Abstract
A preliminary version of the homeopathic prescribing and patient care indicators was available. The instrument was modified further in this study with an intention to address formally its validity and reliability, audit prescriptions, identify areas of sub-optimal prescribing, and highlight target areas for improving the quality of practices. A cross-sectional study with record analysis was conducted on systematically sampled 377 patients of Mahesh Bhattacharyya Homeopathic Medical College and Hospital (MBHMC and H), Howrah, West Bengal, India. The outcome measures were homeopathic prescribing indicators (6 items) and patient care indicators (5 items). Individualized homeopathic prescriptions predominated in the encounters. Areas demanding immediate attention were extremely poor labeling of drugs dispensed from the hospital pharmacy, improper record of case history and disease diagnosis, ongoing therapies, and investigational findings in the prescriptions. Internal consistency of the overall instrument was estimated to be good (Cronbach's alpha: Prescribing indicators 0.752 and patient care indicators 0.791). The prescribing indicators, except items 1 and 3, reflected acceptable item-corrected total correlations – Pearson's r from 0.58 (95% CI: 0.52-0.65) to 0.74 (95% CI: 0.69-0.78). The patient care indicators, except item 2, showed acceptable correlations – Pearson's r from 0.40 (95% CI: 0.31-0.48) to 0.82 (95% CI: 0.78-0.85). The instrument also showed high discriminant validity (prescribing indicators P < 0.0001 and patient care indicators P < 0.0001). Improper prescribing practice was quite rampant and corrective measures are warranted. The developed indicators appeared to be validated and reliable; however, they are amendable for further development.
Keywords: Homeopathy, Patient care indicators, Prescribing indicators, Reliability, Validity
INTRODUCTION
As medical practice has become more complex, the scope of the term “prescription” has been broadened to include clinical outcome assessments, disease diagnosis, and reporting of investigations performed relevant to optimizing the safety or efficacy of medical treatment. [1] In a prescription audit study, these parameters may be evaluated for their presence or absence; the number of absent parameters directly correlates to the inconsistencies in the prescriptions and raises medico-legal concern. The indicators may be used to measure the impact of the interventions undertaken and problems in performance. They can help health planners, managers, and researchers to make basic comparisons between healthcare and prescribing practices in different areas or at different time periods. [2]
A preliminary version of the indicator instrument was developed which was pilot-tested and implemented on 600 samples as well. [3] The instrument was modified further in this study. This study shall address formally the validity and reliability of this newly developed instrument, audit prescriptions, and intend to identify sub-optimal levels of prescribing and highlight target areas for improving the quality of prescribing and patient care practices.
MATERIALS AND METHODS
Setting and design
A cross-sectional, prospective, institutional, observational, prescription and record analysis study was conducted in January 2014 on 377 patients visiting different outpatient clinics of Mahesh Bhattacharyya Homeopathic Medical College and Hospital (MBHMC and H), Howrah, West Bengal, India.
Participation criteria
Inclusion criteria were patients 18 years and above, completing their physician's and pharmacist's consultation, giving written informed consent, and being ready to share their prescription information. Exclusion criteria were patients who were too sick for consultation, unable to read patient information sheets, unwilling to stay after the doctor's visit, and not giving consent to join the survey.
Sample size
The sample size was determined as 377 [margin of error 5%, confidence level 95%, population size 13,500 (monthly average patient turnover of the hospital in 2013), and response distribution estimated to be 50%.] Systematic sampling method was used for recruitment of the patients. Sampling fraction was estimated (and approximated) to be 5/6 (n/N; n = required sample size of 377; N = average number of out-patient patients every day, i.e. 450); 5 was decided as the sampling unit by simple random sampling, and thus every 5th patient was interviewed.
