Abstract
The present study was carried out to evaluate the anticancer, antioxidant, and possible anti-inflammatory properties of diverse medicinal plants frequently used in Indian traditional medication. The selected botanicals such as Soymida fembrifuga (Roxb.) A. Juss. (Miliaceae), Tinospora cordifolia (Willd.) Miers. (Menispermaceae), Lavandula bipinnata (L.) O. Ktze. (Lamiaceae), and Helicteres isora L. (Sterculiaceae) extracted in different solvents were evaluated for their in vitro anticancer and antioxidant activities. The results obtained indicate that H. isora has potent cytotoxic activity toward the selected cancer cells such as HeLa-B75 (34.21 ± 0.24%), HL-60 (30.25 ± 1.36%), HEP-3B (25.36 ± 1.78%), and PN-15 (29.21 ± 0.52%). Interestingly, the selected botanicals selectively inhibited cyclooxygenase-2 (COX-2) more than (COX-1), which are the key enzymes implicated in inflammation. COX-2 inhibition was observed to be in the range of 19.66-49.52% as compared to COX-1 inhibition (3.93-19.61%). The results of the antioxidant study revealed that the selected plants were found to be effective 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl (OH), and superoxide radical (SOR) scavenging agents. High-performance thin layer chromatography (HPTLC) fingerprint of flavonoids was used as a measure of quality control of the selected plant samples. The results of the present findings strengthen the potential of the selected plants as a resource for the discovery of novel anticancer, anti-inflammatory, and antioxidant agents.
Keywords: Anticancer, Anti-inflammatory, Antioxidant, Medicinal plants
INTRODUCTION
Cancer is one of the most life-threatening diseases, with more than 100 different types occurring due to some molecular changes within the cell. It is the third leading cause of death worldwide following cardiovascular and infectious diseases.[1] It is estimated that 12.5% of the population dies due to cancer (WHO, 2004). The disease is widely prevalent, and in the West, almost a third of the population develops cancer at some point of time during their life. Although the mortality due to cancer is high, many advances have been made both in terms of treatment and understanding the biology of the disease at the molecular level.[2]
Breast cancer is the most common form of cancer in women. The incidence of breast cancer is the highest in Pakistan among the South-Central Asian countries. It is the most frequent malignancy in women and accounts for 38.5% of all female cancers. About half (43.7%) of all breast cancers are detected in an advanced stage.[3] Colon cancer is the second most common cause of cancer deaths in the US. Prostate cancer is the most frequently diagnosed cancer among men in the US, and ranks second to skin cancer, with an estimated 180,000 new cases and 37,000 deaths expected to occur by the American Cancer Society each year.[4]
Moreover, it is increasingly being realized that many of today's diseases are due to the “oxidative stress” that results from an imbalance between the formation and neutralization of prooxidants. Oxidative stress is initiated by free radicals, which seek stability through electron pairing with biological macromolecules such as proteins, lipids, and DNA in healthy human cells and cause protein and DNA damage along with lipid peroxidation. These changes contribute to cancer, atherosclerosis, cardiovascular diseases, aging, and inflammatory diseases.[5,6] All cells are exposed to oxidative stress, and thus, oxidation and free radicals may be important in carcinogenesis at multiple tumor sites.
The enzymes cyclooxygenase-1 and -2 (COX-1 and -2) are the key enzymes involved in recruiting inflammation. Nevertheless, the proinflammatory cytokines play a crucial role in the initiation and progression of various cancers.[7] Besides the key role of COX in the initiation and progression of inflammation, overexpression of COX has been considered as one of the culprits in the formation of carcinogenic state in the body.[8] It is this molecular attribute of the COX upregulation that has made it an attractive target for the design and development of anticancer agents also. Free radical induced oxidative stress and its relevance with inflammation and carcinogenesis is well established.[9] Therefore, inflammation, free radicals, and carcinogenesis are closely related with one another. The drug candidates having anti-inflammatory and free radical scavenging activities are more appreciated as anticancer agents.
Due to lack of effective drugs, cost of chemotherapeutic agents, and the side effects of anticancer drugs, cancer can be a cause of death. Therefore, efforts are still being made to search for effective naturally occurring anticarcinogens that would prevent, slow, or reverse cancer development. Medicinal plants have a special place in the management of cancer. It is estimated that plant-derived compounds in one or the other way constitute more than 50% of anticancer agents.[10,11] Numerous cancer research studies have been conducted using traditional medicinal plants in an effort to discover new therapeutic agents that lack the toxic side effects associated with the present chemotherapeutic agents. Taking into consideration the above facts, an attempt has been made to evaluate the anticancer, anti-inflammatory, and antioxidant activities of selective medicinal plants used in Indian traditional medicine system.
