor-1, AP-1 and GATA-2. 28–30 The gene encodes for a larger 203 amino acid precursor peptide called preproendothelin. Preproendothelin is cleaved to a smaller 38 amino acid peptide, big-ET-1 by the enzyme furin convertase. 31 Mature ET-1 is then produced by the action of a further enzyme, endothelin-converting enzyme (ECE) to produce the active 21 amino acid peptide (Figure 3). ECE exists in 3 isoforms, with ECE-1 and 2 being responsible for the formation of ET-1. ECE-1 itself exists as four additional isoforms termed a, b, c and d. 32
Figure 3.
Steps in the biosynthesis of endothelin-1. Modified from Kohan et al. 104
There are multiple factors that can affect the synthesis of ET-1 which include mechanical force (shear stress or pulsatile stretch), hypoxia, oxidised LDL cholesterol, low levels of estrogens, glucose, thrombin, other vasoconstrictors, growth factors, cytokines and adhesion molecules. 33 In contrast, NO, prostacyclin atrial natriuretic peptides and estrogen can all reduce the amounts of ET-1 released. The release of ET-1 from endothelial cells appears to occur preferentially towards the underlying vascular smooth muscle, possibly due to stoichiometric binding of ET-1 to its receptors. 34 This may explain why only low levels of the peptide can be detected in the circulation, which can act as a guide to the amounts being released in certain conditions, but is not indicative to the concentrations present at the receptors in the vessel wall.
Endothelin receptors
The range of effects mediated by ET-1 is achieved via the activation of specific G-protein coupled cell surface receptors. There are two subtypes of ET-1 receptors that have been characterised (Figure 4). Termed ETA- and ETB-receptors, these binding sites consist of single sub-units with a molecular mass in the region of 45-70 kDa and are recognised by ET-1 and when activated transduce the signal to intra-cellular signalling pathways that mediate the response of the cell. 35 However, recent evidence and proposed models of receptor signalling have suggested that the ET-receptor might exist as a heterodimer. 36 Emerging concepts such as receptor cooperation and heterodimerisation are currently being investigated to explain how the dual effects of ET-1 are mediated by its receptors. 37
Figure 4.
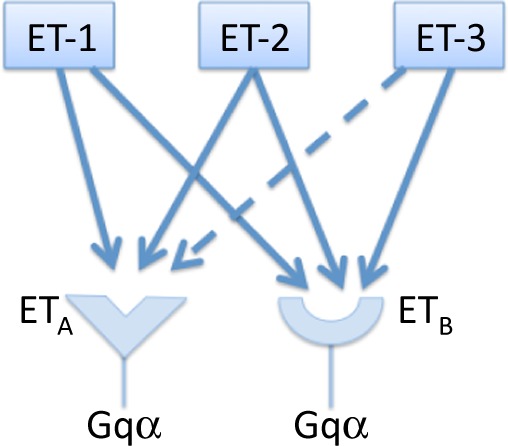
Endothelin receptor agoinsts, receptor subtypes and principal signalling pathways.
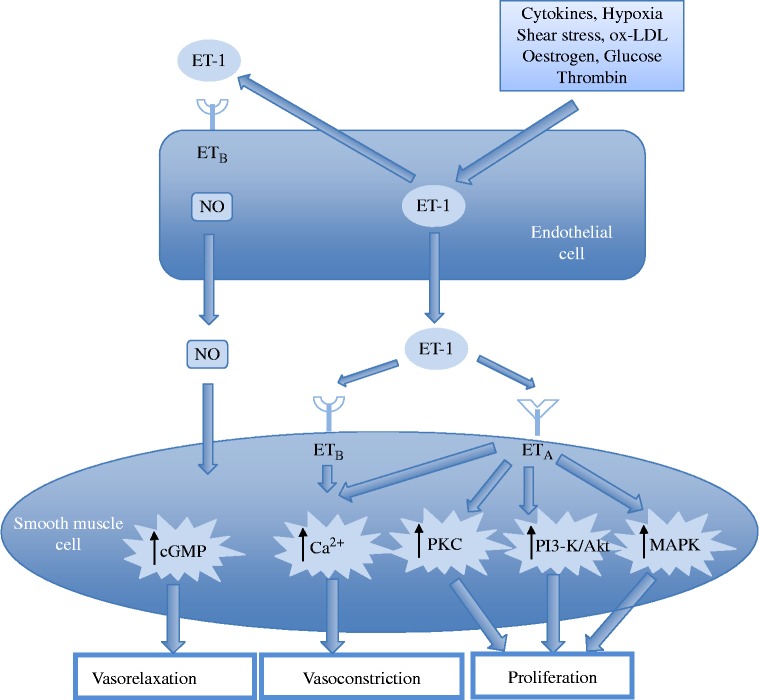
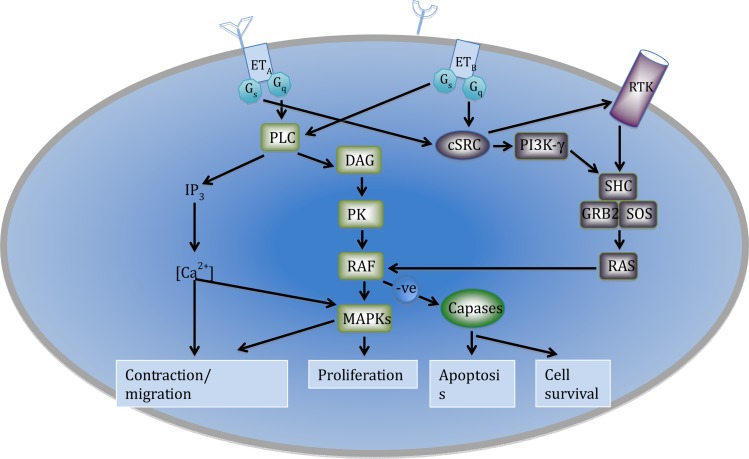
ET-receptors are found on vascular smooth muscle cells and myocytes, while ETB-receptors are also located on vascular smooth muscle cells and endothelial cells. 33 The receptors on the vascular smooth muscle cells both mediate vasoconstrictor responses via the activation of phospholipase C, an increase in inostitol triphosphate and diacylglycerol and a subsequent increase in intra-cellular calcium, leading to contraction of the cell. In contrast, the mitogenic effects of the peptide are mediated by the stimulation of protein kinase C by diacylglycerol and calcium. 38,39 Those ETB-receptors that are located on endothelial cells stimulate the release of nitric oxide and prostacyclin. This effect has a small influence on inducing relaxation of the vessel wall (Figure 5). Additional effects of ETB-receptors are linked to a reduction in ECE expression and inhibition of apoptosis. 40,41 Endothelial ETB-receptors are also believed to be involved with the clearance of ET-1 from the circulation by internalising the receptor complex once ET-1 has bound. Due to the high surface area of the pulmonary vasculature the lung therefore acts to clear ET-1 from the circulation, with an estimated removal of 50% of the circulating ET-1 as the blood passes across the lung. 42,43 This may explain why circulating levels of ET-1 are kept at very low levels (in the picomolar range) and why most of the ET-1 released by the endothelium is directed towards to the underlying smooth muscle cells. In addition to contraction of the vessel wall stimulation of ETA and ETB-receptors may also lead to activation of signalling pathways that mediate cell migration, proliferation, apoptosis or cell survival (Figure 6).
