Abstract
This study aimed at determining the effect of indigenously prepared neem and mango chewing stick mouthwashes on plaque and gingival indices. A sample of 105 children aged 12-15 years was randomized into three groups, namely neem, mango, and chlorhexidine mouthwash groups. All the children were examined at baseline and gingival and plaque indices were recorded. Baseline scores for plaque and gingivitis were fair and moderate, respectively, in all the three groups and there existed no statistically significant difference among them. Ten millilitres each of herbal and chlorhexidine mouthwashes (0.2%) were administered according to the group allocation twice daily for 21 days. Indices were reassessed at 21 days (immediately after intervention) and at 1 month, 2 months, and 3 months after discontinuing the mouthwashes. Statistically significant reduction (P < 0.001) in plaque index was found in all the three mouthwash groups at 21 days and at 1 month from discontinuing the mouthwash. Chlorhexidine additionally showed statistically significant reduction in plaque index at 2 months from discontinuing the mouthwash. Statistically significant reduction (P < 0.001) in gingival index was found in all the three mouthwash groups at 21 days (immediately after discontinuing the mouthwash) and at 1 and 2 months from discontinuing the mouthwash. To conclude, all the three mouthwashes were effective antiplaque and antigingivitis agents. Chlorhexidine and neem possess equivalent efficacy in reducing plaque, while chlorhexidine has superior antigingivitis properties.
Keywords: Azadirachta indica, Gingival index, Mangifera indica, Mouthwash, Plaque index
INTRODUCTION
Research has linked oral microorganisms, particularly those with adherent biofilm properties, to clinically specific oral conditions such as dental caries, periodontal disease, and oral malodor.[1,2] Plaque reduction has been the hallmark of preventive dentistry since the advent of antibiotics and the realization that bacteria are possible causative agents of the major dental diseases, caries, and periodontal disease.[3]
Mechanical hygiene procedures such as tooth brushing, interdental brushing, and dental floss are the key methods of plaque control. However, despite the potential for adequate mechanical plaque control, clinical experience and population-based studies demonstrate that such methods are not being employed sufficiently by large numbers of the population.[2,4]
Chemical methods of reducing plaque, such as mouthwashes, are less technically demanding alternatives to mechanical plaque control.[5] Chlorhexidine is the most popular mouthwash which has been recognized by the pharmaceutical industry as the positive control against which the efficacy of alternative antiplaque agents should be measured, and has earned its eponym of gold standard.[6] But its long-term usage may result in various side effects.[3] An effective substitute to chlorhexidine with all the good qualities and sans its unpleasant effects is highly desirable and has been long awaited.
Plants have been exploited by man for many centuries as sources of chemotherapeutic and other medicinal drugs due to the presence of various bioactive compounds. These herbal products are not only economical, but also have minimal side effects.
Brushing with neem and mango twigs and chewing neem leaves and seeds after a meal have been the traditional dental care practices in India. Stems of Azadirachta indica (neem) contain substances like nimbin and nimbidin which have anti-inflammatory and broad-spectrum antimicrobial activities.[7] The natural C-glucoside xanthone mangiferin, a phenolic compound, has been reported in various parts of Mangifera indica leaves, fruits, stem, bark, heartwood, and roots.[8,9,10,11,12] It is known to possess antioxidant, radioprotective, immunomodulatory, antitumor, anti-allergic, anti-inflammatory, antidiabetic, and antimicrobial properties.[13] Mangiferin has also demonstrated promising therapeutic potential both in the prevention and treatment of periodontitis.[14] In vitro studies indicate that neem and mango stick extracts are inhibitory to oral streptococci which are responsible for various oral diseases.[15,16,17,18] Literature review revealed very few in vivo studies worldwide assessing the effects of neem and mango stick extracts on plaque and gingiva. Hence, the present study was planned to evaluate the effect of neem and mango on plaque and gingival scores in high school children of Belgaum city.
MATERIALS AND METHODS
The present study was a triple-blind randomized controlled field trial conducted to evaluate the effectiveness of two herbal mouthrinses (neem and mango) on plaque and gingival scores of 12-15-year-old school children in Belgaum city. Permission to conduct the study was obtained from the institutional review board of KLE VK Institute of Dental Sciences, Belgaum, Karnataka, India, Deputy Director of Public Instruction (DDPI), Belgaum, and the principal of the selected high school.
A pilot study was conducted on 10 people to determine the acceptability, palatability, and safety of the mouthwashes. The required sample size was estimated based on the difference in the plaque and gingival scores between the study and control groups. Sample size was calculated based on the minimum difference expected between the two groups, which was 0.7.