MATERIALS AND METHODS
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The COX assay was performed by using colorimetric COX (ovine) inhibitor screening assay kit (catalog no. 760111; Cayman Chemical Company, USA). 1,10-Phenanthroline, phenazine methosulfate (PMS), and nitroblue tetrazolium (NBT) were obtained from SD Fine Chem. (Mumbai, India). Nicotinamide adenine dinucleotide (NADH) was purchased from Spectrochem, Pvt Ltd (Mumbai, India). All other chemicals and reagents used were of AR grade and were obtained from commercial sources.
Collection, identification, and authentication of the selected medicinal plants
The selected plants, Soymida fembrifuga (Roxb.) A. Juss. (SRTH-08), Tinospora cordifolia (Willd.) Miers. (SRTH-54), Lavandula bipinnata (L.) O. Ktze. (SRTH-24), and Helicteres isora L. (SRTH-61), were collected from the nearby regions of Nanded district (Maharashtra) during September 2009. The plants were identified and authenticated by RNG, Head, Department of Botany, School of Life Sciences, Swami Ramanand Teerth Marathwada University, Nanded, Maharashtra, India.[12] Voucher specimens of the collected plants were deposited in the herbarium center of the host institute. The shade-dried and powdered plant samples were preserved for further experiments.
Sequential extraction of the plant samples
The shade-dried, powdered plant samples (10 g) were sequentially extracted in hexane, ethanol, and water as per their boiling points which are (69°C), (79°C), and (100°C), respectively, up to 8 h using Soxhlet's apparatus. The extracted samples were evaporated under reduced pressure at room temperature. The yield of the individual plant extract was measured and the dried extracts were preserved at 4°C in a refrigerator for further analysis.
HPTLC analysis
High-performance thin layer chromatography (HPTLC) analysis was performed using the instrument from CAMAG (Germany). Thin layer chromatography (TLC) plates (silica gel >60 F254, 20 cm × 10 cm; Merck) were prewashed with methanol. The plates were activated in an oven at 100°C for 10 min. Ten microliters of individual plant extracts (1 mg/ml) was spotted onto the precoated plates using Linomat 5 application system. Rutin hydrate (50, 100, 200 μg/ml) was used as the marker flavonoid. The flavonoids were separated using ethyl acetate: Formic acid: Glacial acetic acid: Water (100:11:11:27) as the mobile phase. Natural product (NP) reagent was used as the flavonoid derivatizing agent, and the spots developed were visualized under CAMAG UV cabinet (366 nm) and digitized using CAMAG photodocumentation system.
Cell lines and culturing
Human cancer cell lines HeLa-B75, HL-60, HEP-3B, PN-15, and normal liver cell lines were obtained from National Center for Cell Sciences, Pune, Maharashtra. >All cell lines were propagated in Minimum Essential Medium (Eagle) with 2 mM l-glutamine and Earle's BSS (Balanced Salt Solution) adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate 90%, and 10% fetal calf serum. All cell lines were grown in a humidified incubator at 37°C.
MTT cytotoxicity assay for in vitro anticancer study
The cytotoxicity assay was performed according to the microculture MTT method with slight modifications.[13] The cells were harvested (1.5 × 104 cells/well) and inoculated in 96-well microtiter plates. They were washed with phosphate-buffered saline (PBS) and the cultured cells were then inoculated with and without the extract (1 mg/ml). After 72 h of incubation, the medium was aspirated. Ten microliters of MTT solution (5 mg/ml in PBS, pH 7.2) was added to each well and the plates were incubated for 4 h at 37°C. After incubation, 100 μl of dimethyl sulfoxide (DMSO) was added to the wells followed by gentle shaking to solubilize the formazan dye for 15 min. Absorbance was read at 540 nm and the surviving cell fraction was calculated. Suramin (100 μM) was used as the reference standard for anticancer activity, and H2O2 (1 mM) was used as the cytotoxic agent against normal liver cell lines. The inhibition of cell viability and COX was calculated using the formula:
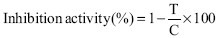
Where T= Absorbance of the test sample
C= Absorbance of the control sample
COX inhibition assay
The assay was performed using colorimetric COX (ovine) inhibitor screening assay kit.[14] Briefly, the reaction mixture contained 150 μl of assay buffer, 10 μl of heme, 10 μl of enzyme (either COX-1 or COX-2), and 10 μl of the plant sample (1 mg/ml). The assay utilizes the peroxidase component of COX. The peroxidase activity was assayed colorimetrically by monitoring the appearance of oxidized N, N, N′, N′-tetramethyl-p-phenylenediamine (TMPD) at 590 nm. Aspirin (acetylsalicylic acid, 1 mM) was used as the reference anti-inflammatory compound.
