Abstract
Our knowledge about land-use impacts on biodiversity and ecosystem functioning is mostly limited to single trophic levels, leaving us uncertain about whole-community biodiversity-ecosystem functioning relationships. We analyse consequences of the globally important land-use transformation from tropical forests to oil palm plantations. Species diversity, density and biomass of invertebrate communities suffer at least 45% decreases from rainforest to oil palm. Combining metabolic and food-web theory, we calculate annual energy fluxes to model impacts of land-use intensification on multitrophic ecosystem functioning. We demonstrate a 51% reduction in energy fluxes from forest to oil palm communities. Species loss clearly explains variation in energy fluxes; however, this relationship depends on land-use systems and functional feeding guilds, whereby predators are the most heavily affected. Biodiversity decline from forest to oil palm is thus accompanied by even stronger reductions in functionality, threatening to severely limit the functional resilience of communities to cope with future global changes.
 Transformation of natural ecosystems into agricultural land is usually accompanied by extensive biodiversity loss. Calculating multitrophic energy fluxes, Barnes et al. report severe reductions of biodiversity and ecosystem functioning from tropical rainforest to oil-palm plantations.
Transformation of natural ecosystems into agricultural land is usually accompanied by extensive biodiversity loss. Calculating multitrophic energy fluxes, Barnes et al. report severe reductions of biodiversity and ecosystem functioning from tropical rainforest to oil-palm plantations.
The transformation from natural ecosystems to agricultural land use and its continued intensification has led to extensive losses in biodiversity and ecosystem services1 resulting in the degradation of human well being2. The transformation of lowland tropical rainforest to oil palm (Elaeis guineensis Jacq.) plantations has gained more recent attention as an especially severe threat to tropical biodiversity3,4. In the last 25 years the total plantation area of oil palm has tripled, with current global estimates of over 15 million hectares3, making this crop one of the world’s most rapidly expanding forms of agriculture5. It is now clear that the expansion of oil palm agriculture is one of the greatest causes of deforestation6,7, and this threat appears to be increasing without respite as Indonesia, one of the world’s leaders in oil palm, makes plans to double production by 2020 (ref. 8). The rapid expansion of such large-scale land-use transformation raises questions about the impending implications for biodiversity and ecosystem functioning in the tropics.
Despite a broad consensus that biodiversity is positively correlated with ecosystem functioning in controlled experiments9,10, there are few real-world examples of such biodiversity–ecosystem functioning relationships11,12. In fact, until now there have been no studies that explore the relationship between biodiversity and ecosystem functioning in ecosystems undergoing agricultural land-use transformation to oil palm. Thus, our knowledge of this globally important land-use conversion is strongly limited. Furthermore, over the past decade there have been important advances towards multitrophic approaches in research investigating biodiversity–ecosystem functioning relationships9,13,14,15,16. Despite these advances, however, we are still substantially limited by the lack of clear approaches to quantify single measures of ecosystem functioning that can be compared among any combination of trophic levels. This has resulted in our inability to directly look at whole-community relationships between entire species assemblages and the respective functional processes carried out in these communities.
Here we use the total energy flux between functional feeding guilds as a measure of multitrophic ecosystem functioning, as many studies have suggested process rates, such as energy fluxes, to be important proxies for ecosystem functioning10,13,17. Depending on the resource pool that the energy flux comes from, these fluxes can be directly related to ecosystem services such as decomposition18,19, plant biomass production20,21 or biocontrol through predation22. These energy flux calculations are based on metabolic scaling theory23 and principles of food-web energy dynamics18. Using individual metabolic rates that are dependent on body mass, environmental temperature and phylogenetic grouping18,24, combined with resource-specific assimilation efficiencies25 and energy loss to predation18, we present this energy flux calculation as a unified measure of multitrophic ecosystem functioning (Fig. 1). Studies that incorporate diversity across trophic levels to test the relationship between biodiversity and ecosystem functioning have predominantly used only biomass as the measure of ecosystem function26. However, the metabolic activity and thus the energy-processing rates of these biomass pools can vary substantially. Integrating over body mass, phylogeny and temperature with their constraints on metabolic rates, and additionally taking into account assimilation efficiencies and loss to predation, our measure of whole-community energy flux inherently incorporates not only biomass but also other important ecosystem attributes enabling the quantification of emergent functional properties of ecosystems that would otherwise remain undetected. As such, our measure of energy flux provides a comprehensive and robust measure of multitrophic ecosystem functioning that can be utilized for modelling biodiversity–ecosystem functioning relationships for any assemblage of taxonomic groups, while incorporating multiple ecological functions.