Two examiners were selected to ensure blind evaluation of the study participants. Examiner 1 (principal investigator) selected the schools, obtained permission from them, did primary screening (examination for inclusion and exclusion criteria, which included baseline clinical examination) and selection, collected baseline data, and administered the mouthwashes for 21 days. Examiner 2 recorded the plaque and gingival scores after 21 days (immediately after the intervention) and 1 month, 2 months, and 3 months after discontinuing the mouthwash. Examiner 2 was blinded to the type of mouthwashes admistered. The statistician remained blinded regarding the subject allocation to the three groups. Both the examiners were trained and calibrated before the start of study in the Department of Public Health Dentistry, KLE VK Institute of Dental Sciences, Belgaum under the guidance of a professor in order to limit the intra-examiner and inter-examiner variability. They were reassessed for satisfactory agreement at various time intervals during the clinical examinations. Recording assistants were trained in documenting the readings accurately. The intra-examiner and inter-examiner variabilities were calculated using Kappa statistics. Inter-examiner and intra-examiner variability (Kappa) for plaque index and gingival index ranged from 0.8 to 0.9 and from 0.7 to 0.8, respectively, during all the assessments.
For obtaining the study sample, two-stage random sampling was done. In the first stage, a list of all the schools was obtained from DDPI, Belgaum. From these schools, one school was selected by lottery method. Written informed consent was obtained from the parents of all the children examined. Children were free to withdraw from the study at any point during the study period.
During the initial phase of study (before selecting the sample), a 29-item self-designed combination of closed and open-ended questionnaire and assessment form was prepared to collect information regarding socio-demographics, oral hygiene practices, and food habits from all the children aged 12-15 years. All the children were also clinically examined for the inclusion and exclusion criteria, as mentioned later. This form was divided into five parts:
First part (13 questions) pertained to socio-demographic data
Second part (9 questions) dealt with self-reported oral hygiene practices of children
Third part (4 questions) was concerned with food habits
Fourth part (3 questions) was concerned with their use of mouthwashes, medication, and presence of any systemic, chronic diseases
Fifth and last part of this form was designed to record DMFT, plaque and gingival indices.
Inclusion and exclusion criteria used were as follows.
Inclusion criteria
Free from systemic diseases
Gingival scores were moderate and plaque scores were fair according to the plaque and gingival indices proposed by Silness and Loe[19] and Loe and Silness,[20] respectively
DMFT scores between 3 and 6
Should not have used mouthwashes for the last 1 month
All the index teeth should be completely erupted
Parents should give informed consent.
Exclusion criteria
Suffering from diseases which might affect the salivary flow
History of antibiotic therapy in the previous 1 month till the start of the study
Retained deciduous teeth
Currently using any mouthwashes or has used mouthwash in the past 1 month
Suffering from any physical disability.
One hundred and five subjects were randomly selected from the eligible population (who fulfilled the inclusion criteria) and randomized into three groups, namely mango, neem, and chlorhexidine mouthwash groups, having 35 participants each. Randomization was done using lottery method by a person not involved with the study proceedings. All the parameters (including food habits, oral hygiene habits, etc.) were assessed statistically. No statistically significant difference was found among the three groups.
All the children were administered mango, neem, and chlorhexidine mouthwashes (0.2%) according to the group they were assigned to, twice daily for 21 days. Measured amount (10 ml) of mouthwashes was poured in plastic cups and given to children. All the children were asked to take mouthwash into their mouths and start swishing the mouthwash upon a prompt from the investigator who stood with a stop watch to record the time. After swishing the mouthwash for 30 sec as recorded in the stop watch, children were asked to spit in the nearby wash basin. They were also asked not to eat or drink anything for 30 min. Children were instructed to use 10 ml of mouthwash as prescribed, under parents’ supervision at night time after dinner. Every participant was provided with 75 ml of their respective mouthwashes for home use on a weekly basis. Before start of the intervention, all the children were instructed to rinse with the mouthwashes given to them in the night, before going to bed, and not to eat or drink anything for atleast half an hour after rinsing. Positive reinforcements were given from time to time. No oral prophylaxis was done prior to commencement of intervention. Children were allowed to follow their individual oral hygiene procedures.
After 21 days of mouthwash administration, plaque index and gingival index were reassessed. Parameters were reassessed at monthly intervals for 3 months, i.e. at 1 month, 2 months, and 3 months after discontinuing the mouthwash. Children were blinded as to which mouthwash they were receiving. Second examiner was blinded to the group allocation, meaning he did not know which child was assigned to which mouthwash group. The statistician remained blinded regarding the subject allocation to the three groups.