Figure 5.
Interaction between endothelial cells and vascualar smooth mucle cells mediated by endothein-1. (NO, nitric oxide; ET-1, endothelin-1; cGMP, cyclic guanosine monophosphate; CA2+, calcium ions; PKC, protein kinase C; PI3-K, phosphatidylinositol 3-kinase; AKt – Protein Kinase B; MAPK, mitogen activated protein kinase). Modified from Kohan et al. 104
Figure 6.
Signalling pathways linked to the conractile, migartory, proliverative and fate of cells mediated by ETA and ETB receptors. (PLC, phospholipase C; IP3, phosphatidylinositol; DAG, diacylglycerol; IP3 Ca2+, calcium ions; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; PLD, phospholipase D; RTK, receptor tyrosine kinase; PI3K, phosphatidylinositol 3-kinase, cSRC, cytosolic tyrosine kinase; SHC, Src homology 2 domain-containing; GRB2, Growth factor receptor-bound protein 2; and SOS, Son of sevenless proetin; RAS, rat sarcoma protein; RAF, Rapidly Accelerated Fibrosarcoma protein).
Pulmonary effects of endothelin-1
ET-1 is able to affect numerous tissues and organs throughout the body. ET-1 is highly expressed in the lung, with levels of ET-1 mRNA being at least 5 times greater than in any other organ. 44 In a similar manner to its actions in other vascular beds, ET-1 in the pulmonary circulation is able to produce an intense and protracted vasoconstriction of the pulmonary arteries and veins at very low concentrations, with its efficacy and potency being greater than 5-hydroxytryptamine, noradrenaline and the thromboxane A2 mimetic, U46619. 45,46 In addition to its effects on pulmonary vascular tone, ET-1 also has a weak mitogenic effect on pulmonary vascular smooth muscle cells and to stimulate matrix production by the vessel wall. These effects are enhanced by the presence of other growth factors such as TGF-b1 and platelet-derived growth factor. 26,47 ET-1 has also been shown to be able to stimulate the proliferation of pulmonary fibroblasts. In addition to these effects in the lung, ET-1 has been shown to be able to have a positive inotropic and chronotropic effect in the myocardium and to stimulate the production of cytokines, growth factors and matrix proteins in a variety of other tissues. 26,33,48-52
Role of endothelin-1 in pulmonary arterial hypertension
The abundance of ET-1 in the lung makes dysregulation of the ET system a prime candidate for involvement in the onset and progression of increased pulmonary vascular resistance (PVR) and pulmonary vascular remodelling. The muscular arteries seen in PAH and vascular endothelial cells have been shown to express greater levels of ET-1 and preproendothelin-1 compared to normal lungs. 53 Expression of ET-1 is also evident in the plexiform lesions that are characteristic of the disease. The levels of expression of ET-1 correlated with the increased levels of PVR, as did the severity of the structural abnormalities found in distal pulmonary arteries (measured by intravascular ultrasound). 53,54 In support of this apparent increased ability of the lung to release ET-1 is the observation that PAH patients have increased circulating levels of ET-1 and that there are increased levels of ET-1 exiting the lung compared to the levels that enter the lung. This effect is most likely due to a combination of increased production and reduced clearance. 55
Those patients who have conditions associated with PAH, such as connective tissue disease, congenital heart defects, pulmonary fibrosis (without connective tissue disease) with left-to-right shunts have elevated levels of plasma ET-1. 56–59 However, some of these patient groups elevated levels of ET-1 occurred in the absence of PAH or did not correlate with haemodynamic changes. 56,60
ET-1 also interacts with ligands at the bone morphogenetic protein receptor-2 (BMPR2). Mutations in BMPR2 have been linked to the familial form of the disease, with inactivating heterozygous mutations, including frameshifts, nonsense and missense mutations, and deletions that could truncate the protein or alter conserved regions, interfering with ligand binding to the BMPR2 or kinase activity. 61–64 The BMPR2 ligand, BMP7, and in part BMP4, were shown to regulate the balance between vasoconstrictor and vasodilator mechanisms via their ability to suppress ET-1 release from smooth muscle cells and inhibit the contractile response of the vascular wall to the peptide. Over-expression of BMPR2 in rats has been shown to protect against the development of PAH in response to hypoxia. Changes in the function of BMPR2 could either directly or indirectly influence the response of different BMPs and thereby the release of and response to ET-1.
This body of evidence identifies the ET-1 system as a possible pharmacological target for the management of patients with PAH. At the forefront of this effort is the quest to identify ET-1 receptor antagonists that have the required potency and efficacy to be effective in patients with PAH.