Antioxidant study
DPPH radical scavenging assay
DPPH radical scavenging assay was carried out as per the method reported earlier, with slight modifications.[15,16] Briefly, 1 ml of the test solution (individual plant extracts) was added to an equal quantity of 0.1 mM solution of DPPH in ethanol. After 20 min of incubation at room temperature, the DPPH reduction was measured by reading the absorbance at 517 nm. Ascorbic acid (1 mM) was used as the reference compound.
Hydroxyl (OH) radical scavenging assay
The OH radical scavenging activity was determined using Fenton reaction.[17] The reaction mixture contained 60 μl of FeCl2 (1 mM), 90 μl of 1,10-phenanthroline (1 mM), 2.4 ml of phosphate buffer (0.2 M, pH 7.8), 150 μl of H2O2 (0.17 M), and 1.5 ml of individual plant extracts (1 mg/ml). The reaction was started by adding H2O2. After 5 min incubation at room temperature, the absorbance was recorded at 560 nm. Ascorbic acid (1 mM) was used as the reference compound.
Superoxide radical scavenging assay
The superoxide anion scavenging assay was performed by the method reported earlier.[18] Superoxide anion radicals were generated in a non-enzymatic PMS–NADH system through the reaction of PMS, NADH, and oxygen. It was assayed by the reduction of NBT. In this experiment, superoxide anion was generated in 3 ml of Tris HCl buffer (100 mM, pH 7.4) containing 0.75 ml of NBT (300 μM), 0.75 ml of NADH (936 μM), and 0.3 ml of the plant sample (1 mg/ml). The reaction was initiated by adding 0.75 ml of PMS (120 μM) to the mixture. After 5 min of incubation at room temperature, the absorbance at 560 nm was measured in a spectrophotometer. Ascorbic acid (1 mM) was used as the reference compound.
RESULTS
Sequential extraction and HPTLC profiling
The powdered plant samples were extracted sequentially in hexane, ethanol, and water. HPTLC fingerprint of the derivatized flavonoids is shown in Figure 1. The plants were found to contain diverse flavonoids. However, none of the plants showed the presence of rutin (used as marker flavonoid).
Figure 1.
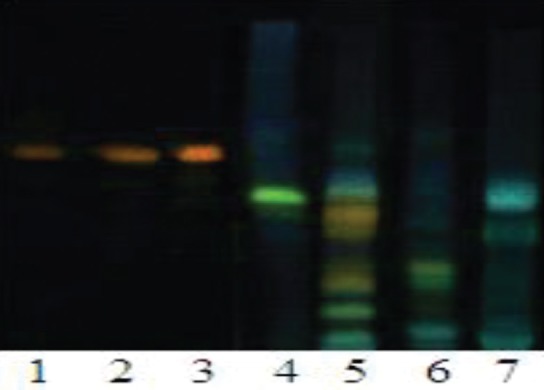
HPTLC profile of the selected medicinal plant extracts. Track No. 1, rutin (50 μg); 2, rutin (100 μg); 3, rutin (200 μg); 4, Tinospora cordifolia; 5, Lavandula bipinnata, 6, Helicteres isora; 7, Soymida fembrifuga
Anticancer activity of the selected medicinal plants
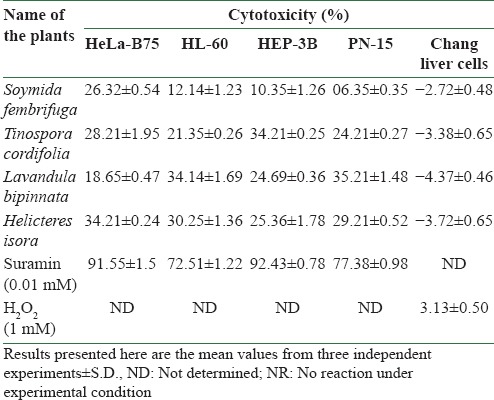
The results of cytotoxicity of the selected plant samples (ethanol extract) against the selected cancer cells are summarized in Table 1. It was observed that the ethanol phytofraction (1 mg/ml) of all the tested plants showed promising anticancer activity toward the selected cancer cell lines. L. bipinnata possessed significant anticancer activity by inhibiting PN-15 (35.21 ± 1.48%), while S. fembrifuga showed moderate inhibition toward PN-15 (06.35 ± 0.35%). All the plants under investigation were found to possess moderate cytotoxic activity toward HeLa-B75 and HL-60 cell lines, showing the activity in the range of 12.14-34.21%. The cytotoxic activity was compared with suramin (0.01 mM), which was used as the standard anticancer drug (HeLa-B75, 91.55%; HL-60, 72.51%; HEP-3B, 92.43%; and PN-15, 77.38%). None of the plant samples showed cytotoxicity toward normal “Chang liver” cells, except the standard H2O2 (3.13%).