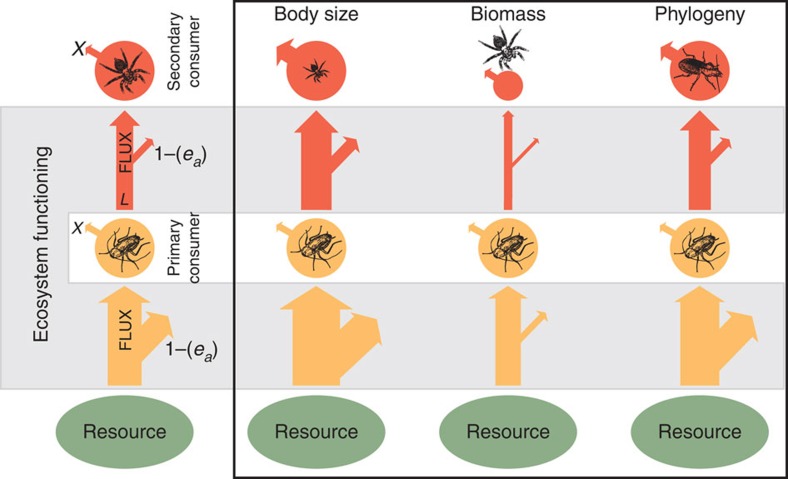
Figure 1. Energy fluxes along a conceptual food chain as a measure of multitrophic ecosystem functioning.
Energy flux between two nodes is calculated as 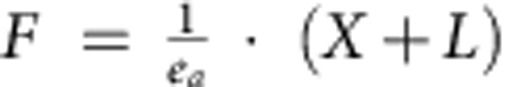 , where F is the total energy flux into the network node of a feeding guild (vertical red and yellow arrows), ea is the diet-specific assimilation efficiency (denoted by diagonal arrows arising from the flux arrows), X is the per-unit-mass metabolic demand of the feeding guild (which is nonlinearly dependent on body sizes, temperature and phylogeny) and L is the loss to predation from the node (for the yellow node, this is equal to the flux to the red secondary consumer node). Here we demonstrate three examples where changes in the mean body size (size of black animal icons), biomass (diameter of red and yellow circles) or phylogeny (black animal icons) on any trophic level (here demonstrated by the secondary consumer guild) can result in nonproportionally altered total energy flux (sum of all arrow widths in the food chain).
, where F is the total energy flux into the network node of a feeding guild (vertical red and yellow arrows), ea is the diet-specific assimilation efficiency (denoted by diagonal arrows arising from the flux arrows), X is the per-unit-mass metabolic demand of the feeding guild (which is nonlinearly dependent on body sizes, temperature and phylogeny) and L is the loss to predation from the node (for the yellow node, this is equal to the flux to the red secondary consumer node). Here we demonstrate three examples where changes in the mean body size (size of black animal icons), biomass (diameter of red and yellow circles) or phylogeny (black animal icons) on any trophic level (here demonstrated by the secondary consumer guild) can result in nonproportionally altered total energy flux (sum of all arrow widths in the food chain).
In the tropical lowland rainforests of Sumatra, Indonesia, which have been undergoing vast land-use transformation to oil palm7, we quantify the impacts of this transformation ranging from tropical secondary rainforest, jungle rubber and intensively managed rubber, to oil palm. We utilize data gathered from 32 sites in Sumatra, Indonesia, comprising 2,415 populations of 871 species. First, we investigate the biodiversity value of jungle rubber, conventional rubber and secondary forest compared with oil palm agriculture by comparing observed species richness, density and biomass of litter-associated macroinvertebrate communities across these systems. Second, as a multitrophic measure of the rate of ecosystem processes carried out by these communities, we calculate total solid fresh mass energy flux in a system by incorporating community metabolism27, resource-specific assimilation efficiencies and biomass loss to predation18 into whole-community energy flux equations (Fig. 1). This provides a quantitative measure of multitrophic ecosystem functioning, defined here as the total flux of energy from any resource pool to consumer trophic levels. In addition, this measure can be attributed to specific functional feeding guilds within communities to look for patterns in ecosystem functioning at different trophic levels. Using the energy-mass flow conversion28, we express energy flux as kilograms per hectare, per year and explore the relationship between total species diversity and energy flux, distinguishing among four transformation systems to test for land-use-dependent biodiversity–ecosystem functioning relationships. Our results demonstrate strong losses in species diversity that, in turn, predict reductions in whole-community energy fluxes. However, these reductions are strongest in oil palm systems, suggesting that land-use conversion from forest to oil palm causes disproportionally strong losses in multitrophic ecosystem functioning.
