Abstract
The objective of the study was to identify the active fraction(s) from AR aqueous extract responsible for promoting angiogenesis using bioassay-guided fractionation. The angiogenic activity was screened by monitoring the increase of sprout number in sub-intestinal vessel (SIV) of the transgenic zebrafish embryos after they were treated with 0.06-0.25 mg/ml of AR aqueous extract or its fraction(s) for 96 h. Furthermore, the angiogenic effect was evaluated in treated zebrafish embryos by measuring the gene expression of angiogenic markers (VEGFA, KDR, and Flt-1) using real-time polymerase chain reaction (RT-PCR), and in human microvascular endothelial cell (HMEC-1) by measuring cell proliferation using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, 3H-thymidine uptake assay, and cell cycle analysis. A major active fraction (P1-1-1), which was identified as glycoproteins, was found to significantly stimulate sprout formation (2.03 ± 0.27) at 0.125 mg/ml (P < 0.001) and up-regulate the gene expression of VEGFA, KDR, and Flt-1 by 2.6-fold to 8.2-fold. Additionally, 0.031-0.125 mg/ml of P1-1-1 was demonstrated to significantly stimulate cell proliferation by increasing cell viability (from 180% to 205%), 3H-thymidine incorporation (from 126% to 133%) during DNA synthesis, and the shift of cell population to S phase of cell cycle. A major AR active fraction consisting of glycoproteins was identified, and shown to promote angiogenesis in zebrafish embryos and proliferation of endothelial cells in vitro.
Keywords: Astragali Radix, Glycoproteins, Traditional Chinese Medicine, Wound healing
INTRODUCTION
Angiogenesis, defined as the formation of new blood vessels from pre-existing vessels, plays crucial roles in many physiological processes.[1] During the wound healing process, angiogenesis is involved in proliferation, migration, and differentiation of endothelial cells with a new basement membrane (Hoeben et al., 2004). These promote wound healing process through the delivery of oxygen and nutrients to the wound site. However, in impaired wound, due to poor circulation and reduced oxygenation, angiogenesis will also be affected.[2,3] The resulting chronic wound formed may eventually lead to ulceration. One of the examples is diabetic foot ulcer.[4,5] If the ulcer is not properly treated, the patient may need amputation which may further lead to morbidity and mortality.[6,7]
Astragali Radix (AR), or the root of Astragalus (黃耆 Huáng Qí) membranaceus (Fisch.) Bunge (Fabaceae), is a common Chinese herb that has been traditionally used in treating various diseases such as anemia, fever, wounds, chronic fatigue, uterine bleeding, diabetes, and immune-related diseases.[8,9] It is also one of the herbs commonly found in Chinese herbal formulae used for ulcer healing.[10] In our previous clinical study, two herbal formulae comprising AR as one of the component herbs were shown to rescue 85% of the legs condemned to amputation due to non-healing chronic diabetic ulcer.[11] In addition, AR was shown to be the major component in our simplified herbal formula (NF3) which was demonstrated to enhance diabetic wound healing in rats through tissue regeneration, pro-angiogenesis, and anti-inflammation and also exhibited pro-angiogenic effect in zebrafish embryo in vivo and rat aortic ring in vitro.[12,13] As a single herb, the research focused on its constituents including polysaccharides, triterpene saponins, isoflavonoids, and trace elements.[14] In our recent study, two major anti-inflammatory AR active fractions, which may enhance wound healing, were identified using bioassay-guided fractionation, and formononetin (an isoflavone) was one of the active ingredients in the active fractions(Lai et al., 2013a). Their anti-inflammatory properties were further confirmed by reducing the release of inflammatory mediators and inactivation of nuclear factor kappa B (NFκB) through mitogen-activated protein kinase (MAPK) signaling pathway(Lai et al., 2013b). In another study, AR extract that is enriched in saponin and isoflavone constituents has been demonstrated to exhibit pro-angiogenic effect in vitro.[15] Calycosin, one of the flavonoids found in AR, induces angiogenesis in human umbilical vein endothelial cell (HUVEC) and zebrafish embryos.[16] However, other AR angiogenic active components might be present, but are not yet identified.
Hence, in this study, we aim to systematically identify active fraction(s) from AR which is/are responsible for the pro-angiogenic effect by bioassay-guided isolation method in in vivo zebrafish model. The isolated fraction(s) will be chemically characterized and its angiogenic effects will also be investigated in zebrafish model as well as in human microvascular endothelial cell (HMEC-1) model.
