Abstract
Natural products are an important source of antioxidant molecules like tannins, phenolic compounds, flavonoids, etc., Helicanthus elastica (Desr.) Danser (Loranthaceae) is one such plant belonging to the category of mistletoe, and grows commonly on the mango trees in India. In the present study, an attempt has been made to assess the antioxidant properties of the plant. Ethanol extract of H. elastica growing on mango tree was studied using different in vitro models. Shade-dried whole plant material was extracted with ethanol by cold percolation. Fifty milligrams of the alcohol extract of H. elastica was weighed and dissolved in 10 ml of methanol. The resultant 5 mg/ml solution was suitably diluted to obtain different concentrations. Total phenol content, reducing power assay, and scavenging of free radicals like nitric oxide, hydroxyl, hydrogen peroxide, and 1,1-diphenyl-2-picrylhydrazyl were studied by standardized in vitro chemical methods using ascorbic acid as the standard. The total phenol content of the plant was found to be 1.89% w/w. The extract showed good reducing power as well as scavenging of free radicals (nitric oxide, hydroxyl, superoxide anion, and hydrogen peroxide) at concentrations ranging from 5 to 100 μg/ml. The study revealed the antioxidant potential of H. elastica.
Keywords: Ascorbic acid, Free radicals, Helicanthus elastica, Loranthaceae, Mango mistletoe, Total phenol content
INTRODUCTION
Antioxidants can be broadly defined as “any substance, which when present at low concentration compared to that of an oxidizable substrate, significantly prevents or delays any oxidation of that substrate.”[1] Oxidizable substrates include almost anything found in foods and living tissues, including proteins, carbohydrates, and DNA. The body has developed several endogenous antioxidant systems to deal with the production of reactive oxygen species (ROS). These systems can be divided into enzymatic and non-enzymatic groups. The enzymatic antioxidants include superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px). The non-enzymatic antioxidants include the lipid-soluble vitamins [vitamin E and vitamin A or provitamin A (β-carotene), the water-soluble vitamin C, and glutathione. When ROS are generated in living system, a wide variety of antioxidants come into play, such as tocopherol, ascorbic acid, SOD, GSH-Px, catalase, ceruloplasmin, flavonoids, uric acid, and several others. The relative importance and efficacy of these depends on which ROS are involved, how and where they are generated, and which target of damage is selected. Thus, an antioxidant may protect against the free radicals in one system but fails to protect other systems. Natural products are an important source of antioxidant molecules. Search for these molecules from traditional herbs is an ongoing process. Helicanthus elastica (Desr.) Danser (Loranthaceae) is one such plant belonging to the category of mistletoe, and grows commonly on the mango trees in India. This hemiparasite was found to be a rich source of phenolic compounds;[2] hence, an attempt was made to assess the antioxidant properties of the plant in the present study. The ethanol extract of H. elastica growing on mango tree was studied using different in vitro models.
MATERIALS AND METHODS
Preparation of extract
Fresh plants of the mistletoe growing on Mangifera indica L. were collected during flowering during August 2009 from Kasaragod District of Kerala. Both host and the mistletoe were authenticated and the voucher specimen of the plant (no. 00637) was deposited at the pharmacognosy department of Captain Srinivasa Murti Drug Research Institute for Ayurveda, Chennai. The shade-dried whole plant material including the parasitic roots found on the surface of host was extracted with 90% ethanol by cold percolation. Fifty milligrams of the alcohol extract of H. elastica was weighed and dissolved in 10 ml of methanol. The resultant 5 mg/ml solution was suitably diluted to obtain different concentrations (50, 100, 150, 250, 500, 1000, 1500, and 2500 μg/ml) of the plant extracts and used for the following studies. Ascorbic acid (vitamin C) was used as the standard for comparison in the present study.