Endothelin receptor antagonists
The profile of ET receptors in the pulmonary vasculature presents a dilemma for devising the best strategy for pharmacological modulation of the effects of ET-1. The effects mediated by ETB-receptors on the endothelium and smooth muscle cells have opposing actions. Those of the smooth muscle, along with the ETA-receptors, contribute to the contractile and remodeling effects of the peptide, which would be advantageous to block in patients with PAH. However, the ETB-receptors on the endothelial cells mediate potentially beneficial effects, namely the release of nitric oxide and prostacyclin and possible removal of endothelin from the circulation. 40,42 The efficacy of compounds designed non-selectively to block all ET-receptors would therefore be limited by the fact they would block endothelial ETB-receptors. Conversely, a selective ETA-receptor antagonist would leave the ETB-receptors on the smooth muscle cells functional and therefore not block all the contractile/remodelling effects of ET-1 on the pulmonary vessel wall. In practice, the success of drug discovery programmes is governed by the ability to identify compounds with selectivity for either of the two receptors. There are currently two ET-receptor antagonists that are in clinical use, Bosentan and Imbrisentan, while drugs like Sitaxsentan, which initially showed favourable results have now been withdrawn due to issues relating to hepatic toxicity. Macitentan is currently in phase III clinical trials (Figure 7).
Figure 7.
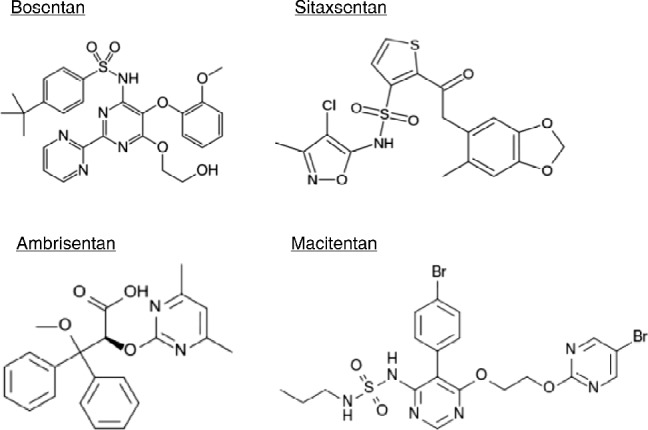
Chemical stucture of clinically used endothein receptor antagonists.
Bosentan
Bosentan (Tracleer®) is a mixed ETA/ETB- receptor antagonist and was the first ET-receptor antagonist to be used clinically. It has a higher affinity for ETA-receptors compared to that for ETB-receptors. Bosentan has a half-life of approximately 7 hours and a 50% bioavailability. 65 Therapy is accompanied with routine liver function tests. It is metabolised by the CYP2C9 and 3A4 isoenzymes of cytochromeP450 and may therefore interact with drugs such as warfarin and digoxin, although their use together in not contraindicated, closer monitoring is recommended. 4,66–68
The first clinical trial with bosentan contained 32 patients treated for 12 weeks, showed in patients with idiopathic PAH or scleroderma-associated PAH to improve performance in the 6-minute walk test by 70 m, improve the cardiac index and reduce the PVR after 8 weeks of treatment. 4 Just under half the patients (49%) improved their NYHA function from class III to class II, while the remaining 51% stayed at class III. This was then followed by the BREATHE-1 (Bosentan Randomised trail of Endothelin Antagonist Therapy) study, which studied effects for 16 weeks in 213 patients (69 in the placebo group and 144 in the bosentan group) with idiopathic PAH or connective tissue-associated PAH and was able to demonstrate a 44 minute improvement in the six-minute walking distance, the Borg dyspnea index and WHO functional class. Patients also saw an increase in the time to clinical worsening. 66 The BREATH-1 study also saw 9% of the patients exhibit liver toxicity, which was associated with the higher dose of the drug (250 mgs compared to 125 mgs). The BREATHE-2 trial studied the effects of bosentan (62.5 mgs b.i.d for 4 weeks followed by 125 mgs b.i.d for the next 12 weeks) in combination with intravenous therapy with epoprostenol (2 ng/kg/min starting dose, titrated up to a maximum dose of 12 to 16 ng/kg/min for up to 16 weeks) in 33 patients (11 in the placebo groups and 22 in the treatment group) with either idiopathic PAH or connective tissue-associated PAH. While improvements were seen in haemodynamics, exercise capacity and functional class in both groups at week 16, the combination of treatment with the two drugs showed no additional significant effect. 68 The BREATHE-3 study provided safety and efficacy data for bosentan in children with PAH treated with or without concomitant prostanoid therapy. 69 Bosentan at a target dose of between 31.25–125 mg twice daily was well tolerated and gave a reduction in mean pulmonary artery pressure of 8.0 mm Hg and a reduction in PVR of 300 dyne.s.m2/cm5. The study concluded that bosentan had a similar pharmacokinetic profile in paediatric patients with PAH as it did in adults with the disease. The BREATHE-4 and BREATHE-5 trials went on to examine the effect of bosantan in patients whose PAH is related to their infection with the human immunodeficiency virus or patients who had Eisenmenger's syndrome (PAH associated with a congenital heart defect). 70,71 The BREATHE-4 trial showed an improvement in exercise capacity, WHO functional class, quality of life and cardiopulmonary haemodynamics, while in the BREATH-5 trial, which contained 54 patients (17 in the placebo group and 37 in the bosentan group), bosentan decreased pulmonary vascular resistance and improved exercise capacity.
Trials in addition to the BREATHE series of studies have also been carried out with bosentan. These have compared the effects of bosentan with the phosphodiesterase-5 inhibitor sildenafil (SERAPH trial, which included idiopathic PAH and connective tissue disease- associated PAH patients) and the selective ETA-receptor antagonist sitaxentan (STRIDE-2 trial, which included idiopathic PAH, connective tissue disease- associated PAH patients and congenital heart disease-associated PAH patients). 72,73 These trials show that while sildenafil and sitaxsentan both show improvements with a range of clinical parameters, there was no significant difference between their effects and those of bosentan.
The major limitation of the use of bosentan is the incidence of hepatatic toxicity. In the BREATHE-1 trial there was a 14% incidence in the elevation of alanine aminotransferase and aspartate aminotransferase with the higher (250 mg) dose used. 66 It is now recommended that with clinical use of the drug, liver enzymes should be monitored on a monthly basis. Indeed, there has been a reported case of a patient developing cirrhosis of the liver after taking bosentan. 74 Other side effects also include a reduction in haemoglobin levels immediately after commencement of therapy, a drop in blood pressure with the intravenous preparation (but not the oral therapy) and peripheral oedema. 4,61,66,68,71,75–77
While the experience of using bosentan is greater than any other ET-receptor antagonist, the profile of adverse side effects is greater than that with other therapies. Thus, as experience grows we will be in a better position to determine which patients groups derived the maximum benefit from the drug and to what extent the side effects of bosentan limit the clinical benefit that can be derived from the drug. 78
Imbrisentan
Imbrisentan (Letairis®, Volibris®) is an ET-receptor antagonist that preferentially blocks the ETA-receptor. It has >4000 times greater affinity at the ETA-receptor compared to that at the ETB-receptor. 79 Imbrisentan has a half life in the region of 15 hours, allowing daily dosing to be used. 80 Unlike bosentan, it is tolerated by the liver, being metabolised via glucuronidation and it has no interaction with warfarin.