MATERIALS AND METHODS
Materials
The raw herb of AR was purchased from mainland China and its voucher specimen was deposited in the museum of the Institute of Chinese Medicine, The Chinese University of Hong Kong, with voucher specimen number 2008-3201. The procedures of authentication and preparation of aqueous crude extract were described in our previous publication(Lai et al., 2013a). Extraction yield of the crude extract was about 30% (w/w). All chemicals and solvents were purchased from Sigma Chemical Company (St. Louis, MO, USA) unless otherwise specified. The transgenic zebrafish line TG (flil: EGFP) with endothelial cells expressing enhanced Green Fluorescent Protein (eGFP) was purchased from Zebrafish International Resource Center (University of Oregon, USA) and was maintained as described in the previous report.[17] The handling of the zebrafish was under the animal licence issued and endorsed by Department of Health, the Government of the Hong Kong Special Administrative Region and the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong, respectively [reference no.: (09-530) in DH/HA and P/8/2/1 Pt. 10]. HMEC-1 was purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were maintained in MCDB 131 medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Invitrogen, CA, USA), 10 ng/ml epidermal growth factor, 1 μg/ml hydrocortisone, penicillin (100 IU/ml), and streptomycin (100 μg/ml). They were grown in 37°C humidified incubator supplied with 5% CO2.
in vivo zebrafish model
Collection of zebrafish embryos and herbal treatment were performed as described in our previous report.[18] Briefly, embryos at 1-4 cell stage were placed into six-well plates with 20-30 embryos per well depending on the assay. AR crude extract and its isolated fractions were dissolved into embryo medium [19.3 mM NaCl, 0.23 mM KCl, 0.13 mM MgSO4‧7H2O, 0.2 mM Ca (NO3)2, 1.67 mM HEPES, pH 7.2] and then filtered. The medium of the wells was replaced with the filtered AR extract and fractions in various concentrations. After incubation at 28°C for 96 h, sprout formation in sub-intestinal vessel (SIV) region of the treated embryos was examined under fluorescence microscope.[16] The number of sprouts formed in each embryo was counted.
Bioassay-guided isolation of active fractions from AR
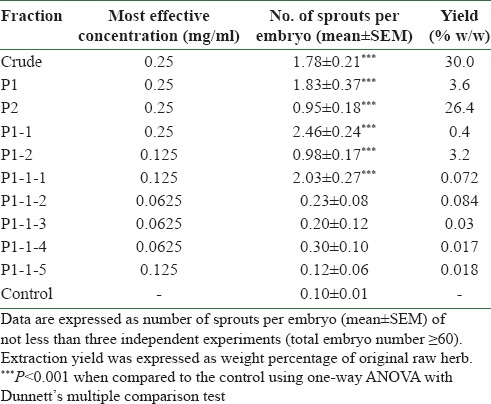
AR aqueous crude extract was used for the isolation of active fractions by bioassay-guided fractionation method. For each fraction, zebrafish model was applied to evaluate the angiogenic activities. The active fractions were selected for further sub-fractionation until the most potent fraction(s) or component(s) were identified and isolated. Finally, an active fraction P1-1-1 was identified. A simplified diagram [Figure 1] shows the isolation of P1-1-1. Firstly, AR aqueous crude extract was re-dissolved in water, and solvent precipitation method using 95% ethanol was employed. The resulting precipitate (P1) and supernatant (P2) were concentrated under reduced pressure and lyophilized to dryness. P1 was re-dissolved in water and further fractionated into two sub-fractions, P1-1 and P1-2, by solvent partition method using chloroform: n-butanol (4:1). Among these sub-fractions, P1-1, which showed the most promising activity [Table 1], was selected for further fractionation into five sub-fractions (P1-1-1, P1-1-2, P1-1-3, P1-1-4, and P1-1-5) by Sephadex G-100 column. According to the result shown in Table 1, P1-1-1 showed the highest angiogenic activity among these five fractions. Thus, in the following experiments, P1-1-1 was used to characterize its biological activities.
Figure 1.