Results
Transformation to oil palm leads to biodiversity loss
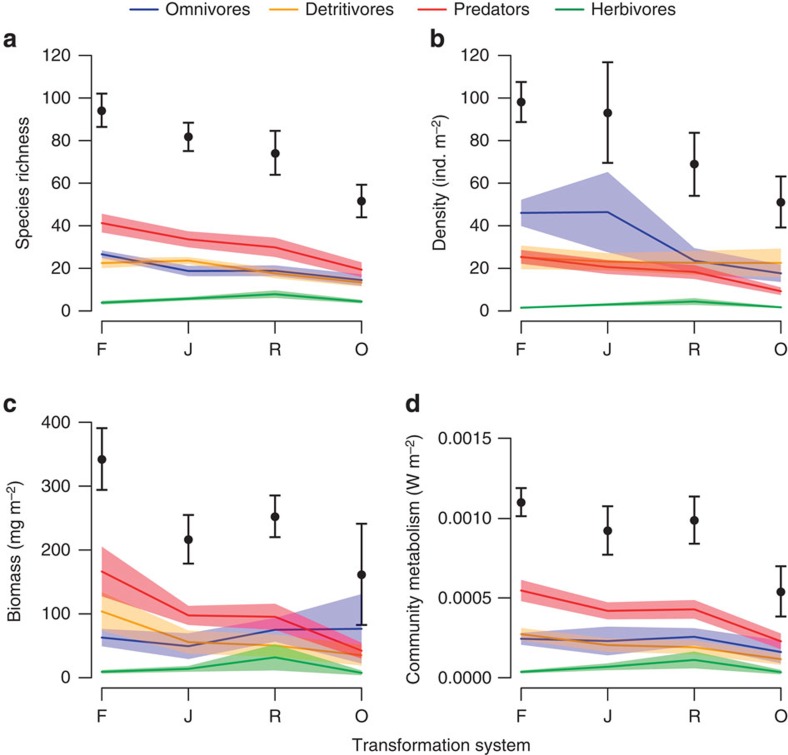
Using generalized linear mixed effects models, we show that transformation of tropical rainforest to oil palm plantations leads to severe losses in species richness (45% decline), animal density (48% decline) and biomass (52% decline; Fig. 2a–c and Supplementary Table 1), supporting previous studies suggesting that land-use transformation to oil palm poses one of the greatest threats to global biodiversity3. Beyond mere diversity effects, land-use transformation altered animal densities and biomass, threatening to not only drive species extinctions but also to eliminate vital ecological functions. The effects of land-use transformation on species richness and animal densities were additionally dependent on functional feeding guilds, with predators decreasing in species richness and density most rapidly (Fig. 2a–c and Supplementary Table 1) as could be expected for higher trophic level feeding guilds29. Such alteration of higher trophic levels is likely to have severe indirect functional impacts on other functional guilds within the trophic network30.
Figure 2. Effects of land-use transformation on macroinvertebrate communities.
The mean (±s.e., n=32) species richness (a), density (b), biomass (c) and community metabolism (d) of the total community (black points) and of each functional feeding guild (coloured lines) for the four land-use transformation systems: forest (F), jungle rubber (J), rubber (R) and oil palm (O).
Community metabolism
Summing up individual metabolic rates, we demonstrate that transformation of forest to oil palm yields a 51% decrease in community metabolism, with jungle rubber and rubber only 16% and 10% below forest levels of community metabolism, respectively. However, all systems yielded significantly higher community metabolism than oil palm (Fig. 2d and Supplementary Table 1). As such, we show that ecosystem energy processing is critically reduced in oil palm plantations. Interestingly, biomass responses to land-use transformation among feeding guilds were not clearly comparable to responses in community metabolism (Fig. 2c,d). This suggests that systematic changes in species composition, body-mass distributions (Supplementary Fig. 1) and biomass exhibited a complex interaction in determining the functional consequences of land-use transformation.