Preparation of mouthwash
Procurement and drying of the tree sticks
Mango and neem trees are available in abundance in India. The small branches of these trees were freshly cut. Leaves from the concerned branches procured were removed and immediately the branches measuring 4 inches in length were cut. These cut sticks were cleaned thoroughly in a disinfecting solution (2% povidone iodine) and washed in running water for about 10-15 min to remove all traces of dirt and extraneous contaminating material. Final wash was done with distilled water. These branches were dried in direct sunlight keeping each variety on separate sheets of double filter paper. Sticks were covered with filter paper during drying to prevent contamination. Drying was carried out till the sticks were completely dehydrated and became easily breakable. Both types of twigs were stored in separate containers and labeling was done to avoid mixing with other branches during the following procedures.
Preparation of neem and mango sticks powder
The extracts of the above chewing sticks were prepared for each one separately, starting with the mango sticks. The dried sticks were cut into smaller pieces using a twig cutter and pulverized to fine powder using a Kenstar® high-speed electric grinder for 15 min. The powder was transferred to separate sterile, airtight plastic containers with lids and each container was labeled with the name of the respective plant. Similar procedure was adopted for the neem sticks. After preparation of powder of each variety, the electric mixer was thoroughly cleaned with distilled water and dried thoroughly before commencing the preparation of the powder of the other variety of chewing sticks. Finally, two containers of the powders of different chewing sticks were obtained which were labeled and kept in cool, dry conditions till further use.
Preparation of mouthwash solution
Cold maceration technique was employed. The obtained powders of mango and neem were weighed individually into 50 g using Digiweigh® electronic weighing machine and put into separate sterile containers to which sterile deionized distilled water was added using a measuring jar to make the final volume of 100 ml. The container was then shaken well manually for 5 min to mix the powder well with water before keeping it in the refrigerator at 4°C. The mixture was allowed to soak for 48 h at 4°C in the refrigerator. After 48 h, the mixture was filtered using a filter paper. Sweetening agent (30% sucralose, code E955) and preservative (0.05% sodium benzoate, code 211 and 0.01% sodium methyl paraben, code 218) were added to obtain the final mouthwash.
Statistical analysis
Data were entered in Microsoft Excel and analyzed using SPSS for Windows, Version 17 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to calculate frequencies, percentages, and mean values. Analysis of variance (ANOVA) was applied to know whether the differences in the plaque index and gingival index of the three groups being compared were statistically significant or not. Tukey's post hoc test was used to know the difference between the pairs of mouthwashes. Student's paired t-test was applied to know whether the differences in the plaque index and gingival index of the three groups before and after intervention were statistically significant or not. A P value of less than 0.05 was taken as statistically significant.
RESULTS
At the onset of the study, there were 105 participants (35 in each group). Final assessment was made on 97 participants as 8 participants dropped out (3 in mango group, 2 in neem group, and 3 in chlorhexidine group). Taste was the main governing factor for children dropping out of herbal mouthwashes group as neem and mango are known for their bitter and astringent taste, respectively (mango = 2, neem = 2). Two participants were excluded from the study on 12th and 16th day, respectively, as they acquired throat infection and were put on antibiotics by their physicians (chlorhexidine = 1, mango = 1). One participant dropped out because he had to take a leave of absence from the school (chlorhexidine = 1). One participant was excluded as he revealed that he was not able to comply with home rinsing regimen for all the days.
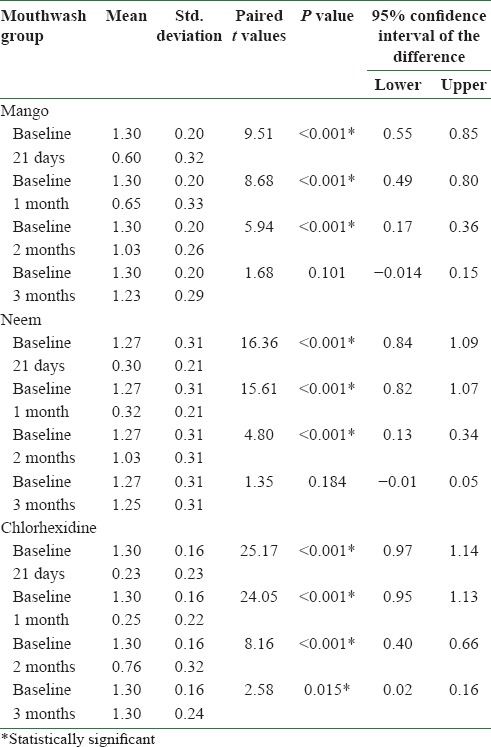
There was no statistically significant difference among the three mouthwash groups at baseline with respect to age, sex, plaque and gingival indices [Tables 1 and 2]. Statistically significant reduction (P < 0.001) in plaque index was found in all the three mouthwash groups at 21 days and at 1 month after discontinuing the mouthwash [Table 3]. Chlorhexidine additionally showed statistically significant reduction in plaque index (P = 0.001) at 2 months of discontinuing the mouthwash. Statistically significant reduction (P < 0.001) in gingival index was found in all the three mouthwash groups at 21 days and at 1 and 2 months after discontinuing the mouthwash [Table 4]. Chlorhexidine sustained statistically significant reduction in gingival index (P = 0.015) at 3 months of discontinuing the mouthwash [Table 4].