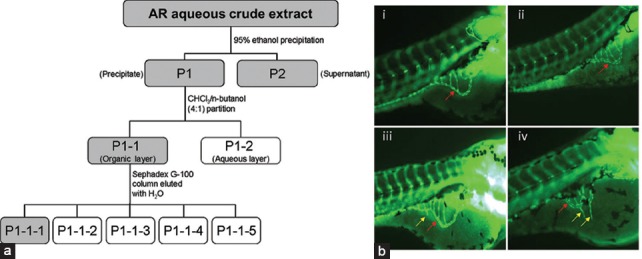
Schematic diagram showing the method for bioassay-guided fractionation of AR aqueous crude extract and representative images of zebrafish study. (a) Active fractions at each level (shown in gray boxes) were selected for further fractionation. P1-1-1 was the fraction finally selected for mechanistic study. (b) Zebrafish embryos treated with (i) embryo medium only and zebrafish embryos treated with P1-1-1 at (ii) 0.03125 mg/ml, (iii) 0.0625 mg/ml, and (iv) 0.125 mg/ml. Red arrows indicate the smooth basket-like structure of sub-intestinal vessel (SIV) appearing at the bottom of each embryo. Yellow arrows indicate the new blood vessels (sprouts) formed on the SIV of embryos treated with P1-1-1 which were observed in (iii) and (iv)
Table 1.
Summary of the angiogenic effects of AR crude extract and isolated fractions on in vivo zebrafish model
Chemical characterization of active fractions
In order to characterize the chemical property of P1-1-1, this fraction was further processed to divide into two sub-fractions, P1-1-1-A and P1-1-1-B, as shown in Figure 1. Molecular weight distribution of the three isolated active fractions (P1-1-1, P1-1-1-A, and P1-1-1-B) was determined by gel filtration chromatography (GFC) using Waters (Milford, MA, USA) ACQUITY ultra performance liquid chromatograph (UPLC) equipped with a calibrated 7.8 mm × 30 cm, 7 μm TSKgel G3000 column (Tosoh Bioscience, Tokyo, Japan) and evaporative light scattering detector (Alltech, Columbia, USA). The column was equilibrated with water at a flow rate of 0.5 ml/min. The molecular mass range of dextran standards (Fluka, Buches, Switzerland) used for calibration was 5-2000 kDa. The retention times plotted as a function of the logarithm of molecular mass and the respective linear regression obtained were used for molecular mass determination. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed with the fractions (10 mg/ml) using 10% (w/v) acrylamide in gels. The reference markers used were the high-range rainbow markers (GE Healthcare, Buckinghamshire, UK). Glycoprotein was detected by the periodic acid-Schiff (PAS) staining of the gel.[14] The carbohydrate and protein content in the fractions were determined by anthrone reagent[19] and bicinchoninic acid (BCA) protein assay, respectively.
Quantitation of mRNA expression level of angiogenic markers by real-time polymerase chain reaction
Total RNA was isolated from 20 zebrafish embryos treated with or without various concentrations of active fraction (P1-1-1) for 72 h using NucleoSpin RNA kit (Macherey-Nagel, Duren, Germany). Reverse transcription and polymerase chain reaction (PCR) amplification were carried out using iScript One-Step real-time polymerase chain reaction (RT-PCR) kit supplied by Bio-Rad (CA, USA). The reaction mixture contained 15 μl of RT-PCR reagent mix, 3 μl of 10 mM primer mix of target gene (VEGFA, Flt-1, or KDR) or house-keeping gene (β-actin), 0.2 μl of reverse transcriptase, 5 μl of RNA template, and 11.8 μl of nuclease-free water. Their primer sequences are as follows: VEGFA, 5′-TCCAGGAGTATCCCGATGAG-3′ and 5′-GCTTTGACTTCTGCCTTTGG-3′; Flt-1, 5′-ATGGGAACAGCAGCACTCTT-3′ and 5′-TTGAAGACGGAGGGACAATC-3′; KDR, 5′-TGTGGTCAGCTATGCTGGAG-3′ and 5′-AGCCTCTCATGCTGTGGACT-3′; β-actin, 5′-CTCTTCCAGCCTTCCTTCCT-3′ and 5′-CTTCTGCATACGGTCAGCAA-3’. RT-PCR was then performed by CFX96 Real-Time System (Bio-Rad, CA, USA). Analysis of expression of VEGFA, Flt-1, and KDR was preformed using β-actin as the control gene for normalization.