Total phenol content
Five grams of the sample was extracted with 50 ml of alcohol. The extract was concentrated and weighed. One milliliter of Folin's reagent was diluted with 1 ml of water. Twenty grams of Na2CO3 was dissolved in 100 ml of water at 70-80°C and cooled overnight. The clear liquid was decanted before use. Ten milligrams of tannic acid was dissolved in 100 ml of water afresh. Standard curve was obtained by taking the standard solution at concentrations of 0.2, 0.4, 0.6, 0.8, and 1 ml. After adding the reagents add sample, make up with water, phenols reagent, followed by Na2CO3), the mixture was incubated at room temperature for 40 min in dark and the blue color developed was read at 725 nm in a UV spectrophotometer. The phenol content was estimated from the calibration curve of standard tannic acid which was obtained by plotting concentration versus absorbance.[3]
Reducing power assay
About 0.75 ml of different concentrations of the extract were mixed with 0.75 ml of phosphate buffer (0.2 M, pH 6.6) and 0.75 ml of potassium ferricyanide (1% v/v) and the mixture was incubated at 50°C for 20 min. The reaction was stopped by adding 0.75 ml of 10% trichloroacetic acid and centrifuged at 800 rpm speed for 10 min. About 1.5 ml of the supernatant was mixed with 1.5 ml distilled water and 0.1 ml ferric chloride (0.1%). This mixture was incubated at room temperature for 10 min and the absorbance was measured at 700 nm with UV-visible spectrophotometer. Higher absorbance of the reaction mixture indicates the greater reducing power.[4]
Free radical scavenging
Nitric oxide scavenging activity
Two milliliters of 10 mM of sodium nitroprusside in 0.5 ml of phosphate-buffered saline (pH 7.4) was mixed with 0.5 ml of varying concentrations of the extract and the mixtures incubated at 25°C for 2½ h. From the incubated mixture, 0.5 ml was taken out and 1 ml of 0.33% sulfanilic acid was added. This was allowed to stand at room temperature for 5 min. Then, 1 ml of 0.1% of naphthyl ethylenediamide dichloride was added, mixed, and incubated at room temperature for 30 min. The absorbance of the mixture was measured at 540 nm with UV-visible spectrophotometer.[5]
Hydroxyl radical scavenging activity
Two series of tubes were taken. In the first set, 60 μl of 1 mM ferrous chloride, 90 μl of 1 mM 1,10-bathophenanthroline, and 2.4 ml of 0.2 M phosphate-buffered saline (pH 7.4) were taken and 150 μl of 0.17 M hydrogen peroxide was added to initiate the reaction. This set was labeled as blank. In the second set, before adding hydrogen peroxide, 1.5 ml of varying concentrations of the extract was added. After incubation at room temperature for 5 min, the absorbance of the mixture was measured at 560 nm with UV-visible spectrophotometer.[6]
Superoxide anion radical scavenging activity
One milliliter of 156 μM nitroblue T and 1 ml of 468 μM of nicotinamide adenine dinucleotide were mixed with 0.5 ml of varying concentrations of the extract. To this mixture was added 100 μl of phenazine methosulfate and the solution was incubated at room temperature for 5 min. The absorbance was measured at 560 nm with UV-visible spectrophotometer. Decreased absorbance of the reaction mixture indicated increased superoxide anion generation.[7]
Hydrogen peroxide radical scavenging activity
One milliliter of varying concentrations of the extract and 1 ml of 0.1 M H2O2 were mixed, followed by addition of two drops of 3% ammonium molybdate, 10 ml of 2 M H2SO4, and 7 ml of 1.8 M potassium iodide. This reaction mixture was titrated with 5.09 mM of sodium thiosulfate until the disappearance of the yellow color.[8]
DPPH scavenging
1,1-Diphenyl-2-picrylhydrazyl (DPPH) is a stable free radial with purple color (absorbs at 517 nm). If free radicals have been scavenged, DPPH will generate yellow color. This assay uses this character to show the free radical scavenging activity. The extract was dissolved in methanol at a concentration of 1 mg/ml, which was then used to determine its antioxidant activity. Blank used was methanol, control was methanol + DPPH, and test was methanol + DPPH + sample [100 μl extract (1 mg/ml)].[9]
RESULTS
The total phenol content of the plant was found to be 1.89% w/w. The results of antioxidant activity of various concentrations ranging from 50 to 2500 μg/ml of the alcoholic extracts of H. elastica showed that the free radical scavenging effect of the tested extract was concentration dependent. The results obtained for the reducing power assay are presented in Figure 1. The alcoholic extract produced marked and concentration-dependent increase in the reducing power [Figure 1]. It was observed that the extract was effective in inhibiting the nitric oxide radical scavenging activity [Figure 2]. The tested extract caused moderate inhibition of NO formation. The observed activity was not concentration dependent-moderate inhibition of NO formation was observed at 25 and 100 μg/ml. The inhibition observed at higher concentration level was less in comparison to the effect observed at lower concentration level. It was observed that H. elastica extract was effective in scavenging hydroxyl radical [Figure 3]. The IC50 value was found to be 37.42 μg/ml. The effect was not concentration dependent at the concentration level studied. Moderate inhibition was observed at 25 μg/ml, whereas at higher concentration level (100 μg/ml), the inhibitory effect was almost absent. The alcoholic extract of H. elastica produced significant superoxide anion radical scavenging activity. The IC50 value was found to be 218.03 μg/ml. The activity was concentration dependent up to a concentration of 800 μg/ml; above this concentration, the scavenging activity was found to be lesser [Figure 4]. The IC50 value was found to be 17.96 μg/ml. The extract exhibited concentration-dependent inhibition of hydrogen peroxide formation [Figure 5]. The tested extract produced remarkable decrease in the free radical formation at the concentration of 1 mg/ml [Figure 6].