Whole-community energy fluxes and ecosystem functioning
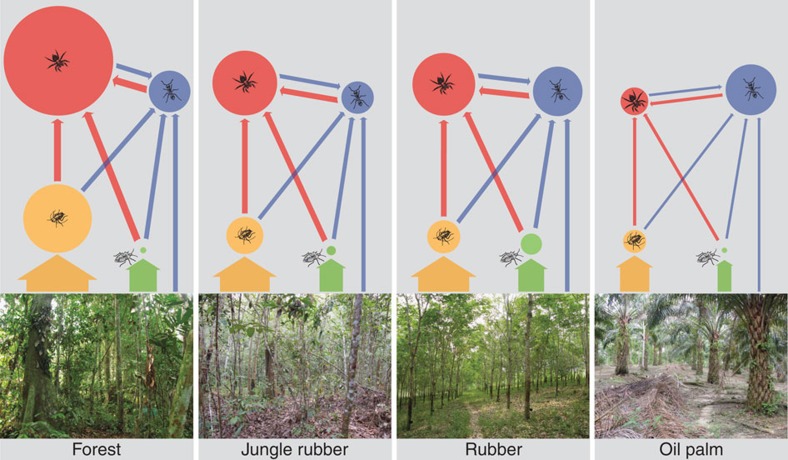
Aiming to visualize the complex interplay between community biomass dynamics and energy flux, we constructed energy networks for the four transformation systems (Fig. 3) based on total energy fluxes as a promising way to quantify multitrophic ecosystem functioning (Fig. 1). In addition to the general decreases in biomass (node sizes in Fig. 3) and energy-processing rates (arrow widths in Fig. 3), we also found a systematic shift from predator to omnivore dominance when comparing forest and oil palm systems. Specifically, we found that predator biomass in oil palm yielded only 25% of their biomass in forest (0.424 and 1.664 kg ha−1, respectively), while the predator-driven energy flux was reduced to 46% of the energy flux driven by predators in forest (30.697 and 66.816 kg ha−1 per year, respectively). In contrast, omnivore biomass in oil palm was 22% higher than in the forest (0.767 compared with 0.629 kg ha−1), while omnivore-driven energy flux in the oil palm was 47% lower than in forest communities (32.531 compared with 61.900 kg ha−1 per year; Supplementary Table 2), suggesting a considerable mismatch of biomass and energy flux, partly dependent on the trophic group in question. In our analyses, this disparity finds its explanation in varying body-mass distributions (Supplementary Fig. 1) and assimilation efficiencies that strongly modify how biomass translates into total resource assimilation rates (Fig. 1). These results suggest that biomass, alone, may be an unsuitable proxy for general ecosystem functioning in animal communities.
Figure 3. Effects of land-use transformation on community energy networks.
Energy networks displaying the relative annual energy flux (coloured arrow width weighted by calculated energy flux (kg ha−1 per year)) and biomass (coloured node diameter weighted by total biomass) among the functional feeding guilds: predators (red), omnivores (blue), detritivores (yellow) and herbivores (green). Each panel represents an energy network for one of the four land-use transformation systems.
Multitrophic biodiversity-ecosystem function relationships
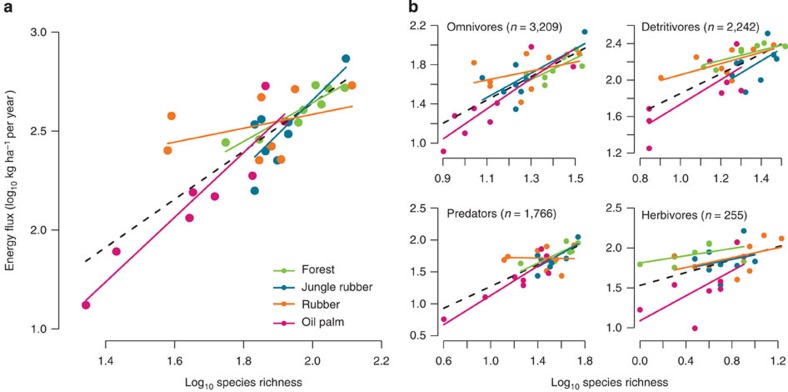
Until now, most studies investigating biodiversity–ecosystem function relationships have focused on single trophic levels31,32. We present a new approach to easily quantify multitrophic ecosystem functioning, requiring only information on body mass, phylogeny, temperature and assimilation efficiencies to overcome previous limitations in biodiversity–ecosystem functioning research. Utilizing this approach, we also investigated the relationship between species richness and ecosystem functioning, identifying a clear linear positive effect of diversity on total energy flux (Fig. 4a and Supplementary Table 3). The relationship between diversity and energy flux was dependent on land-use transformation system, whereby oil palm and jungle rubber showed the strongest decrease in energy flux per unit loss in species richness (Fig. 4a and Supplementary Table 3). Our results suggest that each loss of species in oil palm and jungle rubber therefore would be followed by proportionately higher losses in energy flux, compared with equal species losses in forest and rubber. We found the same pattern as in the overall trend for the predator group, which showed transformation system-dependent relationships between species richness and energy flux (Fig. 4b). However, for omnivores, detritivores and herbivores there was a linear effect of diversity on energy flux driven by these groups; however, this effect was independent of transformation system (Fig. 4b and Supplementary Table 3). This implies that studies focusing on single trophic levels, or even specific species, may fail to detect the alteration of ecosystem processes resulting from land-use transformation. These results call for a wider application of multitrophic approaches that not only measure one ecosystem property, such as total productivity or decomposition, but that also aim to assess whole-community ecosystem processes such as total energy flux.