Measurement of cell viability by MTT assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HMEC-1 cells (5 × 103 cells/well) were seeded in 96-well culture plates and incubated overnight for attachment. Then, the cells were arrested with 0.5% (v/v) FBS in MCDB 131 medium for another 24 h. After further treating them with various concentrations of P1-1-1 for 24 or 48 h, the culture medium was removed and 40 μl of MTT solution [5 mg/ml in phosphate-buffered saline (PBS)] was added to each well. After incubation for 3 h at 37°C, MTT solution was removed and 100 μl of dimethyl sulfoxide was added to dissolve the crystals formed. Then, absorbance at 540 nm was measured using a microplate reader. The percentage cell viability was calculated as [Absorbance (treatment)/Absorbance (negative control)] ×100%.
Measurement of DNA synthesis by 3H-thymidine uptake assay
HMEC-1 cells (5 × 103 cells/well) were seeded in 96-well culture plates and incubated overnight for attachment. Then, the cells were arrested with 0.5% (v/v) FBS in MCDB 131 medium for another 24 h. After further treating them with various concentrations of P1-1-1 for 24 or 48 h, 0.5 μCi of 3H-thymidine in PBS (Invitrogen, USA) was added to each well and the cells were incubated at 37°C for 6 h. Then, DNA was harvested on microfilters with a cell harvester (Beckman Coulter, Brea, CA, USA). The amount of DNA synthesized was determined by measuring the radioactivity of the filter using a microplate scintillation counter (Beckman Coulter).
Cell cycle analysis
HMEC-1 cells (3 × 105 cells/well) were seeded into six-well culture plates and incubated overnight for attachment. After synchronization with 0.5% FBS in MCDB 131 medium for 24 h, the cells were treated with various concentrations of P1-1-1 or vehicle for a further 24 or 48 h. Then, the cells were harvested and fixed in 70% ethanol. Before performing flow cytometry, ethanol was removed and the cells were incubated with RNase (8 μg/ml) and propidium iodide (10 μg/ml) for 30 min. Cell cycle distribution was then detected using a flow cytometer (BD FACSCanto, BD BioSciences, CA, USA), and the results were analyzed using ModfitLT version 3.0 software.
Statistical analysis
All experiments were performed in not less than three replicates and the results were presented as mean ± standard deviation (SD) for in vitro studies or mean ± standard error of the mean (SEM) for in vivo studies. One-way analysis of variance (ANOVA) with Dunnett's multiple comparison test was used for comparisons among various treatment groups and the control group. Results were considered statistically significant when P < 0.05.
RESULTS AND DISCUSSION
Angiogenic effects of AR crude extract and its fractions