Figure 1.
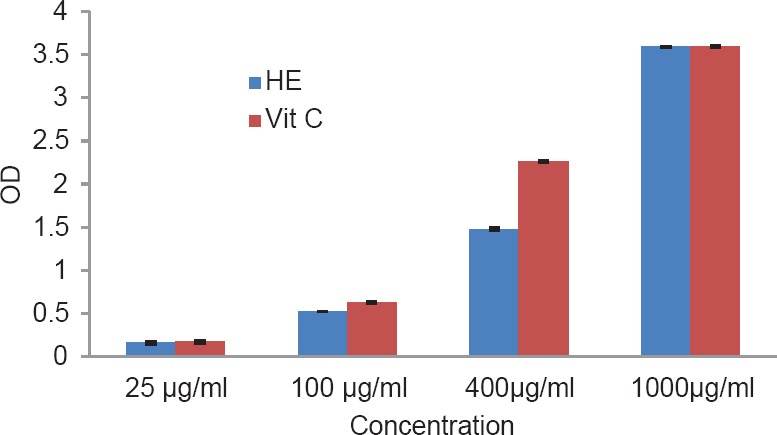
Reducing power assay of alcoholic extract of Helicanthus elastica
Figure 2.
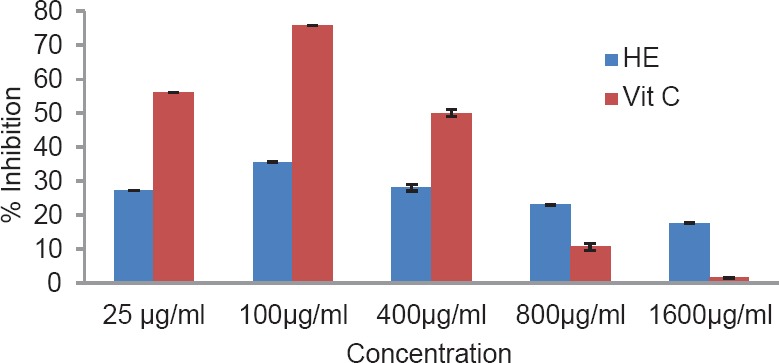
Nitric oxide radical scavenging activity of alcoholic extract of Helicanthus elastica
Figure 3.
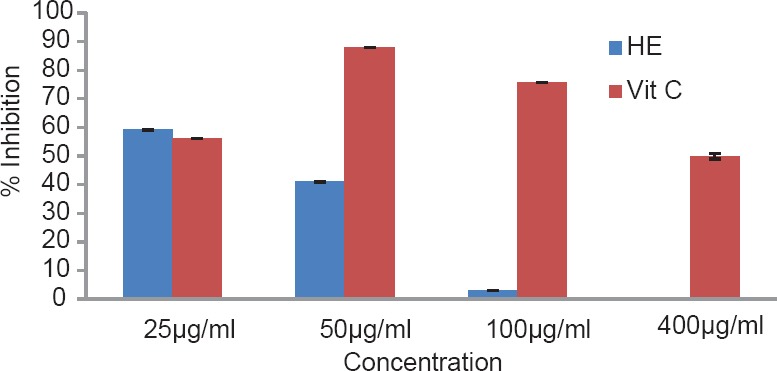
Hydroxyl radical scavenging activity of alcoholic extract of Helicanthus elastica
Figure 4.
