Abstract
Oxidative stress may play a key role in Alzheimer's disease (AD) neuropathology. Pomegranates (石榴 Shí Liú) contain very high levels of antioxidant polyphenolic substances, as compared to other fruits and vegetables. Polyphenols have been shown to be neuroprotective in different model systems. Here, the effects of the antioxidant-rich pomegranate fruit grown in Oman on brain oxidative stress status were tested in the AD transgenic mouse. The 4-month-old mice with double Swedish APP mutation (APPsw/Tg2576) were purchased from Taconic Farm, NY, USA. Four-month-old Tg2576 mice were fed with 4% pomegranate or control diet for 15 months and then assessed for the influence of diet on oxidative stress. Significant increase in oxidative stress was found in terms of enhanced levels of lipid peroxidation (LPO) and protein carbonyls. Concomitantly, decrease in the activities of antioxidant enzymes was observed in Tg2576 mice treated with control diet. Supplementation with 4% pomegranate attenuated oxidative damage, as evidenced by decreased LPO and protein carbonyl levels and restoration in the activities of the antioxidant enzymes [superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione (GSH), and Glutathione S transferase (GST)]. The activities of membrane-bound enzymes [Na+ K+-ATPase and acetylcholinesterase (AChE)] were altered in the brain regions of Tg2576 mouse treated with control diet, and 4% pomegranate supplementation was able to restore the activities of enzymes to comparable values observed in controls. The results suggest that the therapeutic potential of 4% pomegranate in the treatment of AD might be associated with counteracting the oxidative stress by the presence of active phytochemicals in it.
Keywords: Alzheimer's disease, antioxidant, Oman, Oxidative stress, Pomegranate, Tg2576 mouse
INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disorder and the most prevalent form of dementia characterized by a progressive decline in memory, behavior, and cognitive functions in the elderly population.[1] It affects millions of people and has become a major medical and social burden in developed and developing countries.[2] The disease has been the sixth leading cause of death across all ages and the fifth leading cause of death in those aged 65 and above. The neuropathology of AD is characterized at first by the deposition of senile plaques mainly composed of amyloid beta protein (Aβ) and neurofibrillary tangles containing hyperphosphorylated tau protein in the brain and later by the loss of neurons and their processes.[3,4] Cognitive impairment appears to be most closely correlated in time with the loss of neurons and neuronal processes.[5] At present, the etiology of AD is still not well understood. Accumulation of Aβ peptide causes an increase in intracellular reactive free radicals and reactive oxygen species (ROS). The generation of free radicals (ROS) due to the Aβ peptide can induce functional and structural damage to cell membranes through lipid peroxidation (LPO) and protein carbonyl formation which may be involved in the pathogenesis of AD.[6] These abnormal events in cells lead to oxidative neuronal cell death and cognitive decline in patients with AD.[7] However, the relationship between plaque and tangle deposition and the neuronal degeneration that follows it is not clearly understood.
More recently, the interest in the role of dietary antioxidants in human health has prompted research in the field of AD. Fruits are good sources of these bioactives, and there are a number of commercial polyphenol-rich beverages which base their marketing strategies on antioxidant potency. Naturally occurring compounds from plants have been shown to have therapeutic potential f or AD.[8,9,10] Curcumin and Ginkgo biloba extract are such natural compounds that have been shown to be protective against the progression of AD pathology in AD murine models.[11,12] Mediterranean and Middle East countries are the main regions where pomegranate (石榴 Shí Liú) is cultivated and produced.[13,14] Pomegranates (Punica granatum Linn.) contain very high levels of polyphenols, as compared to other fruits and vegetables. They have been extensively used in Unani, Ayurvedic, and Chinese systems of medicine.[15] Different parts of the fruit have been successfully evaluated for various diseases, including peptic ulcer, hepatic damage, and snakebite. The ripe fruit is a tonic, astringent to the bowels, aphrodisiac, and alleged panacea for a myriad of conditions, including biliousness, fever, heart diseases, sore throat, and stomatitis. The rind of the fruit is antihelminthic and useful in diarrhea, dysentery, and ulcer (Ayurveda).[16] Recently, we have reported that the four different pomegranate varieties grown in Oman offer protection to Parkinson's disease like neurotoxicity in human primary neurons.[17] Dietary supplementation of pregnant mice with pomegranate juice was shown to protect against neurodegeneration in neonatal mice subjected to hypoxic–ischemic brain injury.[18] A recent study suggested that 3 months of supplementation with pomegranate may attenuate AD progression by offering the brain anti-inflammatory effects in amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice.[19] But till now, there are no studies conducted to find out the effect of long-term dietary supplementation of pomegranate on oxidative stress status in APPSw/Tg2576 transgenic mouse model. To fill the information gap, we designed this study to find out whether long-term dietary supplementation (15 months) with pomegranate would influence AD-like oxidative stress in an APPsw/Tg2576 mouse model of AD.