The clinical trials with imbrisentan have shown it to be effective in the treatment of patients with PAH. 81,82 The first trial to demonstrate the effect of imbrisentan was conducted on patients with idiopathic PAH or PAH associated with collagen vascular disease, anorexigen use or human immunodeficiency virus infection (HIV). The study was able to show improvements in the 6-minute walk, Borg dyspnea index and WHO functional class test for a concentration range of 1–10 mg for 12 weeks. These clinical benefits were associated with a reduction in mean pulmonary artery pressure of 5 mmHg and increase in cardiac index of 0.33/min/m2. 82 This study was followed by the ARIES series trials. The ARIES-1 study, which contained 201 patients (67 in the placebo group and 67 who received either 5 mg or 10 mg of the drug for 12 weeks) studies 2 doses of imbrisentan (5 mg and 10 mg) in patients with idiopathic PAH, connective tissue disease-associated PAH and a small number of patients with HIV and anorexigenic drug use-associated PAH. The ARIES-2 study was performed over the same time period as the ARIES-1 study and contained 192 patients (65 in the placebo group, 64 who received 2.5 mg and 63 who received 5 mg of the drug). The causes of the PAH were similar to those in the ARIES-1 study.
All treatment groups in the ARIES studies improved their 6-minute walk test by 31 m and 51 m for 5 mg and 10 mg respectively in the ARIES-1 study and 32 m and 59 m for the 2.5 mg and 5 mg respectively in the ARIES-2 study. Improvements in Borg dyspnoea score and BNP levels were seen in both trials, while NYHA functional class improved in ARIES-1, quality of life improvement and a delay in clinical worsening were seen in ARIES-2. As with other ET-receptor antagonists, peripheral oedema was also seen with imbrisentan, but to a greater degree than with bosentan. 83
Sitaxsentan
Sitaxsentan (Thelin) is a highly selective ETA-receptor antagonist with up to 6500 times greater affinity for the ETA-receptor compared to that for ETB-receptors. Like imbrisentan, it has a long half-life (between 5-7 hours). However its interaction with Cytochrome P450, it inhibits CYP2C9, lead to an interaction with drugs such as warfarin. This has been shown to lead up to a 80% reduction in the dose of warfarin needed to maintain the desired INR.
The STRIDE studies have investigated the efficacy of sitaxsentan in the treatment of PAH. The STRIDE-1 included 178 patients and the study involved giving patients with idiopathic PAH and PAH associated with connective tissue disease or congenital heart disease 100 mg or 300 mg daily for 12 weeks. Both doses of sitaxsentan improved the 6-minute walk distance, improved the NYHA functional class, cardiac index and PVR at each dose used. However there was not significant change in the peak VO2 of the patients. 73,84 The STRIDE-2 trial went on to compare 50 mg and 100 mg of sitaxsentan to placebo and patients receiving bosentan over an 18-week period. 247 patients with a similar profile of causes of PAH as studied in the STRIDE-1 trial. As before, sitaxsentan improved the 6-minute walk distance to a degree comparable with the bosentan group. However, there was not sufficient power in the study to make a direct comparison between the two drugs. Sitaxsentan also elevated hepatic transaminase (levels to over 3 times the normal range) in 3-5% of the patients. There was a similar increase in 6% of the placebo group and 11% of patients who were receiving bosentan. This was in contrast to the STRIDE-1 study where 3% and 10% of patients increased their transaminase levels after taking 100 mg or 300 mg respectively. The study concluded that 100 mg daily was the optimal dose of sitaxsentan for the treatment of PAH.
In an extension to the STRIDE-2 trial, the STRIDE-2X trial followed the patients for 52 weeks. 85 Patients receiving sitaxsentan at 100 mg had 96% overall survival and a 34% risk for a clinical worsening and a 15% risk of discontinuation due to adverse events after a year of treatment. Bosentan in comparison had an 88% overall survival, a 40% risk of a clinical worsening and 30% risk of discontinuation due to adverse events. The risk of elevated aminotransferase and/or alanine aminotransferase was 6% of the sitaxsentan group and 14% for the bosentan group. It has been suggested that based on the two STRIDE-2 trials the use of a highly selective ETA-receptor antagonist such as sitaxsentan is advantageous compared to a non-selective ETA/B receptor antagonist such as bosentan. 86
There have been a number of other STRIDE trials. The STRIDE-6 trial compared the effectiveness of sitaxsentan in a group of patients who had to discontinue treatment with bosentan due to a lack of efficacy or skin or liver problems. 87 The 6-minute walk test improved by 15% in 5 out of 15 patients taking 100 mg of sitaxsentan. This was a small study involving only 48 patients and lacked a placebo group. Other STRIDE trials, or long-term extension to trials include the STRIDE-4, STRIDE-1X, STRIDE-3, STRU and STRIDE-X trials. 88
Despite approval for marketing sitaxsentan in Europe, Canada and Australia in 2006, the FDA refused approval due to concerns about the efficacy of the drug in the STRIDE-2 trial. The STRIDE-5 trial was planned to address these issues, as were a series of trials in the USA, designed to compare the efficacy and safety of sitaxsentan with sildenafil (Clinicaltrials.gov identifiers: NCT00795639, NCT00796666 and NCT00796510). However, in 2010 the manufacturer, Pfizer, due to 2 cases of idiosyncratic, fatal hepatic failure associated with sitaxsentan use, terminated these trials. 89 As a consequence the drug was voluntarily withdrawn from worldwide use on the 10th December 2010.