Figure 4. Relationship between species richness and community energy fluxes.
Linear mixed effects models for (a) entire communities and (b) separated into functional feeding guilds. Black dashed lines denote overall model fits and coloured lines indicate different land-use transformation systems.
Discussion
Our study reflects previous findings that the transformation of forest systems to oil palm has severe impacts not only on single animal populations but also on communities as a whole. In particular, species richness and animal biomass are most significantly affected. Furthermore, jungle rubber and rubber appear to represent intermediate steps in land-use intensification. Their higher levels of biodiversity and ecosystem functioning indicate that they potentially provide higher ecological value than oil palm. As such, these rubber land-use systems could present economically viable, lower intensity land-use alternatives.
By taking a multitrophic ecosystem functioning approach we demonstrate that, at the community level, species loss leads to a direct linear decrease in ecosystem functioning. This means that any species loss will be followed by a proportionate loss in function, and this relationship becomes proportionately stronger in more intensive transformation systems such as oil palm plantations. Thus, every one of the few species in high-intensity land-use systems is functionally more important than species in low-intensity systems where functional redundancy is likely to be higher33. Without explicit consideration of multiple trophic levels, such emergent properties are likely to be overlooked. Our study demonstrates the crucial implications of tropical land-use intensification for biodiversity and ecosystem functioning across multiple trophic levels, suggesting that these globally important impacts will likely resonate beyond previously explored trophic boundaries.
Methods
Study site and sampling design
Sampling took place in the Jambi province of Sumatra, Indonesia, a region known as a hotspot for biodiversity, but that has also already undergone extensive deforestation6,34. In the second half of the last century, Sumatra’s forests have experienced vast transformation to rubber and oil palm monocultures35,36. This large-scale land-use conversion has left Sumatra with a very limited area of natural forest mainly restricted to national parks and even here, where logging has been reduced, it has not come to a complete halt37. This severe and extensive land-use transformation, that has progressed already further than in most other tropical landscapes, makes Sumatra a unique and ideal example system for studying the impacts of land-use conversion on biodiversity and ecosystem functioning.
We sampled secondary rainforest, jungle rubber, rubber and oil palm systems, replicated eight times across two landscapes (n=32; Supplementary Fig. 2). Sites were selected by first looking for landscapes in the Jambi province that still contained secondary rainforest. Second, we identified all lowland areas with little or no slope and then randomly selected two landscapes with 16 sites each. Among all of the 32 sampling sites, we maintained a minimum distance of 120 m to insure independence of the epigaeic invertebrate communities sampled. The secondary-forest regions lie within two protected areas, Bukit Duabelas National Park and Harapan Rainforest, and represent the least influenced land-use system. Jungle rubber—forest stands with a high percentage of rubber trees that are still regularly harvested—represents a low-impact agroforestry system38. Rubber and oil palm plantations serve as locally common36 high-impact monocultures. The 32 sites were carefully selected so that they were all of a similar age and from equal elevations close to the sea level. All agricultural systems (jungle rubber, rubber and oil palm) were treated and harvested by their owners with intensities typical for the respective transformation system.
Animal sampling and calculation of response variables
Animal sampling took place between early October and early November 2012. All organisms were collected based on Permit No. 51/KKH-5/TRP/2014 issued by the Indonesian Institute of Sciences and the Ministry of Forestry. In all 32 of the 50 × 50-m sites, we sampled once in each of three 5 × 5-m subplots by sieving the leaf litter from 1 m2 through a coarse sieve of 2-cm-width mesh. In all, 7,472 macroinvertebrates were hand-collected from the sieving samples and stored in 65% ethanol. Specimens were identified to morphospecies and assigned to one of four feeding guilds: omnivores, detritivores, predators and herbivores, based on morphology and literature.