MATERIALS AND METHODS
Collection and preparation
Fresh pomegranate (石榴 Shí Liú) “Helow” (literally, sweet) variety of Oman fruits were purchased from a local farm in Al-Jabal Al-Akhdar, Oman. The fruits were transported to our laboratory in an electric cooler box maintained at 9°C. Then the edible parts were separated and freeze dried at −40°C for 5 days. The samples were then grinded into fine powder by using a KMF grinder (KIKA Werke, Wilmington, Delaware USA) at 6000 rpm. Powders were kept in air-tight plastic containers and stored at −40°C until they were sent for the diet preparation. Before sending, we analyzed the samples for the qualitative presence of polyphenols. Phytochemicals such as anthocycanin, hydroxycinnamic acid derivatives (e.g. caffeic acid, etc.), hydrolyzable tannins (e.g. ellagic acid, quercetin-3-O-glucoside, punicalin, etc.), hydroxybenzoic acids (gallic acid, protocatechuic acid, etc.), hydroxycyclohexane carboxylic acids (quinic acid), and hydroxyphenyls (kaempferol, catechin, etc.) have been reported to be present in pomegranate. This was confirmed by qualitative analysis by high performance liquid chromatography (HPLC; data not included).
Diet preparation for the animals
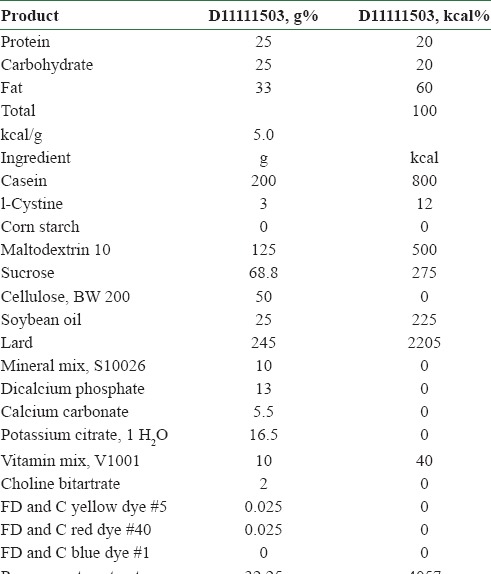
The ground pomegranate samples were sent to USA to prepare the diet for the mice. Based on the our primary dose-dependent study (2, 4, 6, 8 and 10% on the amyloid beta 1-42 induced AD like status in mice) results, we have chosen the 4% (data not shown). The diet was prepared by mixing the pomegranate (4%) with regular diet as per National Institute Health, USA protocol by Research Diet, Inc, NJ, New Brunswick USA. The constituents of the diet are given in Table 1.
Table 1.
Consituents of rodent diet with 60 kcal% fat and 4% pomegranate (prepared by Research Diet, Inc, USA)
Animals and treatment
Twelve transgenic females (APPsw/Tg2576) and six wild control (non-transgenic) mice (Taconic Farm, Hudson St, Manhattan NY, USA) were used. Animals were quarantined for 7 days after shipping and individually housed in plastic cages in an animal room which was maintained at a temperature of 22 ± 2°C, a relative humidity of 50 ± 10%, and a 12-h light/dark automatic light cycle (0800-2000 h). Tap water was offered ad libitum throughout the study. The study was approved by the Animal Care and Use Committee of the Sultan Qaboos University, Oman (SQU/AEC/2010-11/3).
All these animals were free from pathogens and viruses. Experimental period commenced from the age of 4 months. The animals were divided into three groups as follows: Group 1: Wild type (non-transgenic) control of the APPsw mice fed with regular diet; Group 2: AD transgenic mice also fed with regular diet; and Group 3: AD mice fed with 4% pomegranate fruit diet. Dose of pomegranate was designed based on the preliminary study done with different percentages of fruits and 4% showed better effects. These experimental and control mice were examined at the age of 15 months, and oxidative stress, antioxidants and membrane bound enzymes were investigated. All animal experiments in the present study were conducted in compliance with the Animal Care and Use Committee of the Sultan Qaboos University, Oman.
Tissue sample collection
Following the behavioral assessments, the animals were decapitated with the head transferred onto dry ice, followed by rapid dissection of the hippocampus and the cerebral cortex, and homogenized in 9 volumes (1:9 w/v) of cold saline for preparation of a 10% cerebral homogenate in an ice bath, and centrifuged for supernatant collection. Whole brains were rapidly removed simultaneously and chilled in an ice-cold saline solution. The tissue samples were stored at −80°C until assay.