Table 1.
Age and sex distribution in the three mouthwash groups
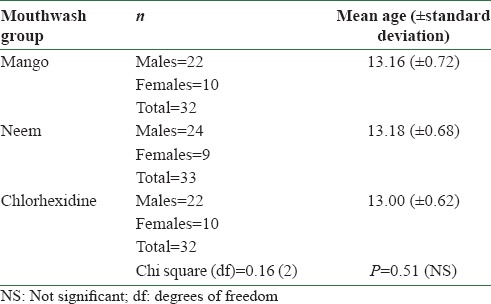
Table 2.
Mean gingival and plaque indices at baseline
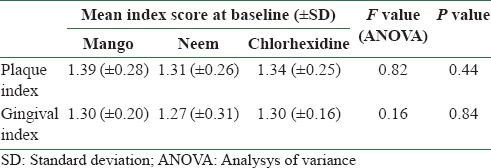
Table 3.
Comparison of plaque scores at baseline with plaque scores at 21 days and at 1 month, 2 months, and 3 months after discontinuing the mouthwash in different mouthwash groups
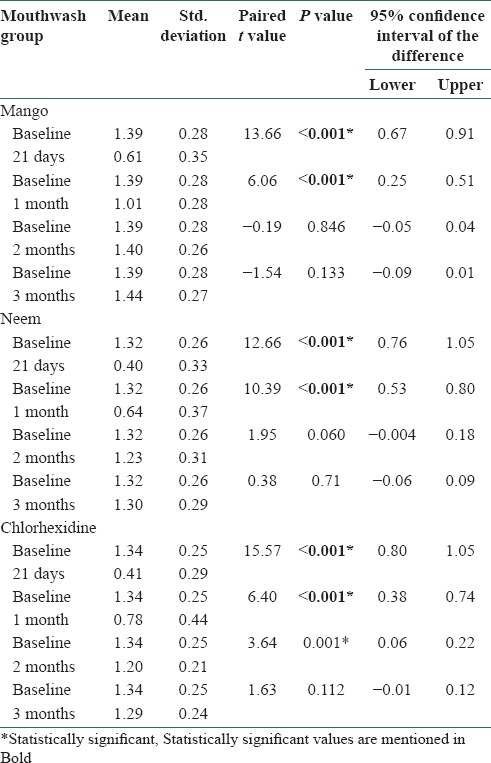
Table 4.
Comparison of gingival scores at baseline with gingival scores at 21 days and at 1 month, 2 months, and 3 months after discontinuing the mouthwash in different mouthwash groups
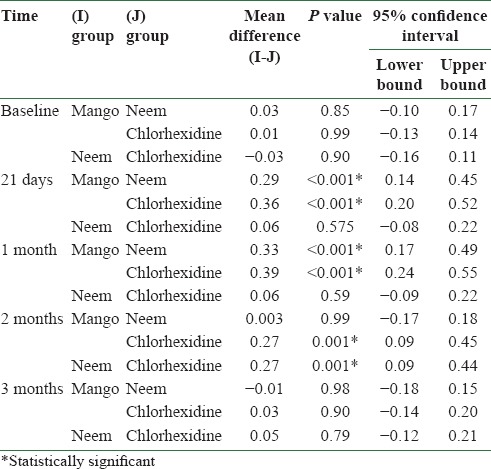
There was a statistically significant difference between the plaque scores of mango and neem mouthwashes at 21 days (P = 0.036), and at 1 month (P ≤ 0.001) and 2 months (P = 0.027). Mango and chlorhexidine also differed significantly at 1 month (P = 0.36) and 2 months (P = 0.01) [Table 5]. There was a statistically significant difference between the gingival scores of mango and neem mouthwashes at 21 days (P < 0.001) and at 1 month (P ≤ 0.001). Mango and chlorhexidine also differed significantly at 21 days (P < 0.001), and at 1 month (P = 0.001) and 2 months (P = 0.001). Gingival scores of neem and chlorhexidine differed significantly at 2 months (P = 0.001) [Table 6].