As biodiversity studies always suffer from undersampling and correlation of sample size with species richness, we compared observed species richness to both extrapolated and rarefied species richness, calculated in the ‘vegan’ package in R39, to assess the accuracy of our species-sampling effort. To extrapolate sampled species richness, we used the nonparametric second-order jacknife estimator40 to calculate extrapolated species richness from the three 1-m2 subsamples at each of the 32 sites, revealing an estimated mean sampling coverage of 56% (s.d. of±2.393%) making the second-order jacknife estimator the most accurate extrapolation method40. In addition, we calculated sample-based rarefaction, whereby rarefaction curves were calculated for each of the 32 sampled sites and then cut off at the sample size of the smallest sample (40 individuals). Because of the very high attrition of data during the rarefaction procedure (a total of 6,192 out of 7,472 individuals, or 83%, were removed), the rarefied species richness yielded very little resemblance to observed species richness when comparing across transformation systems, resulting in almost no pattern of rarefied richness among transformation systems (Supplementary Fig. 3). The jacknife2-extrapolated species richness, however, was extremely closely correlated with observed species richness (Pearson’s ρ=0.993) patterns among transformation systems (Supplementary Fig. 3), suggesting that our observed species richness did in fact accurately capture realistic patterns in total species diversity across the land-use transformation systems.
For each of the 7,472 animals collected, we measured individual body length to an accuracy of 0.1 mm using stage micrometres. We then converted all measured individual body lengths to fresh body mass using length-mass regressions and, where necessary, dry mass-fresh mass relationships from the literature (Supplementary Table 4), yielding an estimated fresh mass in milligrams for every collected individual. Where family-specific relationships were not available or animal body lengths in our collection fell outside of the size ranges of published regressions, we then used regressions from higher-order taxonomic groupings. For heavily damaged individuals that could not be measured for body length, we assigned these individuals a fresh body mass from the median body mass of all animals from the same species or order where only one individual of that species was collected. We then calculated community biomass (mg fresh mass m−2) for each of the 32 communities by summing together all individual body masses calculated from length-mass regressions as derived from the individually measured body lengths.
We calculated individual metabolic rates for all 7,472 animals using body masses, temperature and phylogeny24 (Supplementary Table 5). Temperature was measured over a period of at least 2.5 months at 30-cm depth below the soil surface in each site and averaged for each transformation system in each of the two landscapes. From this, community metabolism was calculated by summing together all individual metabolic rates within each of the 32 sites, providing the total metabolic demand for each of the 32 communities. Using diet-specific assimilation efficiencies25, energy loss to predation and community metabolism, we analytically calculated energy fluxes for each of these communities18 using the formula
 |
where F is the total energy flux into the network node of a feeding guild, ea is the diet-specific assimilation efficiency, X is the metabolic demand of the feeding guild and L is the loss to predation that the feeding guild is subjected to (Fig. 1 and Supplementary Methods). In order to calculate the fluxes between the functional feeding guilds, we constructed a general network of feeding relationships (link structure in Fig. 3) that represents a null model for an energy network structure where no active preferences are assumed. We assumed that, of our four functional feeding guilds, energy fluxes to predators were split up equally into the three animal guilds below them. Energy fluxes to detritivores and herbivores were assumed to come from only detritus and plant material, respectively. Omnivores were assumed to receive energy in equal 25% proportions from the other three functional feeding groups (predators, detritivores and herbivores, making 75%) and the remaining 25% from both plant and detritus material combined (Supplementary Methods).
To assess how these assumptions of feeding preferences might affect the calculations of total energy fluxes, we reconstructed the energy networks so that omnivores were assumed to only consume plant and detritus material (50% derived from each) but with no energy derived from animal material. We then recalculated total energy fluxes and found an overall decrease of up to 54%, which appeared to be highly consistent among the different land-use transformation systems. This consistency between models was especially evident after calculating the loss of energy flux in the three agriculturally used systems compared with the forest system, demonstrating a maximum of only 3% disparity between the two models (Supplementary Fig. 4). This sensitivity analysis indicated that our presented method is highly robust in calculating differences in energy fluxes among different systems. Accordingly, the null model was accepted as the simplest model with the least diet preferences assumed. However, we still suggest that studies adopting this method of energy flux calculation should assign feeding preferences with caution, or employ other techniques such as stable isotope analysis to estimate feeding preferences.