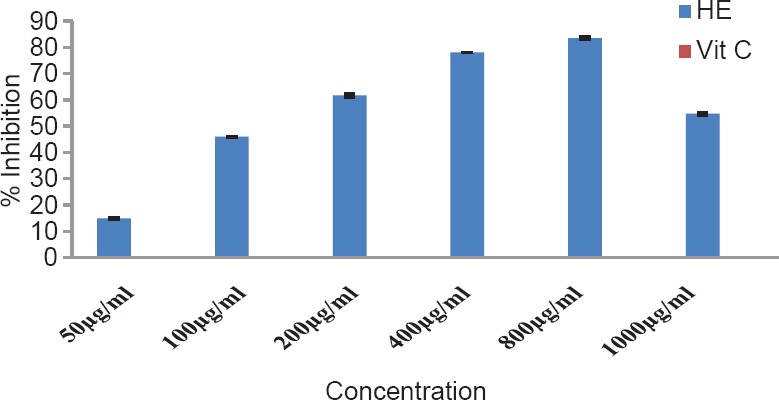
Superoxide anion radical scavenging activity of alcoholic extract of Helicanthus elastica
Figure 5.
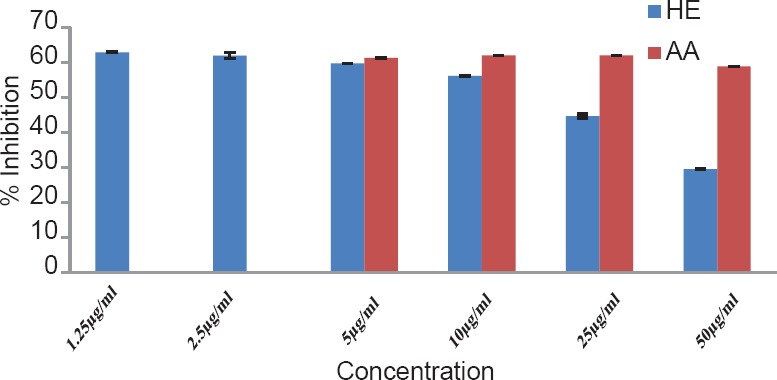
Hydrogen peroxide scavenging assay of alcoholic extract of Helicanthus elastica
Figure 6.
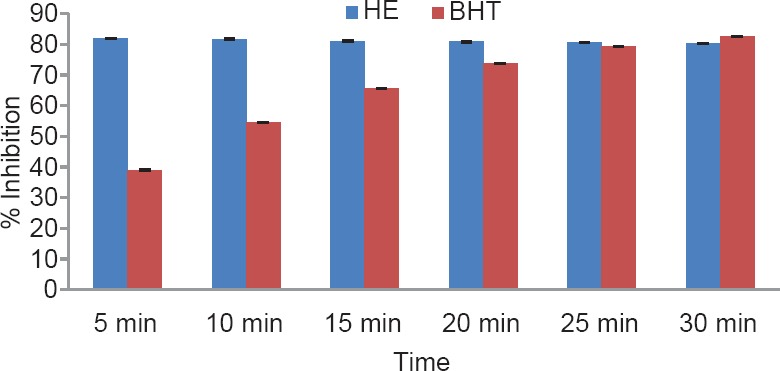
DPPH scavenging assay of alcoholic extract (1 mg/ml) of Helicanthus elastica (BHT, butylated hydroxytoluene)
DISCUSSION
Evaluation of putative drugs, especially plant products, for antioxidant activity is considered important. The antioxidant activity is normally initially screened in in vitro conditions and is followed by evaluation under in vivo conditions. In the present study, the alcoholic extract was subjected to antioxidant property evaluation in a series of test paradigms representing generation of different types of ROS. It is well known that superoxide is generated in cells during oxidative stress, mainly from decomposition of lipid peroxides or by spontaneous dismutation of super oxide.[10] Peroxynitrite is another potent oxidant formed by the interaction of superoxide with nitric oxide under various pathophysiological conditions. Nitric oxide is one of the smallest and most diffusible signal molecules known,[11] and is also a very active molecule involved in many and diverse biological pathways. Nitric oxide can have both positive and negative effects depending on the concentrations reached in the diseases.[12]
Analysis of the results obtained indicates a remarkable and concentration-dependent reducing activity that is almost equal to that of ascorbic acid at 25 μg/ml. This clearly indicates that the extract contains active principle(s) with good antioxidant potential. This was further confirmed in the DPPH assay. In general, the assay systems measured the scavenging activity against free radicals as a whole, followed by assessing the effect on different individual free radicals. The nitric oxide scavenging activity exhibited by the extract was not concentration dependent. Maximum inhibition of around 35.5% was observed at 100 μg/ml concentration, which is almost half of that shown by ascorbic acid. A moderate inhibition of around 59% was obtained for hydroxyl radical formation (56.06% for ascorbic acid), but the IC50 value was found to be lower at 37.42 μg/ml. With regard to superoxide formation, which along with hydroxyl radical is considered as the most reactive of free radicals, good inhibition (up to 80%) was observed, but with comparatively higher IC50 value of 218.03 μg/ml. Ascorbic acid did not show any inhibition in this system. The extract exhibited concentration-dependent inhibition of hydrogen peroxide formation up to a dose of 5 μg/ml, which was almost equivalent to that of ascorbic acid (61.26%), and at a concentration above this, the inhibitory effect was found to be decreased. The results obtained indicate the presence of inhibitory effect on hydrogen peroxide formation at a comparatively low dose level. The results obtained indicate a good potential of antioxidant activity. However, it remains to be ascertained using in vivo systems whether the observed effect would translate into meaningful antioxidant effect in an intact animal.