Assay of oxidative parameters in the brain
The hippocampus and the cerebral cortex were obtained for measurements of oxidative parameters.
Superoxide dismutase activity
Measurements of the superoxide dismutase (SOD) activity were performed as described by Sun et al.[20] Estimation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, both of which react with nitroblue tetrazolium (NTB) to form formazan dye, followed by measurement of SOD activity at 550 nm by the degree of inhibition of this reaction. One unit of enzyme was defined as the amount of enzyme required at the inhibition rate of 50%. The activity of SOD was expressed as units/mg protein.
Determination of catalase
Catalase (CAT) activity was measured according to the method of Aebi.[21] Briefly, 0.1 ml of supernatant was added to a cuvette containing 1.9 ml of 50 mmol/L phosphate buffer (pH 7.0). The reaction was started with the addition of 1 ml of freshly prepared 30 mmol/L H2O2. The variations of decomposition rate of H2O2 were determined by spectrophotometry at 240 nm. The activity of catalase was expressed as units/mg protein.
Determination of glutathione
Glutathione (GSH) concentration was determined by the procedure of Elmann.[22] Briefly, 0.5 ml homogenate was mixed with 1.5 ml 0.15 M KCl and 3 ml deproteinized solution. Each sample was centrifuged at 3000 rpm for 10 min and the resultant supernatant was obtained, followed by the addition of 2 ml phosphate solution and 0.5 ml 5,5’-Dithiobis-2-Nitrobenzoic Acid (DTNB) into 0.5 ml supernatant, with the absorbance read at 412 nm and compared with glutathione standards.
Determination of glutathione peroxidase
Hippocampal and cerebrocortical glutathione peroxidase (GPx) activity was determined based on the protocol developed by Wendel[23] by indirectly measuring the consumption of reduced nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm. The GPx uses GSH to reduce the tert-butyl hydroperoxide, producing glutathione disulfide (GSSG), which is readily reduced to GSH by glutathione reductase (GR) using NADPH as a reducing equivalent donor. SOD activity was assayed spectrophotometrically as described previously.[24] This method is based on the capacity of SOD to inhibit autoxidation of adrenaline to adrenochrome. The color reaction was measured at 480 nm. One unit of enzyme was defined as the amount of enzyme required to inhibit the rate of epinephrine autoxidation by 50%. The enzymatic activity was expressed as units (U) per milligram protein.
Determination of GR
Hippocampal and cerebrocortical GR activity was determined based on the protocol developed by Carlberg and Mannervik.[25] Briefly, GR reduces glutathione disulfide (GSSG) to GSH at the expense of NADPH, whose disappearance was followed at 340 nm.
Determination of malondialdehyde
Malondialdehye (MDA) was measured by the method previously described.[26] The reagents acetic acid 1.5 ml (20%) pH 3.5, 1.5 ml thiobarbituric acid (0.8%), and 0.2 ml sodium lauryl sulfate (8.1%) were added to 0.1 ml supernatant samples and heated at 95°C for 60 min. The mixture was cooled with tap water, followed by centrifugation at 4000 rpm for 10 min, separation of the organic layer, and absorbance measurement at 532 nm by microplate spectrophotometry. Protein estimation was conducted according to Lowry's method and the data were expressed as nmol/mg protein.
Measurement of the protein carbonyl content
The total protein carbonyl content was determined by a previously described method for brain tissue.[27] Briefly, 1 ml aliquots of the homogenates containing 759-800 mg protein/ml in each sample were mixed with 0.2 ml of 2,4-dinitrophenylhydrazine (DNPH, 10 mM) or 0.2 ml HCl (2 M). After incubation at room temperature for 1 h in a dark room, 0.6 ml of denaturing buffer [150 mM sodium phosphate buffer, pH 6.8, containing 3% sodium dodecyl sulfate (SDS)], 1.8 ml of heptane (99.5%), and 1.8 ml of ethanol (99.8%) were added sequentially and mixed by vortex agitation for 40 s; the mixture was then centrifuged for 15 min. Next, the protein that was isolated from the interface was washed two times with 1 ml of ethyl acetate/ethanol 1:1 (v/v) and suspended in 1 ml of denaturing buffer. Each DNPH sample was read at 370 nm in a Hitachi U-2001 spectrophotometer against the corresponding HCl sample (blank); the level of total carbonylation was calculated using a molar extinction coefficient of 22,000 M1 cm1.
