Abstract
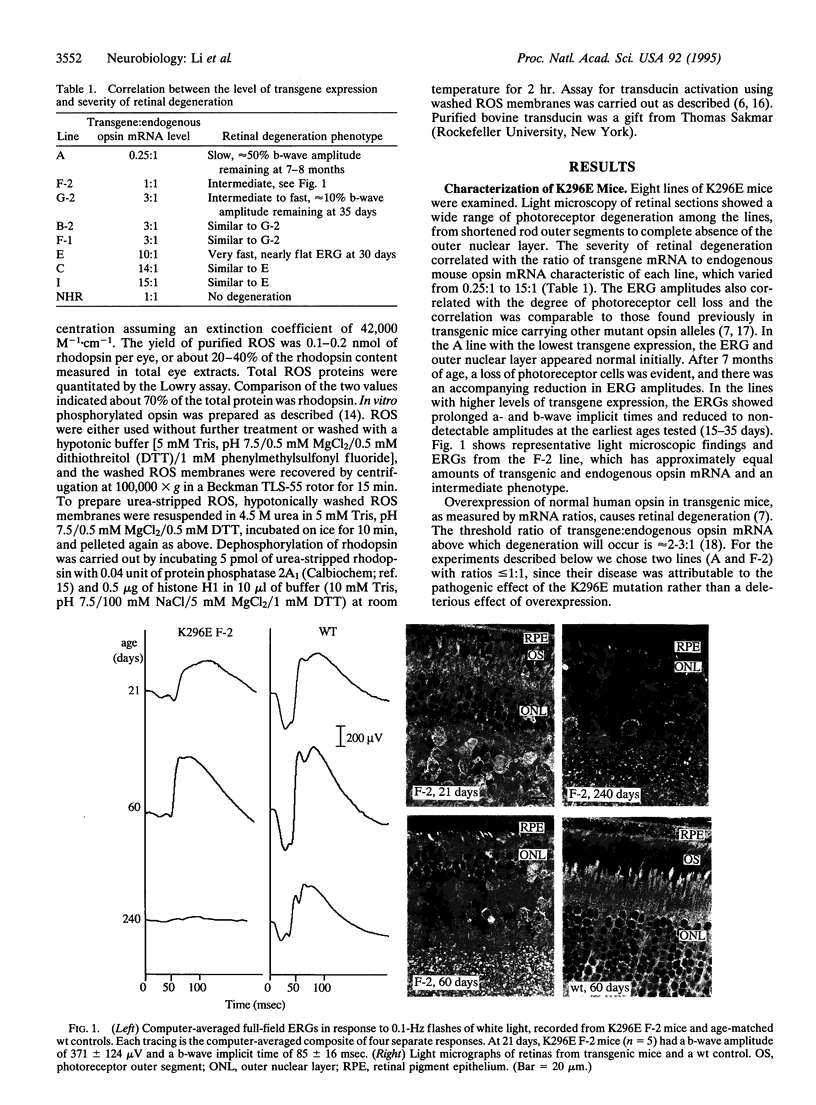
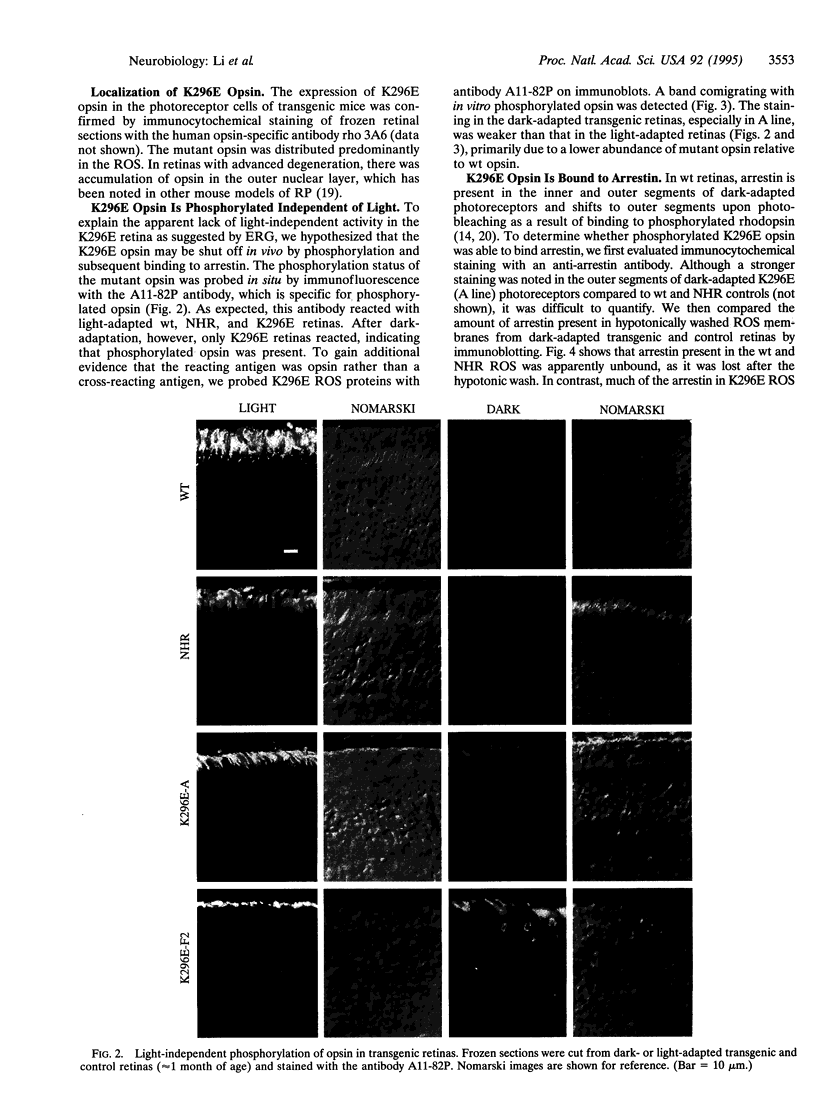
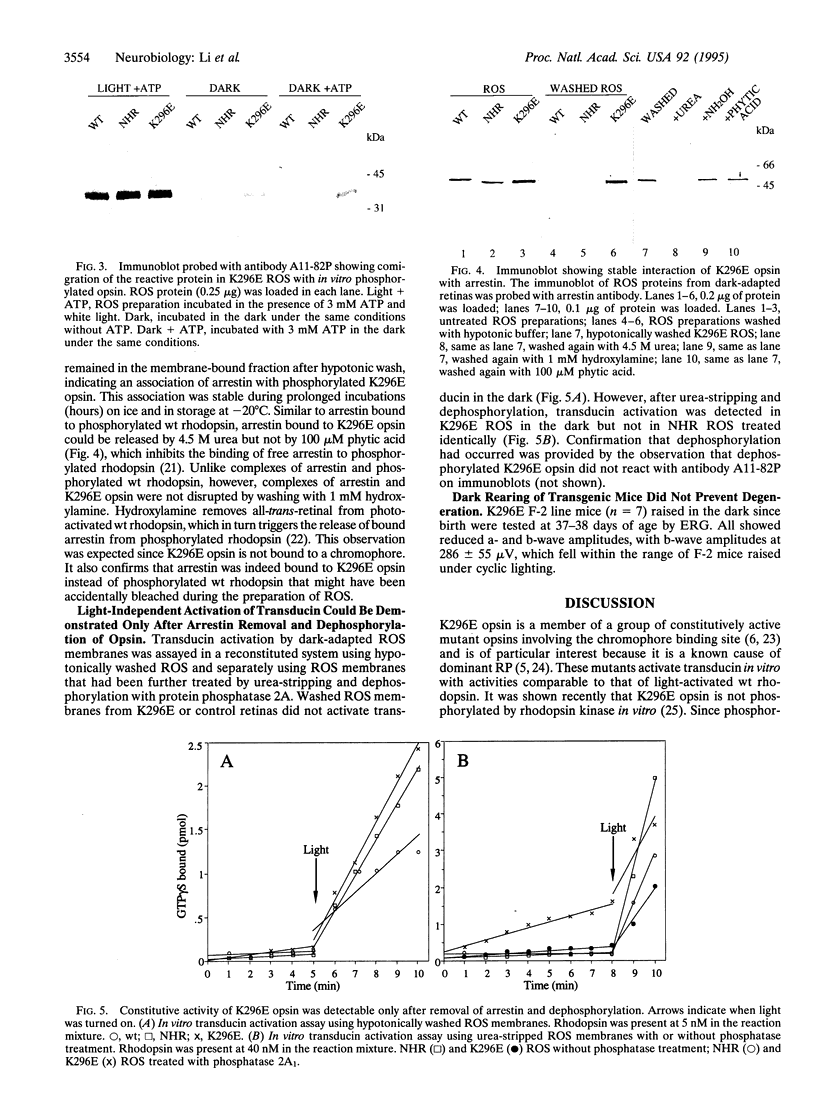
The missense mutation Lys-296-->Glu (K296E) in the rhodopsin gene produces an opsin with no chromophore binding site and therefore is not activated by light. Nevertheless, the mutant opsin constitutively activates transducin in vitro and causes photoreceptor degeneration in vivo, possibly by continuously activating the phototransduction cascade, analogous to constant exposure to environmental light. We studied the K296E mutation in eight lines of transgenic mice. Each line developed photoreceptor degeneration with the rate of degeneration increasing monotonically as the ratio of mutant:wild-type opsin mRNA increased. At no time in the course of degeneration was there endogenous light adaptation in the retina as measured by the electroretinogram. The mutant opsin was found to be invariably phosphorylated and stably bound to arrestin. Light-independent activation of transducin was demonstrated only after the removal of arrestin and dephosphorylation of K296E opsin. Thus, K296E opsin in vivo does not activate the phototransduction cascade because it is shut off by photoreceptor inactivation mechanisms. Our data show that the K296E mutation does not cause photoreceptor degeneration by continuous activation of phototransduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamus G., Zam Z. S., Arendt A., Palczewski K., McDowell J. H., Hargrave P. A. Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 1991;31(1):17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Gunkel R. D. Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol. 1968 Jul;80(1):58–67. doi: 10.1001/archopht.1968.00980050060009. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M., Tolhuizen E. F., Janssen A. P., Winkens H. J. Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res. 1985 May;4(5):613–618. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- Dolph P. J., Ranganathan R., Colley N. J., Hardy R. W., Socolich M., Zuker C. S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993 Jun 25;260(5116):1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Berson E. L., Rao V. R., Oprian D. D. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet. 1993 Jul;4(3):280–283. doi: 10.1038/ng0793-280. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: the equivalent light hypothesis. Exp Eye Res. 1993 Sep;57(3):335–340. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Visual adaptation: its mechanism. Science. 1967 Aug 4;157(3788):583–584. doi: 10.1126/science.157.3788.583. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Heaton R. J., Parker J. M., Molday L., Molday R. S. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988 Aug 25;263(24):11768–11775. [PubMed] [Google Scholar]
- Hofmann K. P., Pulvermüller A., Buczyłko J., Van Hooser P., Palczewski K. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J Biol Chem. 1992 Aug 5;267(22):15701–15706. [PubMed] [Google Scholar]
- Keen T. J., Inglehearn C. F., Lester D. H., Bashir R., Jay M., Bird A. C., Jay B., Bhattacharya S. S. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991 Sep;11(1):199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- Korf H. W., Møller M., Gery I., Zigler J. S., Klein D. C. Immunocytochemical demonstration of retinal S-antigen in the pineal organ of four mammalian species. Cell Tissue Res. 1985;239(1):81–85. doi: 10.1007/BF00214906. [DOI] [PubMed] [Google Scholar]