In the present study, we identified the major angiogenic active fraction that possibly contributed to the wound healing property of AR by bioassay-guided fractionation [Figure 1a] using zebrafish embryo in vivo model. As shown in Table 1, AR crude extract at 0.25 mg/ml was demonstrated to enhance angiogenesis significantly by the increased number of sprouts (1.78 ± 0.21) formed in treated embryos when compared with control (0.10 ± 0.01, P < 0.001). Two downstream fractions, P1 (macromolecules) and P2 (small molecules) separated by ethanol precipitation method, were also found to have significant activities at 0.25 mg/ml and, thus, fractionation was conducted on both fractions. Among seven resulting sub-fractions tested, P1-1 showed the most promising angiogenic effect (2.46 ± 0.24) and, therefore, was further fractionated into five sub-fractions (P1-1-1 to P1-1-5) by Sephadex G-100 column. P1-1-1 was selected as it showed the highest enhancing effect (2.03 ± 0.27) among the five fractions tested. When comparing to the mother fraction (P1-1), the effective concentration of P1-1-1 was lower (0.125 mg/ml). When P1-1-1 was further fractionated into P1-1-1-A and P1-1-1-B, it was found that the activities of these sub-fractions were decreased. Therefore, P1-1-1 was identified as the major active angiogenic fraction of AR. Zebrafish (Danio rerio) embryos were chosen as in vivo model for discovery of bioactive drugs from natural sources due to their high genetic similarity to human, high reproducibility, and short generation time, ease of drug administration, as well as their optical transparency for allowing visualization of drug effects on internal organs and tissues.[20] Additionally, transgenic zebrafish with florescent blood vessels have been extensively used for study of embryonic blood vessel formation in the area of angiogenesis since they offer a less labor-intensive method of visualizing blood vessels in the zebrafish embryo.[21]
Chemical characterization of major active fraction P1-1-1
P1-1-1 was isolated from P1 which was composed of macromolecules. The chemical property of P1-1-1 has been characterized in terms of P1-1-1-A and P1-1-1-B. The results of GFC [Figure 2] showed that three groups of macromolecules (P1-1-1-A1, P1-1-1-B1, and P1-1-1-B2) were present in P1-1-1, and their molecular weights were estimated to be 229-2396 kDa, 5.3-143 kDa, and 1.6 kDa, respectively, with P1-1-1-A1 originating from the sub-fraction P1-1-1-A and the others from P1-1-1-B [Table 2]. Additionally, when P1-1-1 was analyzed by SDS-PAGE, a broad band of large molecular size (>76 kDa) and a band of small molecular size (about 38 kDa) were observed [Figure S1, Supplementary Information]. When compared with the results of GFC, these two bands were identified as P1-1-1-A1 and P1-1-1-B1, respectively [Table 2]. It was suggested by PAS staining that the fractions were glycoproteins [Figure S1, Supplementary Information]. The carbohydrate content of the fractions was analyzed and the results are summarized in Table 2. They were estimated to contain 45.1-70.6% (w/w) of carbohydrate and 11.7-15.9% (w/w) of protein.
Figure 2.
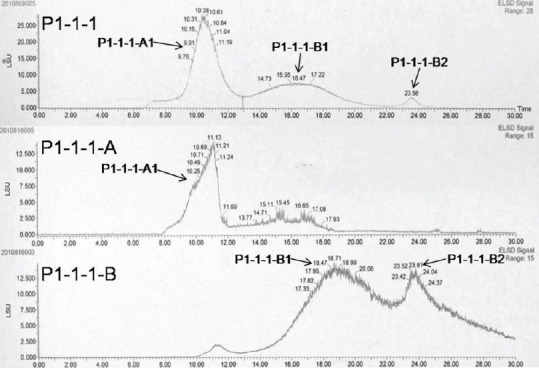
Gel filtration chromatogram (GFC) of the major active fraction P1-1-1 and its sub-fractions (P1-1-1-A and P1-1-1-B). P1-1-1-A1, P1-1- 1-B1, and P1-1-1-B2 were three groups of macromolecules identified in P1-1-1 by GFC
Table 2.
Chemical characterization of P1-1-1, P1-1-1-A, and P1-1-1-B
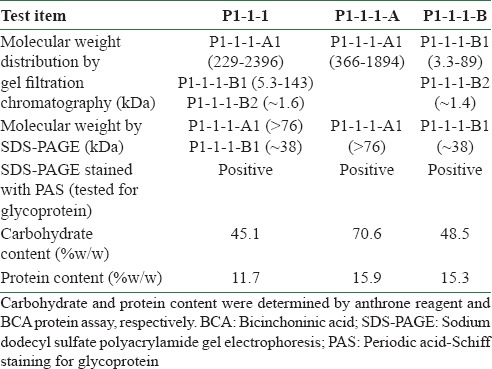
Figure S1.