Assay of acetylcholinesterase activity
The acetylcholinesterase (AChE) activity in the hippocampus and the cerebral cortex was determined according to the method of Ellman.[28] 0.4-ml aliquots of homogenates were added to a cuvette containing 2.6 ml of phosphate buffer (pH 8.0, 0.1 M). Then, 100 μ1 of DTNB was added to the photocell. The absorbance was measured at 412 nm. When the reading had stopped increasing, the photometer slit was opened to set the absorbance to zero. Then, 20 μl of the substrate was added. Changes in absorbance were recorded and calculated as changes in absorbance per minute.
Spectrophotometric analysis of Na+ K+-ATPase activity
The reaction mixture for the Na+ K+-ATPase assay contained 5 mM MgCl2, 80 mM NaCl, 20 mM KCl, 40 mM Tris–HCl buffer, pH 7.4, and purified synaptic membranes (approximately 3 μg of protein) in a final volume of 200 μl. The enzymatic assay was performed at 37°C for 5 min and started by the addition of ATP (disodium salt, vanadium free) to a final concentration of 3 mM. The reaction was stopped by the addition of 200 μl of 10% trichloroacetic acid. Ouabain-insensitive Mg2+-ATPase was assayed under the same conditions with the addition of 1 mM ouabain. Na+ K+-ATPase activity was calculated by determining the difference between the two assays and the inorganic phosphate (Pi) released was measured. Enzyme-specific activities were calculated as nmol of Pi released/min/mg protein and expressed as percentage of controls.[29]
Statistical analysis
Statistical analysis was performed using the software statistical package SPSS 12 (SPSS, Chicago, IL, USA). A univariate analysis of variance was performed using genotype (wild-type and transgenic), treatment (transgenic + pomegranate), and their interactions as between-individuals fixed factors. According to this, differences between treatments and genotype, or differences between transgenic and treatment, were analyzed. For pairwise comparison, Scheffe test (as a post-hoc test) was applied to determine the level of significance between groups. Results are given as mean values ± standard deviation (SD). For all tests, the level of statistical significance was set at P < 0.05.
RESULTS
The effect of 4% pomegranate (石榴 Shí Liú) on LPO and protein carbonyls in APPsw (Tg2576) mice
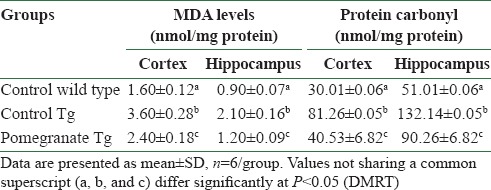
Disease control APPsw (Tg2576) mice showed significant increase in LPO levels in both brain regions studied (cortex and hippocampus), as compared to wild control mice [Table 2]. However, treatment with 4% pomegranate dietary supplementation in APPsw (Tg2576) mice for15 months attenuated LPO levels to values comparable to wild control mice. Table 2 depicts significantly elevated levels of protein carbonyls in control APPsw (Tg2576) mice as compared to wild control mice (cortex and hippocampus). Dietary supplementation with 4% pomegranate significantly attenuated the protein carbonyl levels in APPsw (Tg2576) mice.
Table 2.
Effect of pomegranate on lipid peroxidation and protein carbonyl content in the cortex and hippocampus
Effect of 4% pomegranate on changes in the antioxidant enzymes in APPsw (Tg2576) mice
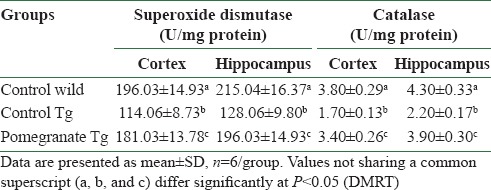
It is evident from the data that there was a significant decrease in the activity of SOD in cerebral cortex and hippocampus in AD mice when compared to wild control mice [Table 3]. However, the SOD activity was significantly elevated by 4% pomegranate dietary supplementation in the cerebral cortex and hippocampus of APPSw (Tg2576) AD mice. Disease control transgenic mice showed significant reduction in CAT activity in the cortex and hippocampus. Dietary supplementation with 4% pomegranate subsequently enhanced the CAT activity both in the cortex and hippocampus [Table 3] in AD mice.
Table 3.