- Kühn H., Hall S. W., Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984 Oct 29;176(2):473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- Naash M. I., Hollyfield J. G., al-Ubaidi M. R., Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. C., Williams D. S. Rhodopsin is the major in situ substrate of protein kinase C in rod outer segments of photoreceptors. J Biol Chem. 1993 Aug 25;268(24):18181–18186. [PubMed] [Google Scholar]
- Olsson J. E., Gordon J. W., Pawlyk B. S., Roof D., Hayes A., Molday R. S., Mukai S., Cowley G. S., Berson E. L., Dryja T. P. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992 Nov;9(5):815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Hargrave P. A., McDowell J. H., Ingebritsen T. S. The catalytic subunit of phosphatase 2A dephosphorylates phosphoopsin. Biochemistry. 1989 Jan 24;28(2):415–419. doi: 10.1021/bi00428a001. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Jakes S., Ingebritsen T. S., Hargrave P. A. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989 Sep 25;264(27):15770–15773. [PubMed] [Google Scholar]
- Palczewski K., Pulvermüller A., Buczylko J., Gutmann C., Hofmann K. P. Binding of inositol phosphates to arrestin. FEBS Lett. 1991 Dec 16;295(1-3):195–199. doi: 10.1016/0014-5793(91)81416-6. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Cohen G. B., Oprian D. D. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994 Feb 17;367(6464):639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Buczyłko J., Ohguro H., Palczewski K. Opsins with mutations at the site of chromophore attachment constitutively activate transducin but are not phosphorylated by rhodopsin kinase. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5411–5415. doi: 10.1073/pnas.91.