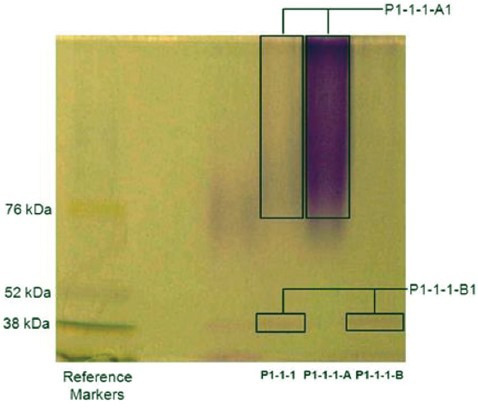
SDS-PAGE analysis of angiogenic active fraction (P1-1-1) and its sub-fractions (P1-1-1-A and P1-1-1-B). The resulting gel was further stained with PAS. The bands (P1-1-1-A1 and P1-1-1-B1) in the lanes of P1-1-1, P1-1-1-A and P1-1-1-B were stained positively with PAS
P1-1-1 stimulated sprout formation in zebrafish embryos by up-regulating expression of angiogenic markers
The angiogenic activity and underlying mechanism of P1-1-1 was further evaluated using zebrafish model. Results indicated the average number of sprouts formed per embryo was significantly increased from 0.76 to 2.03 (P < 0.001) after treating the embryos with 0.0625 mg/ml and 0.125 mg/ml of P1-1-1 and the stimulation was in a concentration-dependent manner [Figure 3a]. The representative pictures of P1-1-1 treated zebrafish embryo and control embryo are shown in [Figure 1b]. In order to identify the molecular targets of the angiogenic effects of P1-1-1 in zebrafish embryos, RT-PCR was used to quantify the mRNA expression levels of selected genes involved in vascular endothelial growth factor (VEGF) signaling pathway. VEGF and its tyrosine kinase receptors (VEGFRs) are the key regulators in angiogenesis and are highly specific to endothelial cells.[22] VEGFA, the most important member of VEGF, binds and activates the two VEGFRs, VEGFR-1 (Flt-1) and VEGFR-2 (KDR), subsequently initiates the main signaling pathway.[23,24] As shown in [Figure 3b-d], 0.125 mg/ml of P1-1-1 was demonstrated to significantly up-regulate mRNA expression of these regulators, 5.7-fold for Flt-1, 2.6-fold for KDR, and 8.2-fold for VEGFA (P < 0.05). Therefore, our results suggested that the angiogenic effect of P1-1-1 observed in zebrafish embryos was at least partly mediated via VEGF signaling pathways.
Figure 3.
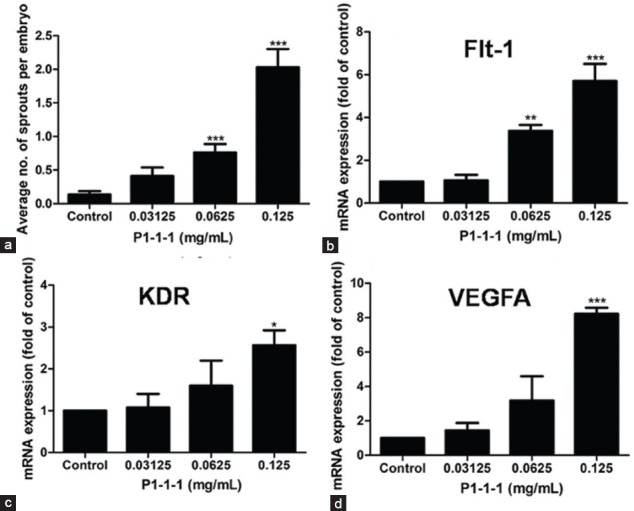
Effects of P1-1-1 on sprout formation and mRNA expression of three angiogenic markers (Flt-1, KDR, VEGFA) in zebrafish embryos. (a) Sprout formation was expressed as number of sprouts per embryo (mean ± SEM) of not less than three independent experiments. For (b) Flt-1, (c) KDR, and (d) VEGFA, the expression levels were normalized and expressed as mean ± SEM of not less than three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant difference when compared to control using one-way ANOVA with Dunnett's multiple comparison test
P1-1-1 stimulated HMEC-1 cell viability, DNA synthesis and cell population in S phase
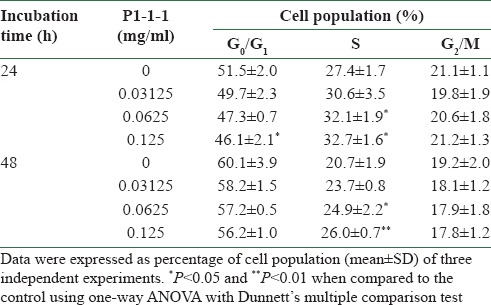
Proliferation of endothelial cells is the first and the primary event in angiogenesis. Therefore, HMEC-1 were used to examine the angiogenic effects of P1-1-1 by measuring cell proliferation using MTT assay, 3H-thymidine uptake assay, and cell cycle analysis. After the cells were incubated with P1-1-1 at a concentration range of 0.03125-0.125 mg/ml for 24 or 48 h, increased cell viability was observed in MTT assay when compared to the control which was set to 100% (P < 0.001) [Figure 4a and b]. In a similar manner, P1-1-1 also significantly accelerated the uptake of 3H-thymidine under the same concentration range (P < 0.01) [Figure 4c and d]. The results indicated that cellular DNA synthesis was enhanced by P1-1-1. This was further verified by the increased S (synthesis) phase population in cell cycle analysis. As shown in Table 3, P1-1-1 at concentrations of 0.0625 mg/ml and 0.125 mg/ml significantly increased the cell population in S phase after 24 h (from 27.4 to 32.7%) and 48 h (from 20.7 to 26.0%) incubation (P < 0.05).