Effect of pomegranate on superoxide dismutase and catalase activities in the brain of mice
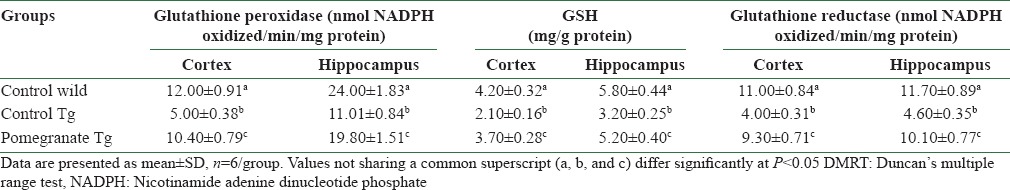
GSH activity of disease control APPsw (Tg2576) mice was significantly lower in the cortex and hippocampus as compared to wild control mice [Table 4]. However, treatment with 4% pomegranate dietary supplementation restored the GSH activity to near-normal levels in the cortex and hippocampus. The data for GPx activity are depicted in Table 4. A significant decrease in GPx activity in the cortex and hippocampus was observed in disease control AD mice. Dietary supplementation with 4% pomegranate was found to protect the GPx activity; the activity was increased in the cortex and hippocampus, respectively. GR activity was significantly reduced in the brain regions, cerebral cortex and hippocampus, in AD mice. The activity was restored with long-term supplementation of 4% pomegranate [Table 4].
Table 4.
Protective effect of pomegranate dietary supplementation on glutathione-dependent antioxidant enzymes
The effect of 4% pomegranate on alterations in membrane-bound enzymes in AD transgenic mice
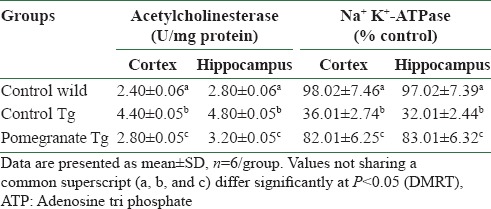
AChE activity significantly increased in the cortex and hippocampus of AD mice and 4% pomegranate dietary supplementation for 15 months attenuated AChE activity in the cerebral cortex and hippocampus. Disease control APPsw (Tg2576) mice showed significantly inhibited activity of Na+ K+-ATPase in the cortex and hippocampus. In 4% pomegranate dietary supplemented mice, there was a significant improvement in the activities of membrane-bound enzymes [Table 5].
Table 5.
Influence of pomegranate on acetylcholinesterase and Na+ K+-ATPase activities in brain
DISCUSSION
Studies demonstrate that the beneficial action of pomegranate (石榴 Shí Liú) is mediated through attenuation of oxidative stress in APPSw (Tg2576) mice. The present study suggests that the anti-AD–like effects of pomegranate grown in Oman might be related to its high antioxidant activities.[30,31,32] ROS can damage essential cellular constituents such as lipids and proteins, which can be measured by identification of their byproducts malondialdehyde and protein carbonyl, respectively.[33] We observed increase in MDA production and protein carbonylation in the cerebral cortex and hippocampus of AD mice, indicating that oxidative stress occurs as a consequence of AD, thereby contributing to brain damage. Dietary supplementation of pomegranate notably inhibited the accumulation of MDA and protein carbonyl levels in the cortex and hippocampus of Tg2576 mice, which is an oxidized byproduct of LPO. In this context, pomegranate has been demonstrated to have direct antioxidant effects,[34,35] and decreased MDA and protein carbonyl content were demonstrated after daily administration of pomegranate juice.[35,36]
GSH is the primary defense in neurons against oxidative stress and maintains cellular redox homeostasis.[37] In our experiment, we observed a significant decrease in the GSH levels in the brain of Tg2576 disease control mice as compared to wild controls. It is known that GSH depletion is the first indicator of oxidative stress during neurodegenerative diseases.[38] Pomegranate supplementation to AD mice was able to reverse the decreased GSH levels. Ajaikumar et al.[39] reported the reversal in GSH levels in aspirin- and ethanol-induced gastric ulceration following pomegranate administration, suggesting the efficacy of pomegranate in preventing the oxidative damage and associated changes.
SOD is responsible for catalyzing the conversion of superoxide anions into hydrogen peroxide[40] which is further decomposed into water and oxygen by CAT.[41] The activities of SOD and CAT were found to be significantly diminished in the cortex and hippocampus of Tg2576 disease control mice. Dietary supplementation of pomegranate to Tg2576 AD mice prevented the reduction in the activities of SOD and CAT. Studies have shown that pomegranate could directly inhibit the superoxide anion formation which could activation of SOD and CAT. This result suggests that the neuroprotective effects of pomegranate might be due to its antioxidant activity.[34,35,36,39,42]
GPx and GR represent a crucial defensive system to protect cells against ROS.[43] The activities of GPx and GR were significantly decreased in the brain regions of AD mice, and a significant decrease in the activities of these enzymes during experimental dementia was reported.[44] On the other hand, Fan et al.[45] have reported decreased GPx and GR activities as a result of oxidative stress in scopolamine-induced amnesia in the hippocampus and cerebral cortex. Pomegranate supplementation significantly attenuated the elevated levels of GPx and GR in the brain regions. Pomegranate has been shown to be successful in increasing the activities of GPx and GR enzymes in animals with aspirin- and ethanol-induced gastric ulceration[39] and improving the antioxidant function in elderly subjects.[36] The mechanism involved in restoration of the activities of these enzymes by pomegranate might include prevention of critical –SH group of these enzymes that is involved in catalysis.