Table 1.
Cytotoxic effect of selected medicinal plant extracts (1 mg/ml) on different cancer cell lines and normal chang liver cells
COX inhibitory potential of the selected medicinal plants
The results of COX inhibitory activity of the ethanolic fraction of the selected medicinal plants are shown in Figure 2. It is interesting to note that all the plant samples preferentially inhibited COX-2 rather than COX-1. L. bipinnata showed the maximum activity by inhibiting COX-2 (50.43 ± 0.39%) more than COX-1 (18.63 ± 0.31%), while all other plant samples inhibited COX-1 and COX-2 in the range of 03.93–23.53% and 19.66-50.43%, respectively. Aspirin (COX-1, 08.83 ± 0.37% and COX-2, 08.83 ± 0.37%) was used as the standard anti-inflammatory agent.
Figure 2.
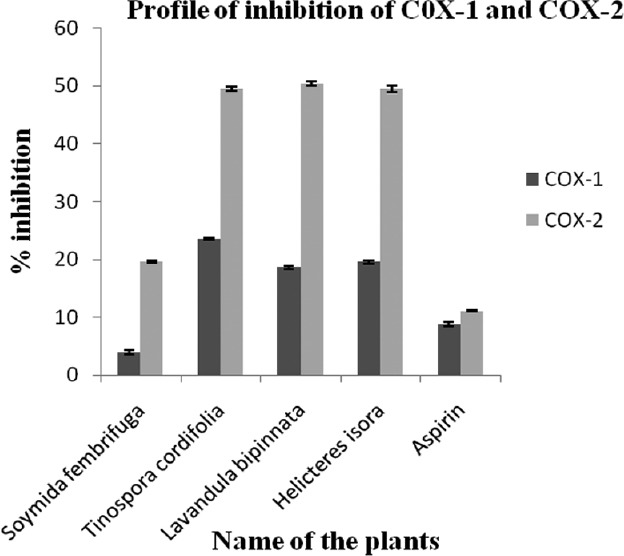
Inhibition of COX-1 and COX-2 by the selected medicinal plants. Data are shown as mean ± SD of three similar experiments
Antioxidant study
DPPH radical scavenging activity
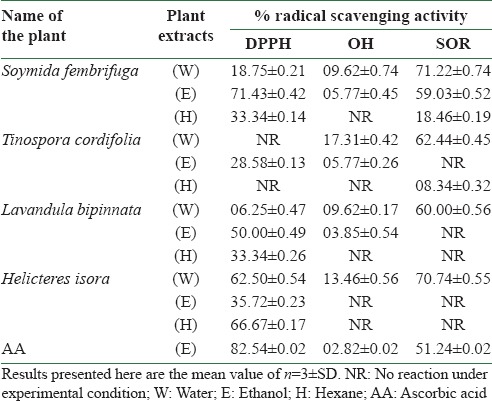
The DPPH radical scavenging assay is used for preliminary screening of the plant extracts for their antioxidant activity. The proton radical scavenging action is known to be an important mechanism of antioxidants. The results of this assay are summarized in Table 2. Overall, it was observed that the ethanolic fraction of all the selected plants was more potent in stabilizing DPPH radicals. Moreover, the ethanol extract of S. fembrifuga (71.43 ± 0.42%) possessed the highest DPPH radical scavenging ability, while for the rest of the plants, it was found to be in the range of 06.25-66.67%, as compared to ascorbic acid (82.54 ± 0.02%).