Table 5.
Comparison of mean plaque scores of different mouthwash groups at baseline, 21 days, and at 1 month, 2 months, and 3 months after discontinuing the mouthwash
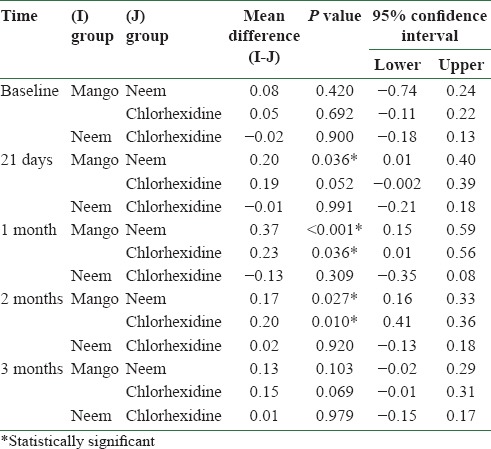
Table 6.
Comparison of mean gingival scores of different mouthwash groups at baseline, 21 days, and at 1 month, 2 months, and 3 months after discontinuing the mouthwash
DISCUSSION
Various studies have shown a high caries and periodontal disease prevalence in India.[21,22,23,24,25,26,27,28] This high prevalence of oral diseases may be ascribed to the fact that majority of population gives a low priority to oral health, and basic oral health education and simple interventions are not available, especially to rural and underprivileged strata of the society.[29]
Traditionally, in all parts of the world, mechanical removal of plaque is the most common method for preventing orodental diseases. But at the same time, evidence suggests that mechanical cleaning methods are inadequate.[30,31,32,33] Chemical antiplaque agents are a newer concept, but it is gradually taking roots. To large percentages of even the most affluent and developed societies, wholesale use of more expensive chemical antiplaque formulations would be quite restrictive due to high expense or ignorance.[34] The World Health Organization estimates that 65-80% of the world's population uses traditional medicine as the primary form of health care.[35] This study was an attempt to investigate if indigenously prepared mouthwashes from neem and mango chewing sticks can effectively reduce plaque and gingival scores in the selected population.
The age group selected to carry out this study was 12-15 years. Children in this age group are vulnerable to both caries and gingival and periodontal problems due to the changes in dietary habits and lifestyle.[36] Herbal mouthwashes were prepared based on the findings of an in vitro study conducted by Prashant et al.[18] In this study, 50% extracts of mango and neem chewing sticks were found to be most effective in inhibiting Streptococcus mutans. Identical extract was made to be used as a mouthwash in the present study. Artificial sweetening agent was added to make the taste pleasant. No flavoring agent was added as most of the flavoring agents like thymol, menthol, etc., are essential oils which might act as confounders in plaque and gingival assessment, as the essential oils are excellent plaque inhibitors and just as popular as chlorhexidine.[37] Sodium benzoate is the sodium salt of benzoic acid. It has long been used as a preservative in foods and other products, and its safety has been established. In the US, sodium benzoate is used at a concentration of 0.03-0.1%.[38] Methyl paraben is a methyl ester of p-hydroxybenzoic acid. It is a stable, non-volatile compound that has been used as an antimicrobial preservative in foods, drugs, and cosmetics for over 50 years.[39] Both the preservatives were used at a much lower concentration than what was found to inhibit oral bacteria.[40,41,42,43] Literature suggests that both neem and mango stick extracts have slightly acidic pH, but the pH is lesser than the tooth critical pH.[13,17] The preservative methyl paraben is effective in a wide range of pH (4-8), while sodium benzoate is most effective at pH 4-4.5.[40,42]
A study done by Siswomihardjo et al., showed that neem stick extract had higher antibacterial properties than the leaves extract.[44] Hence, chewing sticks were preferred to prepare the mouthwash over leaves.
Chlorhexidine mouthwash was employed as control mouthwash in the present study. Children were made to rinse with 10 ml of mouthwashes for 21 days twice daily – once in the morning in school and then again in the night at home after dinner. The time interval between these two rinsings was roughly 12 h. This time table conformed well to the standardized regimen of chlorhexidine mouthwash which has to be used at 12-hourly intervals as it has been shown to suppress salivary bacterial counts for over 12 h.[45]
Plaque scores
After rinsing with the respective mouthwashes, statistically significant differences were found between neem, chlorhexidine, and mango at 21 days, 1 month, and 2 months intervals. Neem and chlorhexidine showed no difference in the mean plaque scores at these time intervals. This implies that neem was equally effective in inhibiting plaque as chlorhexidine. Similar results were obtained by Botelho et al., in which mouthwash prepared from neem leaves demonstrated similar efficacy to that of chlorhexidine mouthwash.[46] In contrast to our findings, neem extract gel was found to be more effective than chlorhexidine mouthwash in a study done by Pai et al.[7] In another study conducted by Patel et al.,[47] neem showed better efficacy in reducing human plaque culture and gram-negative bacteria, compared to commercially available toothpaste. In another study conducted by Sharma et al.,[48] neem mouthwash reduced plaque and gingival indices, but was not as effective as chlorhexidine.