Figure 4.
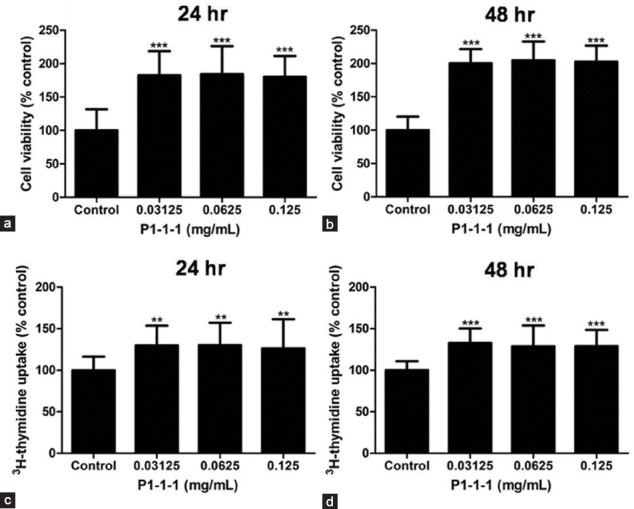
Effect of P1-1-1 on HMEC-1 cell viability and DNA synthesis. The cells were treated with P1-1-1 (0.03125-0.125 mg/ml). (a, b) MTT assay and (c, d) 3H-thymidine uptake assay were performed after 24 and 48 h. Data were expressed as mean ± SD of not less than three independent experiments. **P < 0.01 and **P < 0.001 indicate significant difference when compared to control using one-way ANOVA with Dunnett's multiple comparison test
Table 3.
Effects of P1-1-1 on cell cycle distribution of HMEC-1 cells
Previous in vitro and in vivo studies of AR extract and its constituents (calycosin, astragaloside IV, and polysaccharides) have confirmed their pro-angiogenic activities.[15,16] However, very few biological studies of its glycoprotein were found, except a pathogenesis-related class 10 protein (PR-10), a glycoprotein isolated from AR, which was shown to exhibit ribonuclease activity.[14] It was found that the isolated AR active fraction P1-1-1, which consisted of glycoproteins, stimulated angiogenesis in zebrafish embryos via up-regulated expression of VEGF and its tyrosine kinase receptors, and proliferation of HMEC-1 cells by increasing cell viability, DNA synthesis and the shift of cell population to S phase of cell cycle in which DNA replication was stimulated. Taken together, it was suggested that P1-1-1 was a glycoprotein-containing active fraction which partially contributed to the angiogenic activity of AR.
The analysis of gene expression of VEGFA, KDR, and Flt in HMECs, as well as cell migration and differentiation will be included in our future study.
CONCLUSION
In the present work is presented the isolation of an angiogenic active fraction of AR by bioassay-guided fractionation using in vivo model. The fraction comprising glycoproteins exhibited angiogenic activity in zebrafish embryos via VEGF signaling pathway, and stimulated the proliferation of HMEC-1 cells by increasing cell viability and DNA synthesis.
ACKNOWLEDGMENT
This study was supported by an Area of Excellence (AoE) grant from University Grants Committee (UGC) of the Hong Kong SAR, entitled “Chinese Medicine Research and Further Development” (reference no.: AoE/B-10/01) led by Institute of Chinese Medicine, the Chinese University of Hong Kong.