AChE is an acetylcholine hydrolyzing enzyme that is responsible for the termination of cholinergic response.[46] The AChE activity was found to be markedly elevated in the brain regions of Tg2576 AD mice. This observation collaborates with previous reports whereby Intracerebrobentricular (i.c.v.) administration of streptozotocin at sub-diabetogenic dose has been shown to induce memory deficits, along with an increase in oxidative stress and AChE activity.[47] Koladiya et al.[48] have reported that AChE activity was significantly increased in the hippocampus in L-methionine–induced model of vascular dementia. The activity of AChE depends largely on the membrane characteristics, since the enzyme is membrane bound. Amyloid beta peptides can induce Ca2+ influx that leads to increased activity of AChE which is attributed to Ca2+-mediated oxidative stress.[49] In our study, long-term pomegranate supplementation thorugh diet was found to inhibit AChE activity. Previous studies supporting the leaf extracts of pomegranate were obtained for the AChE inhibitory activity and antioxidant effects.[50] The mechanism of anticholinesterase activity of pomegranate appears to be complicated and needs further investigation.
Modification in Na+ K+-ATPase activity may induce neuronal death with features of both apoptosis and necrosis.[51] In the current study, the activity of Na+ K+-ATPase was found to be decreased in Tg2576 AD mice, which is in line with the other studies reporting reduced enzyme activity during aging.[52,53] Na+ K+-ATPase is known to be highly susceptible to changes in the membrane lipids, which may be further attributed to the progressive increase in the LPO.[54,55] ROS overproduction inhibits the activity of ATPase via thiol- and lipid-dependent mechanisms.[56] It has been demonstrated that the reduced activity of Na+ K+-ATPase caused by oxidative stress cannot drive the ion pumps to maintain depolarization of neurons and, thus, may become lethal to neurons.[57] The mechanism of action of pomegranate in improving Na+ K+-ATPase activity is uncertain, as its multiple active compounds such as anthocyanins, ascorbic acid, ellagic acid, gallic acid, fumaric acid, caffeic acid, catechin, Epigallocatechingallate (EGCG), quercetin, rutin, tannins, alkaloids, and flavanoids have multiple functions, making it pharmacologically complex. However, the antioxidant properties of pomegranate have been well documented, which include free radical scavenging and inhibition of LPO as well as enhancement of antioxidant status[30,31,32] and neuroprotection.[17,18,19,58] The results of our present study are supported by the previous studies on short-term oral juice supplementation in AD mice.[17,18,19,58]
We recently reported that chronic dietary term supplementation of fruits could be beneficial for behavior and oxidative stress related changes in AD transgenic mice[59,60,61,62] which also support our present findings.
CONCLUSION
Pomegranates grown in Oman provide possible protection against the oxidative stress and have antioxidant effects in Tg2576 AD mice brain, and the mechanism of protection may be related to their antioxidant activity and phenolic constituents. These results warrant further exploration of how the anti-ROS property of pomegranate affords such beneficial effects on the AD mice brain. This study also supports an important concept that the onset of neurodegenerative disease may be delayed or mitigated by the use of dietary preventive agents that protect against AD by reducing the oxidative stress.
ACKNOWLEDGMENT
The project was supported by the Research Council, Oman (Grant #RC/AGR/FOOD/11/01), which is gratefully acknowledged. Post doctoral fellowship offered to Dr. Subash Selvaraju from the Research Council Oman is higly acknowledged (RC/AGR/FOOD/11/01).