When compared with baseline, all the three mouthwashes showed statistically significant decrease in the plaque indices at 21 days and at 1 month after stopping the mouthwash. Only chlorhexidine sustained substantial plaque inhibiting effect till 2 months when compared to the herbal mouthwashes. Plaque indices returned to baseline levels at 3 months evaluation in all the mouthwashes. Neem mouthwash was shown to be effective in reducing plaque indices in the studies conducted by Botelho et al. and Sharma et al.[46,48] Neem gel was effective in reducing plaque scores in 3 and 6 weeks of evaluation in a study conducted by Pai et al.[7] Prashant et al., Wolinsky et al., Siswomihardjo et al., Bhuiyan et al., Almas et al., and Subramaniam et al. have carried out in vitro studies which showed the effectiveness of neem extract against plaque-forming bacteria.[15,16,17,18,38,49]
Gingival scores
After using the mouthwashes, significant differences in gingival indices were found at 21 days and till 2 months from stopping the mouthwash. Neem and chlorhexidine mouthwashes had equivalent effect on gingival scores at 21 days and at 1 month evaluation, subsequent to which neem showed significantly higher gingival scores at 2 months evaluation when compared to chlorhexidine. Botelho et al., conducted a study in which neem and chlorhexidine mouthwashes showed similar improvements in gingival indices after 7 days and 1 month from stopping the mouthwashes.[46] In contrast to our findings, Sharma et al., found that neem was not as effective as chlorhexidine in reducing gingival indices.[48] Another study conducted by Bhat et al., found that toothpaste containing neem extract reduced plaque and gingivitis significantly at the end of the 3-month study period.[50] No human in vivo study examined the effect of mango extract on gingivitis. Carvalho et al. found that mangiferin derived from mango prevents periodontitis in Wistar rats.[14]
Limitations
Present study was a short-term study employing a crude extract of neem and mango twigs as mouthrinse. Though significant results were obtained at 21 days and 1 month in the herbal groups, long-term clinical efficacy (6 months as prescribed by American Dental Association) and adverse effects associated with long-term usage could not be assessed.[51]
CONCLUSION
This study provided sufficient data to suggest that neem and mango extract mouthwashes have a beneficial effect on oral health. Plaque and gingival scores were reduced in both the experimental mouthwash groups to such an extent that it warrants an in-depth evaluation of both mango and neem extracts through long-term studies.
REFERENCES
- 1.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 2.Bascones A, Morante S, Mateos L, Mata M, Poblet J. Influence of additional active ingredients on the effectiveness of non-alcoholic Chlorhexidine mouthwashes: A randomized controlled trial. J Periodontol. 2005;76:1469–75. doi: 10.1902/jop.2005.76.9.1469. [DOI] [PubMed] [Google Scholar]
- 3.Fine DH. Chemical agents to prevent and regulate plaque development. Periodontol 2000. 1995;8:87–107. doi: 10.1111/j.1600-0757.1995.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 4.Yap AU, Tan BW, Tay LC, Chang KM, Loy TK, Mok BY. Effect of mouthrinses on microhardness and wear of composite and compomer restoratives. Oper Dent. 2003;28:740–6. [PubMed] [Google Scholar]
- 5.Barnett ML. The role of therapeutic antimicrobial mouthrinses in clinical practice: Control of supragingival plaque and gingivitis. J Am Dent Assoc. 2003;134:699–704. doi: 10.14219/jada.archive.2003.0255. [DOI] [PubMed] [Google Scholar]
- 6.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel--a 6-week clinical study. J Ethnopharmacol. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Desai PD, Ganguly AK, Govindachari TR, Joshi BS, Kamat VN, Manmade AH, et al. Chemical investigation of some Indian plants Part II. Indian J Chem. 1966;4:457–549. [Google Scholar]
- 9.El Ansari MA, Reddy KK, Sastry KNS, Nayudamma Y. Dicotyledonae, anacardiaceae polyphenols of Mangifera indica. Phytochemistry. 1971;10:2239–41. [Google Scholar]
- 10.Bhatia VK, Ramanathan JD, Seshadri TR. Constitution of mangiferin. Tetrahedron. 1967;23:1363–8. [Google Scholar]
- 11.Ramanathan JD, Seshadri TR. Constitution of mangiferin. Curr Sci. 1960;29:131–2. [Google Scholar]