REFERENCES
- 1.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 2.Chabbert-Buffet N, LeDevehat C, Khodabandhelou T, Allaire E, Gaits JP, Tribout L, et al. Evidence for associated cutaneous microangiopathy in diabetic patients with neuropathic foot ulceration. Diabetes Care. 2003;26:960–1. doi: 10.2337/diacare.26.3.960. [DOI] [PubMed] [Google Scholar]
- 3.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 4.Levin E. Preventing amputation in the patient with diabetes. Diabetes Care. 1995;18:1383–94. doi: 10.2337/diacare.18.10.1383. [DOI] [PubMed] [Google Scholar]
- 5.Kalish J, Hamdan A. Management of diabetic foot problems. J Vasc Surg. 2010;51:476–86. doi: 10.1016/j.jvs.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–4. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 7.Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, et al. Major lower extremity amputation: Outcome of a modern series. Arch Surg. 2004;139:395–9. doi: 10.1001/archsurg.139.4.395. [DOI] [PubMed] [Google Scholar]
- 8.Ryu M, Kim EH, Chun M, Kang S, Shim B, Yu YB, et al. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J Ethnopharmacol. 2008;115:184–93. doi: 10.1016/j.jep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Hoo RL, Wong JY, Qiao CF, Xu A, Xu HX, Lam KS. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr Metab (Lond) 2010;7:67–79. doi: 10.1186/1743-7075-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu GW, Lauda DP. Beijing: Huaxia Publishing House; 2002. Development of Formulae of Chinese Medicine. [Google Scholar]
- 11.Leung PC, Wong MW, Wong WC. Limb salvage in extensive diabetic foot ulceration: An extended study using a herbal supplement. Hong Kong Med J. 2008;14:29–33. [PubMed] [Google Scholar]
- 12.Tam JC, Lau KM, Liu CL, To MH, Kwok MH, Lai KK, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol. 2011;134:831–8. doi: 10.1016/j.jep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Tse HY, Hui MN, Li L, Lee SM, Leung AY, Cheng SH. Angiogenic efficacy of simplified 2-herb formula (NF3) in zebrafish embryos in vivo and rat aortic ring in vitro. J Ethnopharmacol. 2012;139:447–53. doi: 10.1016/j.jep.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Yan QJ, Qi XW, Jiang ZQ, Yang SQ, Han L. Characterization of a pathogenesis-related class 10 protein (PR-10) from Astragalus mongholicus with ribonuclease activity. Plant Physiol Biochem. 2008;46:93–9. doi: 10.1016/j.plaphy.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Hu G, Lin HC, Hong SJ, Deng YH, Tang JY, et al. Radix Astragali extract promotes angiogenesis involving vascular endothelial growth factor receptor-related phosphatidylinositol 3-kinase/Akt-dependent pathway in human endothelial cells. Phytother Res. 2009;23:1205–13. doi: 10.1002/ptr.2479. [DOI] [PubMed] [Google Scholar]
- 16.Tang JY, Li S, Li ZH, Zhang ZJ, Hu G, Cheang LC, et al. Calycosin promotes angiogenesis involving estrogen receptor and mitogen-activated protein kinase (MAPK) signaling pathway in zebrafish and HUVEC. PLoS One. 2010;5:1–14. doi: 10.1371/journal.pone.0011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng SH, Wai AW, So CH, Wu RS. Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ Toxicol Chem. 2000;19:3024–31. [Google Scholar]
- 18.Liu CL, Cheng L, Kwok HF, Ko CH, Lau TW, Koon CM, et al. Bioassay-guided isolation of norviburtinal from the root of Rehmannia glutinosa, exhibited angiogenesis effect in zebrafish embryo model. J Ethnopharmacol. 2011;137:1323–7. doi: 10.1016/j.jep.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Trevelyan WE, Forrest RS, Harrison JS. Determination of yeast carbohydrates with the anthrone reagent. Nature. 1952;170:626–7. doi: 10.1038/170626a0. [DOI] [PubMed] [Google Scholar]
- 20.Mandrekar N, Thakur NL. Significance of the zebrafish model in the discovery of bioactive molecules from nature. Biotechnol Lett. 2009;31:171–9. doi: 10.1007/s10529-008-9868-1. [DOI] [PubMed] [Google Scholar]
- 21.Crawford AD, Esguerra CV, de Witte PA. Fishing for drugs from nature: Zebrafish as technology platform for nature product discovery. Planta Med. 2008;74:624–32. doi: 10.1055/s-2008-1034374. [DOI] [PubMed] [Google Scholar]
- 22.Schenone S, Bondavalli F, Botta M. Antiangiogenic agents: An update on small molecule VEGFR inhibitors. Curr Med Chem. 2007;14:2495–516. doi: 10.2174/092986707782023622. [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 24.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–54. [PubMed] [Google Scholar]