REFERENCES
- 1.Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: The implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–78. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RA. Measuring the spatial arrangement patterns of pathological lesions in histological sections of brain tissue. Folia Neuropathol. 2006;44:229–37. [PubMed] [Google Scholar]
- 5.Price JL, Morris JC. Tangles and plaques in nondemented aging and preclinical Alzheimer's disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer's disease. J Neurochem. 1997;68:2061–9. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 8.Essa MM, Guillemin GJ, Al-Adawi S, Al-Asmi A, Vaishnav R, Nandhagopal N, Memon MA. Manickavasagan A, Essa MM, Sukumar E. The Dates – Genous Phoneix“. UK: CRC Press; 2012. Anti amyloidogenic effect of dates with reference to their protection against Alzheimer's disease; pp. 397–403. [Google Scholar]
- 9.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer's Disease. Neurochem Res. 2012;37:1829–42. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 10.Muthaiyah B, Essa MM, Chauhan V, Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011;36:2096–103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–20. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 13.Jbir R, Hasnaoui N, Mars M, Mohamed M, Mokhtar T. Characterization of Tunisian pomegranate (Punica granatum L.) cultivars using amplified fragment length polymorphism analysis. Sci Hortic. 2008;115:231–7. [Google Scholar]
- 14.Melgarejo P, Martínez JJ, Hernández FC, Martínez R, Legua P, Oncina R, et al. Cultivar identification using 18S-18S rDNA intergenic spacer–RFLP in pomegranate (Punica granatum L) Sci Hortic. 2009;120:500–3. [Google Scholar]
- 15.Wang R, Wang W, Wang L, Liu R, Yi Ding, Du L. Constituents of the flowers of Punica granatum. Fitoterapia. 2006;77:534–7. doi: 10.1016/j.fitote.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Kirthikar KR, Basu D. Indian Medicinal Plants. Vol. 5. Delhi, India: Sri Satguru Publications; 2000. Punica granatum; pp. 1508–13. [Google Scholar]
- 17.Braidy N, Selvaraju S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, et al. Neuroprotective effects of a variety of Pomegranate Juice extracts (PJE) against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid Med Cell Longev 2013. 2013 doi: 10.1155/2013/685909. 685909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr Res. 2005;57:858–64. doi: 10.1203/01.PDR.0000157722.07810.15. [DOI] [PubMed] [Google Scholar]
- 19.Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J Nutr. 2013;143:597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–33. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- 24.Kakkar ZY, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 25.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–90. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 26.Niehaus WG, Jr, Samuelsson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Schneider Oliviera M, Flávia Furian A, Freire Royes LF, Rechia Fighera M, de Carvalho Myskiw J, Gindri Fiorenza N, et al. Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience. 2004;128:721–8. doi: 10.1016/j.neuroscience.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 29.Tsakiris S, Deliconstantinos G. Influence of phosphatidylserine on (Na+ + K+)-stimulated ATPase and acetylcholinesterase activities of dog brain synaptosomal plasma membranes. Biochem J. 1984;220:301–7. doi: 10.1042/bj2200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tezcan F, Gultekin-Ozguven M, Diken T, Özçelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009;115:873–7. [Google Scholar]
- 31.Zhang L, Fu Q, Zhang Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011;127:1444–9. [Google Scholar]
- 32.Jing P, Ye T, Shi H, Sheny Y, Slavin M, Gao B, et al. Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem. 2012;132:1457–64. doi: 10.1016/j.foodchem.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Souza AC, Luchese C, Santos Neto JS, Nogueira CW. Antioxidant effect of a novel class of telluroacetilene compounds: Studies in vitro and in vivo. Life Sci. 2009;84:351–7. doi: 10.1016/j.lfs.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Sudheesh S, Vijayalakshmi NR. Flavonoids from Punica granatum-potential antiperoxidative agents. Fitoterapia. 2005;76:181–6. doi: 10.1016/j.fitote.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Pirinccioglu M, Kızıl G, Kızıl M, Kanay Z, Ketani A. The protective role of pomegranate juice against carbon tetrachloride-induced oxidative stress in rats. Toxicol Ind Health. 2012:1–9. doi: 10.1177/0748233712464809. [DOI] [PubMed] [Google Scholar]