- 12.Nigam SK, Mitra CR. Constituents of mango (Mangifera indica) roots. Indian J Chem. 1964;2:378–9. [Google Scholar]
- 13.Wauthoz N, Balde A, Balde ES, Van Damme M, Duez P. Ethnopharmacology of Mangifera indica L. bark and pharmacological studies of its main C-glucosylxanthone, magiferin. Int J Biomed Pharm Sci. 2007;1:112–9. [Google Scholar]
- 14.Carvalho RR, Pellizzon CH, Justulin L, Jr, Felisbino SL, Vilegas W, Bruni F, et al. Effect of mangiferin on the development of periodontal disease: Involvement of lipoxin A4, anti-chemotaxic action in leukocyte rolling. Chem Biol Interact. 2009;179:344–50. doi: 10.1016/j.cbi.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Wolinsky LE, Mania S, Nachnani S, Ling S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J Dent Res. 1996;75:816–22. doi: 10.1177/00220345960750021301. [DOI] [PubMed] [Google Scholar]
- 16.Bhuiyan MM, Nishimura M, Matsumura S, Shimono T. Antibacterial effects of the crude Azadirachta indica Neem bark extract on Streptococcus sobrinus. Pediatr Dent J. 1997;7:61–4. [Google Scholar]
- 17.Almas K. The antimicrobial effects of extracts of Azadirachta indica (Neem) and Salvadora persica (Arak) chewing sticks. Indian J Dent Res. 1999;10:23–6. [PubMed] [Google Scholar]
- 18.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of Mango and Neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 19.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 20.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 21.Moses J, Gurunathan D. Prevalence of dental caries, socio-economic status and treatment needs among 5 to 15 year old school going children of Chidambaram. J Clin Diagn Res. 2011;5:146–51. [Google Scholar]
- 22.Shourie KL. Dental caries in Indian children. Indian J Med Res. 1941;29:709–21. [Google Scholar]
- 23.Tewari A, Chawla HS. Study of prevalence of dental caries in an urban area of India (Chandigarh) J Indian Dent Assoc. 1977;49:231–9. [Google Scholar]
- 24.Dash JK, Sahoo PK, Bhuyan SK, Sahoo SK. Prevalence of dental caries and treatment needs among children of Cuttack (Orissa) J Indian Soc Pedod Prev Dent. 2002;20:139–43. [PubMed] [Google Scholar]
- 25.Dhar V, Jain A, Van Dyke TE, Kohli A. Prevalence of dental caries and treatment needs in the school going children of rural areas in Udaipur District. J Indian Soc Pedod Prev Dent. 2007;25:119–21. doi: 10.4103/0970-4388.36560. [DOI] [PubMed] [Google Scholar]
- 26.Saravanan S, Kalyani V, Vijayarani MP, Jayakodi P, Felix J, Arunmozhi P, et al. Caries prevalence and treatment needs of rural school children in Chidambaram Taluk, Tamil Nadu, South India. Indian J Dent Res. 2008;19:186–90. doi: 10.4103/0970-9290.42948. [DOI] [PubMed] [Google Scholar]
- 27.Antia FE. The dental caries experience of school going children in the City of Bombay. J Indian Dent Assoc. 1962;39:325. [Google Scholar]
- 28.National Oral Health Survey and fluoride mapping 2002-2003. Dental Council of India, New Delhi. 2004 [Google Scholar]
- 29.Shaju JP, Zade RM, Das M. Prevalence of periodontitis in the Indian population: A literature review. J Indian Soc Periodontol. 2011;15:29–34. doi: 10.4103/0972-124X.82261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addy M, Griffiths G, Dummer P, Kingdon A, Shaw WC. The distribution of plaque and gingivitis and the influence of brushing hand in a group of 11-12 year old school children. J Clin Periodontol. 1987;14:564–72. doi: 10.1111/j.1600-051x.1987.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 31.De La Rosa MR, Guerra JZ, Johnson DA, Radike AW. Plaque growth and removal with daily toothbrushing. J Periodontol. 1979;50:661–4. doi: 10.1902/jop.1979.50.12.661. [DOI] [PubMed] [Google Scholar]
- 32.Frandsen A. Mechanical oral hygiene practices. In: Loe H, Kleinman DI, editors. Dental plaque control measures and oral hygiene practices. Oxford: IRL Press; pp. 93–116. [Google Scholar]
- 33.MacGregor ID, Rugg-Gunn AJ. A survey of toothbrushing sequence in children and young adults. J Periodont Res. 1979;14:225–30. doi: 10.1111/j.1600-0765.1979.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 34.Moran JM. Chemical plaque control-prevention for the masses. Periodontol 2000. 1997;15:109–17. doi: 10.1111/j.1600-0757.1997.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Traditional medicine. 2008. [Last accessed on 2011 Sep 28]. Available from: http://www.who.int/mediacentre/factsheets/fs134/en .