Macitentan
Macitentan (Opsumit) is a mixed ETA/ETB receptor antagonist that was developed by modifying the structure of bosentan to make sulphonamide derivatives. 90 The tert-butyl benzene sulfonamide part of the initial compound was then replaced by a series of simple alkyl sulfamide moieties (Figure 8). This yielded a series of compounds with differing efficacy at the ETA and ETB receptor. One of these compounds, macitentan, emerged has having improved tissue penetration and high affinity for both receptors. 91 Doses required to achieve the similar effect of bosentan were an order of magnitude lower for macitentan. The efficacy of the drug is contributed to by the formation of its metabolite ACT-132577, formed by oxidative depropylation, which also has antagonistic activity at ETA and ETB receptors. 91 Macitentan is slowly absorbed and has a half-life of between 6–8.5 and 14–18.5 hours depending on dose. In contrast, ACT-132577 has a half-life of approximately 48 hours, indicating that daily dosing is applicable. There was no effect of macitentan on bile salts indicating no detrimental effect on hepatic function. 92,93
Figure 8.
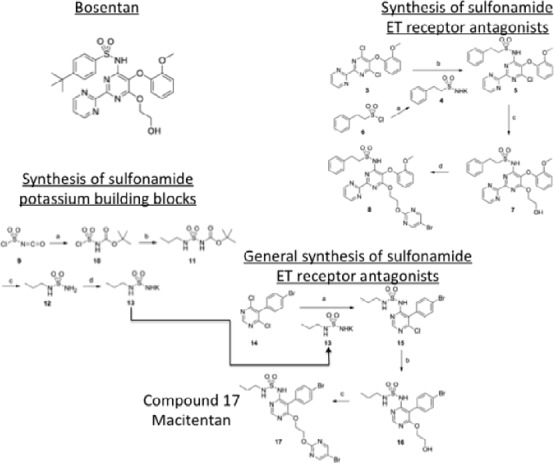
Principal steps in the chemical synthesis of macitentan from bosentan.
The clinical benefit of macitentan was recently demonstrated in the SERAPHIN trial that involved 742 patients. The placebo group of 250 patients were compared to 250 patients who received 3 mg daily and 242 patients received 10 mg daily of the drug. Patients with either idiopathic or heritable PAH or PAH associated with connective-tissue disease, congenital left-to-right shunts, HIV infection, or drug/toxin use/exposure participated in the trial. Macitentan significantly improved the morbidity and mortality of patients with PAH irrespective of whether they had previously received treatment for the disease or not. Improvements in the 6-minute walk test, WHO functional class and reductions of PVR were seen for both concentrations of macitentan. A number of patients withdrew from the trial due to adverse effects that included worsening of PAH, upper respiratory tract infection, peripheral oedema and right ventricular failure. Compared to patients in the placebo group, higher percentages of patients in the two macitentan groups had nasopharyngitis, headache and anaemia. There was no significant incidence in elevations of liver enzymes in any of the three groups. The SERAPHIN trial is discussed in greater detail by Karim Said elsewhere in this issue.
Macitentan is now approved for use in the treatment of PAH in the USA and Canada and it has received a positive opinion from regulatory authorities in Europe.
Combination therapy with ET-receptor antagonists
In addition to ET-1 receptor blockade, there are a number of other established and new therapies used for the treatment of PAH. These include prostacyclin analogues (epoprostenol, treprostinil, iloprost, beraprost), phosphodiesterase-5 (PDE-5) inhibitors (sildenafil, tadalafil, vardenafil) and more recently activators of cGMP (Riociguat). 94
There have been several small studies that have specifically examined the benefits of combinations of some of these agents. These studies have shown that combining bosentan with sildenafil is safe and effective in patients with PAH and that the beneficial effects of sildenafil are maintained despite the reduced bioavailability of the PDE-5 inhibitor caused by bosentan. 95,96 Combining bosentan with prostacyclin analogues was also shown to be safe and effective, with additional improvements seen with bosentan when added to poprostenol or treprostinil therapy. 97,98 One case report showed recovery over a 6-month period of a woman suffering from progressive right heart failure and severe PAH after treatment was commenced with a combination of bosentan, tadalafil, and beraprost. 99 However, more recently the FREEDOM-C trial, which contained 350 patients studied over a 16-week period, compared the addition of oral treprostinil to patients that were stable on background ET-receptor antagonist and/or a PDE-5 inhibitor. Addition of treprostinil therapy gave no additional benefit in the 6-minute walk test or Borg dyspnea score. 100 The FREEDOM-C study used the same profile of combination therapy as reported for the FREEDOM-C trial, but used a differing dosing regime for treprostinil since it had been shown that the original dosing regime for triprostinil to be sub-optimal. 101 While there was no change in the profile of adverse events, the combination therapy failed to yield any further additional benefits. 100 It was suggested that this may have been due to the relatively short duration of the study (16 weeks) and that longer-term studies are warranted.
Future directions
The data from the clinical trials with ET-receptor antagonists and clinical practice has shown that blocking the effects of ET-1 are beneficial in the treatment of patients with PAH. While the effects of highly selective ETA-receptor antagonists, such as sitaxsentan, are limited by hepatic toxicity, there appears to be no obvious advantage in selectively blocking one receptor subtype compared to the non-selective actions of drugs like bosentan and macitentan. The structure/activity relationship studies made during the development of macitentan represent the most effective way forward in the development of additional compounds with high affinity at ET receptors and potentially and less deleterious side effects. 90
In identifying the ET system as a therapeutic target in PAH, attempts would be made to assess the efficacy of other targets of pharmacological intervention. In a similar manner to the way angiotensin receptor blockers and angiotensin converting enzyme inhibitors are used to modulate the actions of angiotensin II, inhibitors of ECE represent potential pharmacological tools to limit the effects of ET-1. Compounds such as SLV-306 (daglutril), which inhibit ECE and neutral endopeptidase, has been shown to reduce circulating ET-1 levels in healthy volunteers, reduce systolic blood pressure and reduce pulmonary and right atrial pressures in patients with congestive heart failure. 102,103 However this latter study failed to show a true dose-response relationship for the drug. As newer ECE inhibitors are developed, time will tell if this represents a viable pharmacological strategy for the treatment of PAH that will rival existing drugs.