Table 2.
Antioxidant activity of the selected medicinal plants
OH radical scavenging activity
The OH radicals are the most hyperreactive amongst the relative oxygen species and affect every type of molecule found in the living system. Physiologically important biomolecules such as sugar, amino acids, phospholipids, DNA bases, and organic acids may undergo reaction with OH radicals and may change the normal physiological function of cells. The OH radical scavenging activity of the selected medicinal plants is shown in Table 2. The water and ethanol extracts showed moderate OH radical scavenging activity in the range of 03.85-17.31%, but not the hexane extract of the individual plant samples. Ascorbic acid (02.82 ± 0.02%) was used as the reference compound.
SO radical scavenging activity
The results of the SO radical scavenging activity of the selected medicinal plants are shown in Table 2. Water extract of all the selected plants was found to be an excellent SO radical scavenger, showing the activity in the range of 60.00-71.22%. However, S. fembrifuga extracts showed promising SO radical scavenging activity in the order hexane, ethanol, and water (18.46, 59.03, and 71.22%, respectively). A poor activity was observed in the hexane extract of T. cordifolia (08.34%).
DISCUSSION
Over the past decade, herbal medicines have been appreciated and accepted all over the world and they have made an impact on both global health and international trade. Hence, medicinal plants continue to play an important role in the healthcare system of a majority of the world's population.[19] Traditional medicine is widely used in India. Even in the US, the use of plants and phytomedicines has increased dramatically in the last two decades, and as a result, a National Centre for Complementary and Alternative Medicine has been established there. The herbal products have been classified under “dietary supplements” and are included with vitamins, minerals, amino acids and other products intended to supplement the diet.[20] In fact, there are several medicinal plants all over the world, including India, which are being used traditionally for the prevention and treatment of cancer. However, only a few medicinal plants have attracted the interest of scientists to investigate the remedy for neoplasm (tumor or cancer).
The plants consist of various phytochemicals which dissolve in specific solvents. According to this, the polar (ethanol, water) and nonpolar (hexane) solvents were selected for the extraction purpose. While describing the mechanism of anticancer, anti-inflammatory, and antioxidant activities of the medicinal plants, various phytochemicals seem to be associated with these activities. In particular, the phytochemicals such as vitamins (A, C, E, K), carotenoids, terpenoids, flavonoids, polyphenols, alkaloids, tannins, saponins, pigments, enzymes, and minerals have been found to elicit antioxidant activities.[21,22] With regard to the management of cancers, ellagic acid and a whole range of flavonoids, carotenoids, and terpenoids present in Fragaria vesca (strawberries) and Rubus idaeus (raspberries) have been reported to be responsible for the antioxidant activity. These chemicals block various hormonal actions and metabolic pathways that are associated with the development of cancer.[23,24] A whole variety of phenolic compounds, in addition to flavonoids, are widely distributed in grains, fruits, vegetables, and herbs. Phenolic compounds such as caffeic and ferulic acids, sesamol, and vanillin have been reported to exhibit antioxidant and anticarcinogenic activities and inhibit atherosclerosis.[25,26]
The severe side effects of the presently used nonsteroidal anti-inflammatory drugs (NSAIDs) have resulted in either withdrawal or replacement of these drugs (nimesulide, bromfenac, ibufenac, and benoxaprofen) from the pharmaceutical market. Selective COX-2 inhibitors were described to be very effective in the management of inflammatory disorders. However, nonselective COX-1 inhibition by these agents imposed restrictions on the usage of selective COX-2 inhibitors on health grounds. In an attempt to discover novel anti-inflammatory agents targeting COX, the drug candidates selectively inhibiting COX-2 and skipping COX-1 are more appreciated as safe anti-inflammatory drugs.[27] It is interesting to note that the selected plants preferentially inhibited COX-2 activity more than that of COX-1. This finding indicates the significance of the selected plants as a potential resource for the discovery of novel leads for maneuvering them as effective and safe anti-inflammatory/anticancer agents.
CONCLUSION
It can be summarized that the plants selected in the present study having importance in traditional medicine can be considered as a source for the isolation, identification, and development of novel and effective anticancer, anti-inflammatory, and antioxidant agents. Nevertheless, the research data of the present findings may serve as a guideline for the standardization and validation of natural drugs containing the selected medicinal plants as ingredients.