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. R., Cohen G. B., Zhukovsky E. A., Oprian D. D. Constitutively active mutants of rhodopsin. Neuron. 1992 Oct;9(4):719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- Roof D. J., Adamian M., Hayes A. Rhodopsin accumulation at abnormal sites in retinas of mice with a human P23H rhodopsin transgene. Invest Ophthalmol Vis Sci. 1994 Nov;35(12):4049–4062. [PubMed] [Google Scholar]
- Roof D., Hayes A., Hardenbergh G., Adamian M. A 52 kD cytoskeletal protein from retinal rod photoreceptors is related to erythrocyte dematin. Invest Ophthalmol Vis Sci. 1991 Mar;32(3):582–593. [PubMed] [Google Scholar]
- Sitaramayya A., Liebman P. A. Phosphorylation of rhodopsin and quenching of cyclic GMP phosphodiesterase activation by ATP at weak bleaches. J Biol Chem. 1983 Oct 25;258(20):12106–12109. [PubMed] [Google Scholar]
- Steele F., O'Tousa J. E. Rhodopsin activation causes retinal degeneration in Drosophila rdgC mutant. Neuron. 1990 Jun;4(6):883–890. doi: 10.1016/0896-6273(90)90141-2. [DOI] [PubMed] [Google Scholar]
- Vaithinathan R., Berson E. L., Dryja T. P. Further screening of the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. Genomics. 1994 May 15;21(2):461–463. doi: 10.1006/geno.1994.1301. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Allosteric behavior in transducin activation mediated by rhodopsin. Initial rate analysis of guanine nucleotide exchange. J Biol Chem. 1987 Mar 15;262(8):3697–3705. [PubMed] [Google Scholar]
- Zhukovsky E. A., Robinson P. R., Oprian D. D. Transducin activation by rhodopsin without a covalent bond to the 11-cis-retinal chromophore. Science. 1991 Feb 1;251(4993):558–560. doi: 10.1126/science.1990431. [DOI] [PubMed] [Google Scholar]