CONCLUSION
The extract showed good reducing power as well as efficient scavenging of free radicals (nitric oxide, hydroxyl, superoxide anion, and hydrogen peroxide) at concentrations ranging from 5 to 100 μg/ml. The study revealed the antioxidant potential of H. elastica which is attributed to its high phenol content.
ACKNOWLEDGMENT
Authors are highly grateful to the Director General, CCRAS, New Delhi for providing facilities. Help by Mrs. Joy Sugandhan is gratefully acknowledged.
REFERENCES
- 1.Halliwell B. Food derived antioxidants-evaluating their importance in food and in vivo. Food Sci Agric Chem. 1999;1:67–109. [Google Scholar]
- 2.Sunil Kumar KN. PhD Thesis. Chennai: The University of Madras; 2011. Pharmacognostical, Phytochemical and Medicinal Activity Profile of Helicanthus elastica (Desr.) Danser (Mango Mistletoe)-Loranthaceae; pp. 45–63. [Google Scholar]
- 3.1st ed. Vol. 2. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH); 2008. The Ayurvedic Pharmacopoeia of India. Part II (Formulations) p. 263. Appendices 1 to 5. [Google Scholar]
- 4.Oyaizee M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 5.Garret DC. Japan: Chapman and Hall Ltd; 1964. The Quantitative analysis of Drugs; pp. 456–8. [Google Scholar]
- 6.Yu W, Zhao Y, Shu B. The radical scavenging activities of Radix purariae isoflavanoids: A chemiluminescence study. Food Chem. 2004;86:525–9. [Google Scholar]
- 7.Liu F, Ool VE, Chang ST. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 1997;60:763–71. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XY. Beijing: Chema Science Press; 2000. Principles of chemical analysis; pp. 275–6. [Google Scholar]
- 9.Patel RM, Patel NJ. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res. 2011;1:52–68. [Google Scholar]
- 10.Kamat JP, Sarma HD, Devasagayam TP, Nesaratnam K, Basiron Y. Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol Cell Biochem. 1997;170:131–7. doi: 10.1023/a:1006853419214. [DOI] [PubMed] [Google Scholar]
- 11.Moroz LL, Kohn AB. On the comparative biology of nitric oxide (NO) synthetic pathways: Parallel evolution of NO-mediated signaling. Adv Exp Biol. 2007;1:1–44. [Google Scholar]
- 12.Hu DE, Brindle KM. Immune cell-induced synthesis of NO and reactive oxygen species in lymphoma cells causes their death by apoptosis. FEBS Lett. 2005;579:2833–41. doi: 10.1016/j.febslet.2005.03.099. [DOI] [PubMed] [Google Scholar]