ACKNOWLEDGMENTS
Authors are thankful to University Grant Commission (UGC), New Delhi (India), for financial assistance [F. No. 33-157/2007 (SR)]. RUS thanks UGC for the JRF.
REFERENCES
- 1.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2008;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 2.Doll R, Peto R. 4th ed. USA: Oxford University Press; 2003. Malignant diseases Text Book of Medicine; pp. 483–4. [Google Scholar]
- 3.Soliman AS, Samad S, Banerjee M, Chamberlain RM, Robert M, Aziz Z. Brief Continuing Medical Education (CME) Module Raises Knowledge of Developing Country Physicians International Electronic. J Health Educ. 2006;9:31–41. [Google Scholar]
- 4.American Cancer Society, Facts and figures. 1999 [Google Scholar]
- 5.Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–81. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–61. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Warren JS. Interleukins and tumor necrosis factor in inflammation. Crit Rev Clin Lab Sci. 1990;28:37–59. doi: 10.3109/10408369009105897. [DOI] [PubMed] [Google Scholar]
- 8.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, et al. Increased expression of cyclooxygenase-2 occurs frequently in human lung cancers, specifically adenocarcinomas. Cancer Res. 1998;58:3761–4. [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 10.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 11.Nipun D, Vijay S, Jaykumar B, Kirti SP, Richard L. Antitumor Activity of Dendrophthoe falcata against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Pharma Crops. 2011;2:1–7. [Google Scholar]
- 12.Naik VN. 1, 2. Aurangabad, (MS), India: Amrut Prakashan; 1998. Flora of Marathwada. [Google Scholar]
- 13.Kawase M, Motohashi N, Satoh K, Sakagami H, Nakashima H, Tan S, et al. Biological activity of Persimmon (Diospyros kaki) peel extracts. Phytother Res. 2003;17:495–500. doi: 10.1002/ptr.1183. [DOI] [PubMed] [Google Scholar]
- 14.Maurias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, et al. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure activity relationship. Bioorg Med Chem. 2004;12:5571–8. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Roesler R, Malta LG, Carrasco LC, Pastore G. Evaluation of the antioxidant Properties of the Brazilian Cerrado fruit Annona crassiflora (Araticum) J Food Sci. 2006;71:102–7. [Google Scholar]
- 16.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 17.Rollet-Labelle E, Grange MJ, Elbim C, Marquetty C, Gougerot-Pocidalo MA, Pasquier C. Hydroxyl radicals as a potantial intracellular mediator of polymorpho nuclear neutrophil apoptosis. Free Radic Biol Med. 1998;24:563–72. doi: 10.1016/s0891-5849(97)00292-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Ooi VE, Chang ST. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 1997;60:763–71. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 19.Akerele O. Medicinal plants and primary health care: An agenda for action. Fitoterapia. 1988;59:355–63. [Google Scholar]
- 20.Rao KV, Schwartz SA, Nair HK, Aalinkeel R, Mahajan S, Chawda R, et al. Plant derived products as a source of cellular growth inhibitory phytochemicals on PC-3M, DU-145 and LNCaP prostate cancer cell lines. Curr Sci. 2004;87:1585–8. [Google Scholar]
- 21.Kathiresan K, Boopathy NS, Kavitha S. Coastal vegetation-an underexplored source of anticancer drugs. Nat Prod Radiol. 2006;5:115–9. [Google Scholar]
- 22.Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–9. [PubMed] [Google Scholar]
- 23.Caragay AB. Cancer-preventative foods and ingredients. Food Technol. 1992;46:65–8. [Google Scholar]
- 24.Steinmetz KA, Potter JD. Vegetables, fruits and cancer, I. Epidemiology. Cancer Causes Control. 1991;2:325–57. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 25.Craig WJ. Phytochemicals: Guardians of our Health. J Am Diet Assoc. 1997;97(10 Suppl 2):S199–204. doi: 10.1016/s0002-8223(97)00765-7. [DOI] [PubMed] [Google Scholar]
- 26.Decker EA. The role of phenolics, conjugated linoleic acid, carnosine, and pyrroloquinoline quinone as nonessential dietary antioxidants. Nutr Rev. 1995;53:49–58. doi: 10.1111/j.1753-4887.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 27.Bandgar BP, Patil SA, Gacche RN, Korbad BL, Hote BS, Kinkar SN, et al. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg Med Chem Lett. 2010;20:730–3. doi: 10.1016/j.bmcl.2009.11.068. [DOI] [PubMed] [Google Scholar]