References
- 1.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2009;54(1 Suppl):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. The European respiratory journal. 2007;30(1):104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. American journal of respiratory and critical care medicine. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 4.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 5.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, de Groote P, Humbert M. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis and rheumatism. 2005;52(12):3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 6.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Annals of the rheumatic diseases. 2003;62(11):1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado RF, Gladwin MT. Pulmonary hypertension in hemolytic disorders: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):30S–38S. doi: 10.1378/chest.09-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. The European respiratory journal. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ. Pulmonary hypertension: past, present and future. Annals of thoracic medicine. 2008;3(1):1–4. doi: 10.4103/1817-1737.37832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clinics in chest medicine. 2007;28(1):23–42, vii. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O. Pulmonary arterial hypertension. Orphanet journal of rare diseases. 2013;8(1):97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Journal of the American College of Cardiology. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Luu TN, Chester AH, O'Neil GS, Tadjkarimi S, Yacoub MH. Effects of vasoactive neuropeptides on human saphenous vein. British heart journal. 1992;67(6):474–477. doi: 10.1136/hrt.67.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzimiri N, Chester AH, Allen SP, Duran C, Yacoub MH. Vascular reactivity of arterial coronary artery bypass grafts – implications for their performance. Clinical cardiology. 1996;19(3):165–171. doi: 10.1002/clc.4960190307. [DOI] [PubMed] [Google Scholar]
- 15.Naeije R, Chesler N. Pulmonary circulation at exercise. Comprehensive Physiology. 2012;2(1):711–741. doi: 10.1002/cphy.c100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 17.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. The New England journal of medicine. 1992;327(2):70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 18.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. American journal of respiratory and critical care medicine. 1999;159(6):1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Experimental physiology. 2008;93(1):141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 20.Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Annals of internal medicine. 2002;136(7):515–522. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G, Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. The New England journal of medicine. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 22.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 23.Fraisse A, Wessel DL. Acute pulmonary hypertension in infants and children: cGMP-related drugs. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11(2 Suppl):S37–S40. doi: 10.1097/PCC.0b013e3181c8e6e9. [DOI] [PubMed] [Google Scholar]
- 24.Mathew R. Pulmonary hypertension: current therapy and future prospects. Cardiovascular & hematological agents in medicinal chemistry. 2011;9(3):165–182. doi: 10.2174/187152511797037501. [DOI] [PubMed] [Google Scholar]
- 25.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102(19):2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 27.Masaki T. The discovery of endothelins. Cardiovascular research. 1998;39(3):530–533. doi: 10.1016/s0008-6363(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 28.Benatti L, Fabbrini MS, Patrono C. Regulation of endothelin-1 biosynthesis. Annals of the New York Academy of Sciences. 1994;714:109–121. doi: 10.1111/j.1749-6632.1994.tb12035.x. [DOI] [PubMed] [Google Scholar]
- 29.Dorfman DM, Wilson DB, Bruns GA, Orkin SH. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. The Journal of biological chemistry. 1992;267(2):1279–1285. [PubMed] [Google Scholar]
- 30.Morey AK, Razandi M, Pedram A, Hu RM, Prins BA, Levin ER. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. The Biochemical journal. 1998;330(Pt 3):1097–1105. doi: 10.1042/bj3301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denault JB, Claing A, D'Orléans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R. Processing of proendothelin-1 by human furin convertase. FEBS letters. 1995;362(3):276–280. doi: 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- 32.Valdenaire O, Lepailleur-Enouf D, Egidy G, Thouard A, Barret A, Vranckx R, Tougard C, Michel JB. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. European journal of biochemistry/FEBS. 1999;264(2):341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 33.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovascular research. 2004;61(2):227–237. doi: 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Frelin C, Guedin D. Why are circulating concentrations of endothelin-1 so low? Cardiovascular research. 1994;28(11):1613–1622. doi: 10.1093/cvr/28.11.1613. [DOI] [PubMed] [Google Scholar]
- 35.Simonson MS. Endothelins: multifunctional renal peptides. Physiological reviews. 1993;73(2):375–411. doi: 10.1152/physrev.1993.73.2.375. [DOI] [PubMed] [Google Scholar]
- 36.Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Experimental biology and medicine. 2006;231(6):840–846. [PubMed] [Google Scholar]
- 37.Thorin E, Clozel M. The cardiovascular physiology and pharmacology of endothelin-1. Advances in pharmacology. 2010;60:1–26. doi: 10.1016/B978-0-12-385061-4.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9(12):1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 39.Ohlstein EH, Arleth A, Bryan H, Elliott JD, Sung CP. The selective endothelin ETA receptor antagonist BQ123 antagonizes endothelin-1-mediated mitogenesis. European journal of pharmacology. 1992;225(4):347–350. doi: 10.1016/0922-4106(92)90109-9. [DOI] [PubMed] [Google Scholar]
- 40.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. The Journal of clinical investigation. 1993;91(4):1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30(5):1198–1203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- 42.Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. Journal of applied physiology. 1996;81(4):1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- 43.Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;94(7):1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto H, Suzuki N, Onda H, Fujino M. Abundance of endothelin-3 in rat intestine, pituitary gland and brain. Biochemical and biophysical research communications. 1989;164(1):74–80. doi: 10.1016/0006-291x(89)91684-7. [DOI] [PubMed] [Google Scholar]
- 45.Lal H, Williams KI, Woodward B. Chronic hypoxia differentially alters the responses of pulmonary arteries and veins to endothelin-1 and other agents. European journal of pharmacology. 1999;371(1):11–21. doi: 10.1016/s0014-2999(99)00174-0. [DOI] [PubMed] [Google Scholar]