Statistical analyses
Using mixed effects models (GLMM’s), we tested the effects of ‘transformation system’ and its interaction with functional feeding guild on community responses, with ‘landscape’ as a random effect. ‘Density’, ‘biomass’ and ‘community metabolism’ were log10-transformed to meet assumptions of normality and ‘species richness’ (overdispersed poisson-distributed data) was modelled on a negative binomial distribution. We additionally explored biodiversity–ecosystem functioning relationships by first testing for linearity of relationships using untransformed data. Once linearity was established, we then tested for the effects of log10-transformed ‘species richness’ and its interaction with ‘transformation system’ on ‘energy flux’ for overall data and repeated again for data from separate feeding guilds. In addition, because we suspected that our analyses could be affected by spatial autocorrelation, we calculated Moran’s I values for each model’s residuals and tested for spatial autocorrelation using the Moran’s I standard deviate41 in the ‘spdep’ package in R 3.0.2 (ref. 39). Results from these tests provided no support for the spatial autocorrelation of variation in any of the response variables tested (all Moran’s I test results yielded P>0.4).
For all GLMM’s, we applied a backward stepwise selection procedure to obtain the model of best fit, based on the Akaike Information Criterion (AIC). In this procedure, we constructed full models that contained all possible predictors and their interactions (‘transformation system’ and ‘feeding guild’ for general community response models; ‘species richness’ and ‘transformation system’ for biodiversity–ecosystem functioning models) and compared these full models and the model of the backward selection procedure to a null, intercept-only model. The model that yielded the lowest AIC score, with a minimum ΔAIC of 2 units, was selected as the model of best fit. All analyses were conducted with the ‘nlme’ and ‘lme4’ packages in R 3.0.2 (ref. 39).
Author contributions
A.D.B., M.J. and U.B. designed the study; A.D.B., M.J. and S.M. carried out the field and laboratory work; A.D.B. and M.J. prepared and analysed the data; all authors interpreted the results and wrote the paper.
Additional information
How to cite this article: Barnes, A. D. et al. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 5:5351 doi: 10.1038/ncomms6351 (2014).
Supplementary Material
Supplementary Figures 1-4, Supplementary Tables 1-5, Supplementary Methods and Supplementary References
Acknowledgments
We thank Megawati, Rizky Nazarreta, Keisha Disa Putirama, Rosario Reza Valentino Lasse for assistance in the field and laboratory. Roswitha Ehnes provided additional metabolic rate regression parameters and Christian Guill assisted in solving energy flux equations. Ana Meijide, Alexander Knohl, Oleg Panferov and team provided climate data. We also thank the village leaders, local site owners, PT REKI and Bukit Duabelas National Park for granting us access to their properties. This study was financed by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the collaborative German—Indonesian research project CRC990.
References
- Gibbs H. K. et al. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl Acad. Sci. USA 107, 16732–16737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S., Fargione J., Chapin F. S. & Tilman D. Biodiversity loss threatens human well-being. PLoS Biol. 4, e277 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N. Palm-oil boom raises conservation concerns. Nature 487, 14–15 (2012). [DOI] [PubMed] [Google Scholar]
- Koh L. P. & Wilcove D. S. Cashing in palm oil for conservation. Nature 448, 993–994 (2007). [DOI] [PubMed] [Google Scholar]
- Fitzherbert E. B. et al. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 23, 538–545 (2008). [DOI] [PubMed] [Google Scholar]
- Wilcove D. S., Giam X., Edwards D. P., Fisher B. & Koh L. P. Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol. Evol. 28, 531–540 (2013). [DOI] [PubMed] [Google Scholar]
- Koh L. P., Miettinen J., Liew S. C. & Ghazoul J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl Acad. Sci. USA 108, 5127–5132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh L. P. & Ghazoul J. Spatially explicit scenario analysis for reconciling agricultural expansion, forest protection, and carbon conservation in Indonesia. Proc. Natl Acad. Sci. USA 107, 11140–11144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale B. J. et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992 (2006). [DOI] [PubMed] [Google Scholar]
- Hooper D. U. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (2005). [Google Scholar]