- 36.Guo C, Wei J, Yang J, Xu J, Pang W, Jiang Y. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr Res. 2008;28:72–7. doi: 10.1016/j.nutres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–6. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 38.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64:1037–48. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 39.Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punica granatum L. (pomegranate) methanolic extract. J Ethnopharmacol. 2005;96:171–6. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 41.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalase. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Shen KK, Ji LL, Chen Y, Yu QM, Wang ZT. Influence of glutathione levels and activity of glutathione-related enzymes in the brains of tumor-bearing mice. Biosci Trends. 2011;5:30–7. doi: 10.5582/bst.2011.v5.1.30. [DOI] [PubMed] [Google Scholar]
- 44.Ishrat T, Hoda MN, Khan MB, Yousuf S, Ahmad M, Khan MM, et al. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer's type (SDAT) Eur Neuropsychopharmacol. 2009;19:636–47. doi: 10.1016/j.euroneuro.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Fan Y, Hu J, Li L, Yang Z, Xin X, Wang J, et al. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;21(374):222–6. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 46.Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. ScientificWorldJournal. 2006;28:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma SS, Kumar A, Kaundal RK. Protective effects of 4-amino1,8-napthalimide, a poly (ADP-ribose) polymerase inhibitor in experimental diabetic neuropathy. Life Sci. 2008;12(82):570–6. doi: 10.1016/j.lfs.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Koladiya RU, Jaggi AS, Singh N, Sharma BK. Ameliorative role of Atorvastatin and Pitavastatin in L-Methionine induced vascular dementia in rats. BMC Pharmacol. 2008;8:14. doi: 10.1186/1471-2210-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbosa J, Jr, Ferreira LT, Martins-Silva C, Santos MS, Torres GE, Caron MG, et al. Trafficking of the vesicular acetylcholine transporter in SN56 cells: A dynamin-sensitive step and interaction with the AP-2 adaptor complex. J Neurochem. 2002;82:1221–8. doi: 10.1046/j.1471-4159.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 50.Bekir J, Mars M, Souchard JP, Bouajila J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem Toxicol. 2013;55:470–5. doi: 10.1016/j.fct.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 51.Lees AJ. Dopamine agonists in Parkinson's disease: A look at apomorphine. Fundam Clin Pharmacol. 1993;7:121–8. doi: 10.1111/j.1472-8206.1993.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaur J, Sharma D, Singh R. Acetyl-L-carnitine enhances Na+K+-ATPase glutathione-S-transferase and multiple unit activity and reduces lipid peroxidation and lipofuscin concentration in aged rat brain regions. Neurosci Lett. 2001;301:1–4. doi: 10.1016/s0304-3940(01)01576-2. [DOI] [PubMed] [Google Scholar]
- 53.Arivazhagan P, Panneerselvam C. Alpha-lipoic acid increases Na+K+-ATPase activity and reduces lipofuscin accumulation in discrete brain regions of aged rats. Ann NY Acad Sci. 2004;1019:350–4. doi: 10.1196/annals.1297.060. [DOI] [PubMed] [Google Scholar]
- 54.Rauchova H, Ledvinkova J, Kalous M, Drahota Z. The effect of lipid peroxidation on the activity of various membranebound ATPases in rat kidney. Int J Biochem Cell Biol. 1995;27:251–5. doi: 10.1016/1357-2725(94)00083-n. [DOI] [PubMed] [Google Scholar]
- 55.Dobrota D, Matejovicova M, Kurella EG, Boldyrev AA. Na/K-ATPase under oxidative stress: Molecular mechanisms of injury. Cell Mol Neurobiol. 1999;19:141–9. doi: 10.1023/A:1006928927480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebbel RP, Shalev O, Foker W, Rank BH. Inhibition of erythrocyte Ca2+-ATPase by activated oxygen through thiol- and lipid-dependent mechanisms. Biochim Biophys Acta. 1986;862:8–16. doi: 10.1016/0005-2736(86)90463-3. [DOI] [PubMed] [Google Scholar]
- 57.Cohadon F, Rigoulet M, Guerin B, Vandendriessche M. Vasogenic cerebral oedema. Changes in membrane ATPases. Correction by a phospholipid precursor (author's transl) Nouv Presse Med. 1979;8:1589–91. [PubMed] [Google Scholar]
- 58.Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–15. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Subash S, Essa MM, Al-Asmi A, Al-Adawi S, Vaishnav R, Guillemin GJ. Effect of dietary supplementation of dates in Alzheimer's disease APPsw/2576 transgenic mice on oxidative stress and antioxidant status. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000134. In Press. [DOI] [PubMed] [Google Scholar]
- 60.Subash S, Essa MM, Al-Asmi A, Al-Adawi S, Vaishnav R. Chronic dietary supplementation of 4% figs on the modification of oxidative stress in Alzheimer's disease transgenic mouse model. Biomed Res Int 2014. 2014 doi: 10.1155/2014/546357. 546357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subash S, Essa MM, Braidy N, Al-Jabri A, Vaishnav R, Al-Adawi S, et al. Consumption of fig fruits grown in Oman can improve memory, anxiety, and learning skills in a transgenic mice model of Alzheimer's disease. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000131. In press. [DOI] [PubMed] [Google Scholar]
- 62.Subash S, Braidy N, Essa MM, Al-Buraiki Z, Vaishnav R, Al-Adawi S, et al. Long term (15 months) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioural deficts in a transgenic mice model of Alzheimer's disease. Nutrition. 2014 doi: 10.1016/j.nut.2014.06.004. [DOI] [PubMed] [Google Scholar]