- 46.Kemp BK, Smolich JJ, Cocks TM. Evidence for specific regional patterns of responses to different vasoconstrictors and vasodilators in sheep isolated pulmonary arteries and veins. British journal of pharmacology. 1997;121(3):441–450. doi: 10.1038/sj.bjp.0701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambers C, Roth M, Zhong J, Campregher C, Binder P, Burian B, Petkov V, Block LH. The interaction of endothelin-1 and TGF-beta1 mediates vascular cell remodeling. PloS one. 2013;8(8):e73399. doi: 10.1371/journal.pone.0073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seccia TM, Belloni AS, Kreutz R, Paul M, Nussdorfer GG, Pessina AC, Rossi GP. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. Journal of the American College of Cardiology. 2003;41(4):666–673. doi: 10.1016/s0735-1097(02)02860-7. [DOI] [PubMed] [Google Scholar]
- 49.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. The Journal of investigative dermatology. 2001;116(3):417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 50.Jozsef L, Khreiss T, Fournier A, Chan JS, Filep JG. Extracellular signal-regulated kinase plays an essential role in endothelin-1-induced homotypic adhesion of human neutrophil granulocytes. British journal of pharmacology. 2002;135(5):1167–1174. doi: 10.1038/sj.bjp.0704561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishikawa T, Yanagisawa M, Kimura S, Goto K, Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. The American journal of physiology. 1988;255(4 Pt 2):H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa T, Yanagisawa M, Kimura S, Goto K, Masaki T. Positive chronotropic effects of endothelin, a novel endothelium-derived vasoconstrictor peptide. Pflugers Archiv: European journal of physiology. 1988;413(1):108–110. doi: 10.1007/BF00581239. [DOI] [PubMed] [Google Scholar]
- 53.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. The New England journal of medicine. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 54.Bressollette E, Dupuis J, Bonan R, Doucet S, Cernacek P, Tardif JC. Intravascular ultrasound assessment of pulmonary vascular disease in patients with pulmonary hypertension. Chest. 2001;120(3):809–815. doi: 10.1378/chest.120.3.809. [DOI] [PubMed] [Google Scholar]
- 55.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Annals of internal medicine. 1991;114(6):464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 56.Filep JG, Bodolay E, Sipka S, Gyimesi E, Csipo I, Szegedi G. Plasma endothelin correlates with antiendothelial antibodies in patients with mixed connective tissue disease. Circulation. 1995;92(10):2969–2974. doi: 10.1161/01.cir.92.10.2969. [DOI] [PubMed] [Google Scholar]
- 57.Uguccioni M, Pulsatelli L, Grigolo B, Facchini A, Fasano L, Cinti C, Fabbri M, Gasbarrini G, Meliconi R. Endothelin-1 in idiopathic pulmonary fibrosis. Journal of clinical pathology. 1995;48(4):330–334. doi: 10.1136/jcp.48.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993;341(8860):1550–1554. doi: 10.1016/0140-6736(93)90694-c. [DOI] [PubMed] [Google Scholar]
- 59.Jia B, Zhang S, Chen Z, Li Z, Li X, Hui W, Ye M. Plasma endothelin 1 concentrations in children with congenital heart defects. Minerva pediatrica. 1998;50(4):99–103. [PubMed] [Google Scholar]
- 60.Gorenflo M, Bettendorf M, Brockmeier K, Ulmer HE. Pulmonary vasoreactivity and vasoactive mediators in children with pulmonary hypertension. Zeitschrift fur Kardiologie. 2000;89(11):1000–1008. doi: 10.1007/s003920070151. [DOI] [PubMed] [Google Scholar]
- 61.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. Journal of medical genetics. 2000;37(10):741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.International PPHC. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nature genetics. 2000;26(1):81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 63.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, 3rd, Newman J, Williams D, Galiè N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. American journal of human genetics. 2001;68(1):92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. American journal of human genetics. 2000;67(3):737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert opinion on investigational drugs. 2002;11(7):991–1002. doi: 10.1517/13543784.11.7.991. [DOI] [PubMed] [Google Scholar]
- 66.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. The New England journal of medicine. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 67.Weber C, Banken L, Birnboeck H, Schulz R. Effect of the endothelin-receptor antagonist bosentan on the pharmacokinetics and pharmacodynamics of warfarin. Journal of clinical pharmacology. 1999;39(8):847–854. doi: 10.1177/00912709922008380. [DOI] [PubMed] [Google Scholar]
- 68.Humbert M, Nunes H, Sitbon O, Parent F, Herve P, Simonneau G. Risk factors for pulmonary arterial hypertension. Clinics in chest medicine. 2001;22(3):459–475. doi: 10.1016/s0272-5231(05)70284-7. [DOI] [PubMed] [Google Scholar]
- 69.Barst RJ, Ivy D, Dingemanse J, Widlitz A, Schmitt K, Doran A, Bingaman D, Nguyen N, Gaitonde M, van Giersbergen PL. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clinical pharmacology and therapeutics. 2003;73(4):372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 70.Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, Fourme T, Humbert M, Delfraissy JF, Simonneau G. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2004;170(11):1212–1217. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 71.Galie N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, Chiossi E, Landzberg M. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114(1):48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 72.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, Westwood MA, Stefanidis A, Ng LL, Pennell DJ, Mohiaddin RH, Nihoyannopoulos P, Gibbs JS. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. American journal of respiratory and critical care medicine. 2005;171(11):1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 73.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N, STRIDE-2 Study Group Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. Journal of the American College of Cardiology. 2006;47(10):2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 74.Segal ES. Tracleer (bosentan) 2006 Available from: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM153430.pdf . [Google Scholar]
- 75.Dingemanse J, van Giersbergen PL. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clinical pharmacokinetics. 2004;43(15):1089–1115. doi: 10.2165/00003088-200443150-00003. [DOI] [PubMed] [Google Scholar]