- Foster W. A. et al. Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366, 3277–3291 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S. B., Berlow E. L., Rank N. E., Smiley J. & Brose U. Predator diversity and identity drive interaction strength and trophic cascades in a food web. Ecology 89, 134–144 (2008). [DOI] [PubMed] [Google Scholar]
- Duffy J. E. Biodiversity and ecosystem function: the consumer connection. Oikos 99, 201–219 (2002). [Google Scholar]
- Petchey O. L. et al. Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 104, 467–478 (2004). [Google Scholar]
- Schneider F. D., Scheu S. & Brose U. Body mass constraints on feeding rates determine the consequences of predator loss. Ecol. Lett. 15, 436–443 (2012). [DOI] [PubMed] [Google Scholar]
- Schneider F. D. & Brose U. Beyond diversity: how nested predator effects control ecosystem functions. J. Anim. Ecol. 82, 64–71 (2013). [DOI] [PubMed] [Google Scholar]
- Srivastava D. S. & Vellend M. Biodiversity-ecosystem function research: Is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 36, 267–294 (2005). [Google Scholar]
- De Ruiter P. C., Neutel A. M. & Moore J. C. Modelling food webs and nutrient cycling in agro-ecosystems. Trends Ecol. Evol. 9, 378–383 (1994). [DOI] [PubMed] [Google Scholar]
- Handa I. T. et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509, 218–221 (2014). [DOI] [PubMed] [Google Scholar]
- Enquist B. J. et al. A general integrative model for scaling plant growth, carbon flux, and functional trait spectra. Nature 449, 218–222 (2007). [DOI] [PubMed] [Google Scholar]
- Tilman D., Reich P. B. & Knops J. M. H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632 (2006). [DOI] [PubMed] [Google Scholar]
- Cardinale B. J., Harvey C. T., Gross K. & Ives A. R. Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 6, 857–865 (2003). [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M. & West G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- Ehnes R., Rall B. & Brose U. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000 (2011). [DOI] [PubMed] [Google Scholar]
- De Ruiter P. C., Veen J. a., Moore J. C., Brussaard L. & Hunt H. W. Calculation of nitrogen mineralization in soil food webs. Plant Soil 157, 263–273 (1993). [Google Scholar]
- Duffy J. E. et al. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538 (2007). [DOI] [PubMed] [Google Scholar]
- Ehnes R. et al. Lack of energetic equivalence in forest soil invertebrates. Ecology 95, 527–537 (2014). [DOI] [PubMed] [Google Scholar]
- Peters R. H. The Ecological Implications of Body Size Cambridge Studies in Ecology329 (1983). [Google Scholar]
- Purvis A., Gittleman J. L., Cowlishaw G. & Mace G. M. Predicting extinction risk in declining species. Proc. Biol. Sci. 267, 1947–1952 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum M., Schneider F. D., Crowe T. P., Brose U. & O’Gorman E. J. Climate-induced changes in bottom-up and top-down processes independently alter a marine ecosystem. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367, 2962–2970 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balvanera P. et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156 (2006). [DOI] [PubMed] [Google Scholar]
- Ives A. R., Cardinale B. J. & Snyder W. E. A synthesis of subdisciplines: predator-prey interactions, and biodiversity and ecosystem functioning. Ecol. Lett. 8, 102–116 (2004). [Google Scholar]
- Laliberte E. et al. Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol. Lett. 13, 76–86 (2010). [DOI] [PubMed] [Google Scholar]
- Sodhi N. S., Koh L. P., Brook B. W. & Ng P. K. L. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660 (2004). [DOI] [PubMed] [Google Scholar]
- Wilcove D. S. & Koh L. P. Addressing the threats to biodiversity from oil-palm agriculture. Biodivers. Conserv. 19, 999–1007 (2010). [Google Scholar]
- Laumonier Y. et al. Eco-floristic sectors and deforestation threats in Sumatra: identifying new conservation area network priorities for ecosystem-based land use planning. Biodivers. Conserv. 19, 1153–1174 (2010). [Google Scholar]
- Gaveau D., Wandono H. & Setiabudi F. Three decades of deforestation in southwest Sumatra: have protected areas halted forest loss and logging, and promoted re-growth? Biol. Conserv. 134, 495–504 (2007). [Google Scholar]
- Gouyon A., Foresta H. & Levang P. Does jungle rubber deserve its name? An analysis of rubber agroforestry systems in southeast Sumatra. Agrofor. Syst. 22, 181–206 (1993). [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Found. Stat. Comput. (2014) available at http://www.r-project.org/. [Google Scholar]
- Brose U., Martinez N. D. & Williams R. J. Estimating species richness: sensitivity to sample coverage and insensitivity to spatial patterns. Ecology 84, 2364–2377 (2003). [Google Scholar]
- Dormann C. F. et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-4, Supplementary Tables 1-5, Supplementary Methods and Supplementary References