- 36.Bimstein E, Matsson L. Growth and development considerations in the diagnosis of gingivitis and periodontitis in children. Pediatr Dent. 1999;21:186–91. [PubMed] [Google Scholar]
- 37.Claffey N. Essential oil mouthwashes: A key component in oral health management. J Clin Periodontol. 2003;30:22–4. doi: 10.1034/j.1600-051x.30.s5.8.x. [DOI] [PubMed] [Google Scholar]
- 38.Heydaryinia A, Veissi M, Sadadi A. A comparative study of the effects of the two preservatives, sodium benzoate and potassium sorbate on Aspergillus niger and Penicillium notatum. Jundishapur J Microbiol. 2011;4:301–7. [Google Scholar]
- 39.Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem Toxicol. 2002;40:1335–73. doi: 10.1016/s0278-6915(02)00107-2. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg D, Hirschfeld Z, Tayeb I, Ben-Yosef S, David A, Friedman M. The effect of parabens in a mouthwash and incorporated into a sustained release varnish on salivary bacteria. J Dent. 1999;27:101–6. doi: 10.1016/s0300-5712(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 41.Doron S, Friedman M, Falach M, Sadovnic E, Zvia H. Antibacterial effect of parabens against planktonic and biofilm Streptococcus sobrinus. Int J Antimicrob Agents. 2001;18:575–8. doi: 10.1016/s0924-8579(01)00436-8. [DOI] [PubMed] [Google Scholar]
- 42.Ostergaard E. Evaluation of the antimicrobial effects of sodium benzoate and dichlorobenzyl alcohol against dental plaque microorganisms. An in vitro study. Acta Odontol Scand. 1994;52:335–45. doi: 10.3109/00016359409029031. [DOI] [PubMed] [Google Scholar]
- 43.Danielsen B, Fejerskov O, Scheie AA. The effects of a mouthrinse containing sodium benzoate and alcohol on the quantity and metabolism of dental plaque. Int Dent J. 1996;46:91–6. [PubMed] [Google Scholar]
- 44.Siswomihardjo W, Badawi SS, Nishimura M. The difference of antibacterial effect of Neem leaves and stick extracts. Int Chin J Dent. 2007;7:27–9. [Google Scholar]
- 45.Moshrefi A. Chlorhexidine. J West Soc Periodontol Periodontal Abstr. 2002;50:5–9. [PubMed] [Google Scholar]
- 46.Botelho M. Efficacy of a mouthrinse based on leaves of the Neem tree (Azadirachta indica) in the treatment of patients with chronic gingivitis: A double blind, randomized controlled trial. J Med Plant Res. 2008;2:341–6. [Google Scholar]
- 47.Patel VK, Venkatakrishna-Bhatt H. Folklore therapeutic indigenous plants in periodontal disorders in India (review, experimental and clinical approach) Int J Clin Pharmacol Ther Toxicol. 1988;26:176–84. [PubMed] [Google Scholar]
- 48.Sharma S, Saimbi CS, Koirala B, Shukla R. Effect of various mouthwashes on the levels of interleukin-2 and interferon-gamma in chronic gingivitis. J Clin Pediatr Dent. 2008;32:111–4. doi: 10.17796/jcpd.32.2.u01p135561161476. [DOI] [PubMed] [Google Scholar]
- 49.Subramaniam SK, Siswomihardjo A, Sunarintyas S. The effect of different concentrations of Neem (Azadiractha indica) leaves extract on the inhibition of Streptococcus mutans (In vitro) Maj Ked Gigi (Dent J) 2005;38:176–9. [Google Scholar]
- 50.Subraya B, Singh V, Bhat KM. The effect of low cost chalk based dentifrices containing Neem extract, on plaque and gingivitis, amongst users of natural products. J Indian Dent Assoc. 2000;71:229–32. [Google Scholar]
- 51.Council on scientific Affairs. Acceptance Program Guidelines-Chemotherapeutic Products for Control of Gingivitis. [Internet]: ADA. 2008. [Last cited on 2011 Sep 25]. Available from: http://www.ada.org/sections/scienceAndResearch/pdfs/guide_chemo_ging.pdf .