- 76.Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacology & therapeutics. 2006;110(3):386–414. doi: 10.1016/j.pharmthera.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Denton CP, Pope JE, Peter HH, Gabrielli A, Boonstra A, van den Hoogen FH, Riemekasten G, De Vita S, Morganti A, Dölberg M, Berkani O, Guillevin L, TRacleer Use in PAH associated with Scleroderma and Connective Tissue Diseases (TRUST) Investigators Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Annals of the rheumatic diseases. 2008;67(9):1222–1228. doi: 10.1136/ard.2007.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valerio CJ, Coghlan JG. Bosentan in the treatment of pulmonary arterial hypertension with the focus on the mildly symptomatic patient. Vascular health and risk management. 2009;5:607–619. doi: 10.2147/vhrm.s4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng JW. Ambrisentan for the management of pulmonary arterial hypertension. Clinical therapeutics. 2008;30(5):825–833. doi: 10.1016/j.clinthera.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vascular health and risk management. 2007;3(1):11–22. [PMC free article] [PubMed] [Google Scholar]
- 81.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ, Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 82.Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S, Olschewski H, Rubin LJ. Ambrisentan therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology. 2005;46(3):529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 83.Opitz CF, Ewert R, Kirch W, Pittrow D. Inhibition of endothelin receptors in the treatment of pulmonary arterial hypertension: does selectivity matter? Eur Heart J. 2008;29(16):1936–1948. doi: 10.1093/eurheartj/ehn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR, STRIDE-1 Study Group Sitaxsentan therapy for pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2004;169(4):441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 85.Benza RL, Barst RJ, Galie N, Frost A, Girgis RE, Highland KB, Strange C, Black CM, Badesch DB, Rubin L, Fleming TR, Naeije R. Sitaxsentan for the treatment of pulmonary arterial hypertension: a 1-year, prospective, open-label observation of outcome and survival. Chest. 2008;134(4):775–782. doi: 10.1378/chest.07-0767. [DOI] [PubMed] [Google Scholar]
- 86.Langleben D, Cacoub P. A review of STRIDE-2 and STRIDE-2X: the case for selective endothelin receptor blockade. European journal of clinical investigation. 2009;39 Suppl 2:27–31. doi: 10.1111/j.1365-2362.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 87.Benza RL, Mehta S, Keogh A, Lawrence EC, Oudiz RJ, Barst RJ. Sitaxsentan treatment for patients with pulmonary arterial hypertension discontinuing bosentan. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2007;26(1):63–69. doi: 10.1016/j.healun.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 88.Boniface S, Reynaud-Gaubert M. Endothelin receptor antagonists – their role in pulmonary medicine. Revue des maladies respiratoires. 2011;28(8):e94–e107. doi: 10.1016/j.rmr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 89. Agency TEM Thelin (sitaxentan) to be withdrawn due to cases of unpredictable serious liver injury 2010. Available from: http://www.ema.europa.eu/ema/index.jsp?curl = pages/news_and_events/news/2010/12/news_detail_001161.jsp&mid = WC0b01ac058004d5c1 [Google Scholar]
- 90.Bolli MH, Boss C, Binkert C, Buchmann S, Bur D, Hess P, Iglarz M, Meyer S, Rein J, Rey M, Treiber A, Clozel M, Fischli W, Weller T. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-p ropylsulfamide (Macitentan), an orally active, potent dual endothelin receptor antagonist. Journal of medicinal chemistry. 2012;55(17):7849–7861. doi: 10.1021/jm3009103. [DOI] [PubMed] [Google Scholar]
- 91.Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, Weller T, Bolli MH, Boss C, Buchmann S, Capeleto B, Hess P, Qiu C, Clozel M. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. The Journal of pharmacology and experimental therapeutics. 2008;327(3):736–745. doi: 10.1124/jpet.108.142976. [DOI] [PubMed] [Google Scholar]
- 92.Sidharta PN, van Giersbergen PL, Halabi A, Dingemanse J. Macitentan: entry-into-humans study with a new endothelin receptor antagonist. European journal of clinical pharmacology. 2011;67(10):977–984. doi: 10.1007/s00228-011-1043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sidharta PN, van Giersbergen PL, Dingemanse J. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macitentan, an endothelin receptor antagonist, in an ascending multiple-dose study in healthy subjects. Journal of clinical pharmacology. 2013;53(11):1131–1138. doi: 10.1002/jcph.152. [DOI] [PubMed] [Google Scholar]
- 94.Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. European respiratory review: an official journal of the European Respiratory Society. 2012;21(126):321–327. doi: 10.1183/09059180.00004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hatano M, Yao A, Kinugawa K, Hirata Y, Nagai R. Acute effect of sildenafil is maintained in pulmonary arterial hypertension patients chronically treated with bosentan. International heart journal. 2011;52(4):233–239. doi: 10.1536/ihj.52.233. [DOI] [PubMed] [Google Scholar]
- 96.Hoeper MM, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. The European respiratory journal. 2004;24(6):1007–1010. doi: 10.1183/09031936.04.00051104. [DOI] [PubMed] [Google Scholar]
- 97.Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest. 2008;134(1):139–145. doi: 10.1378/chest.07-2111. [DOI] [PubMed] [Google Scholar]
- 98.Akagi S, Matsubara H, Miyaji K, Ikeda E, Dan K, Tokunaga N, Hisamatsu K, Munemasa M, Fujimoto Y, Ohe T. Additional effects of bosentan in patients with idiopathic pulmonary arterial hypertension already treated with high-dose epoprostenol. Circulation journal: official journal of the Japanese Circulation Society. 2008;72(7):1142–1146. doi: 10.1253/circj.72.1142. [DOI] [PubMed] [Google Scholar]
- 99.Maki H, Yao A, Inaba T, Shiga T, Hatano M, Kinugawa K, Yamashita T, Aizawa T, Nagai R. Initial and programmed combination therapy with oral drugs for severe idiopathic pulmonary arterial hypertension. International heart journal. 2011;52(5):323–326. doi: 10.1536/ihj.52.323. [DOI] [PubMed] [Google Scholar]
- 100.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K, Sigman J, Arneson C, Galiè N. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 101.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, Xu KF, Yehle D, Laliberte K, Arneson C, Rubin LJ. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127(5):624–633. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 102.Seed A, Kuc RE, Maguire JJ, Hillier C, Johnston F, Essers H, de Voogd HJ, McMurray J, Davenport AP. The dual endothelin converting enzyme/neutral endopeptidase inhibitor SLV-306 (daglutril), inhibits systemic conversion of big endothelin-1 in humans. Life sciences. 2012;91(13-14):743–748. doi: 10.1016/j.lfs.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 103.Parvanova A, van der Meer IM, Iliev I, Perna A, Gaspari F, Trevisan R, Bossi A, Remuzzi G, Benigni A, Ruggenenti P, Daglutril in Diabetic Nephropathy Study Group Effect on blood pressure of combined inhibition of endothelin-converting enzyme and neutral endopeptidase with daglutril in patients with type 2 diabetes who have albuminuria: a randomised, crossover, double-blind, placebo-controlled trial. The lancet Diabetes & endocrinology. 2013;1(1):19–27. doi: 10.1016/S2213-8587(13)70029-9. [DOI] [PubMed] [Google Scholar]
- 104.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiological reviews. 2011;91(1):1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]