Study instrument
The prescribing indicators consisted of six items – a single item (single individualized medicine per encounter) provided with “yes”/“no” options and five items provided with a 5-point agreement Likert scale (strongly agree: 5; agree: 4; uncertain: 3; disagree: 2; strongly disagree: 1; does not apply: 0), which were proper record of case history and disease diagnosis, proper record of patient identification, good legibility of prescription, proper record of ongoing therapy (if any), and proper record of investigations (if any). There were five patient care indicators – drugs properly dispensed as per prescription, drugs adequately labeled, patient understands the directions given in prescription and has a knowledge of correct dosage and follow-up, patient understands what to do in adverse events, and patients satisfied with the care they received – all ascribed with similar 5-point Likert scale to assess agreement. Agreement ratings were arrived at by a consensus among the six research assistants. Maximum obtainable score for prescribing indicators was either 26 or 16 and that of patient care indicators was 25.
Methodology
The audit involved documentation of current drug regimens and analysis of case notes. No identifiable information of the patients was required, ensuring anonymized protection of patient's privacy. The modified version of the instrument was pilot-tested on 10 randomly selected patients for length, clarity, language, relevance, overall adequacy, and whether the content reflected what it purports to assess. The instrument appeared to be satisfactory and ready for field-testing.
The study protocol was approved by the Institutional Ethics Committee of MBHMC and H. Patient information sheets were provided to the participants to achieve full cooperation. Though the survey did not intend to intervene anyway with the treatment being provided by the institutional doctors, written consent was obtained from all the participants. The survey matter was also explained verbally to all the participants by the research assistants. The filled-in questionnaires by the research assistants were concealed by putting those inside opaque envelopes, which were sealed at the survey site. All these were subjected to data analysis.
Statistical analysis
Different computational websites were used for the purpose.
Descriptive analysis was presented in the form of absolute values, percentages, and mean values. P values less than 0.05 for a two-tailed test were considered as statistically significant. The instrument was tested for item-corrected total correlations (Pearson's r), internal consistency or reliability (Cronbach's alpha coefficient), and discriminant validity [by comparing the mean scores obtained by the different indicators of the instrument using one-way analysis of variance (ANOVA)].
RESULTS
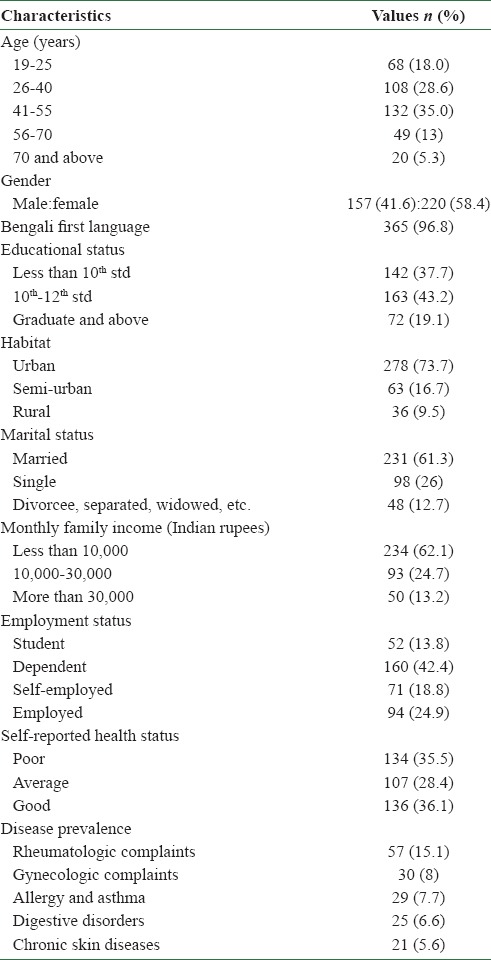
Survey participants mostly spanned the age group of 41-55 years (n = 132, 35%). Most of the participants were females (n = 220, 58.4%), had a level of education of 10th -12th standard (n = 163, 43.2%), were urban residents (n = 278, 73.7%), married (n = 231, 61.3%), had a monthly family income of less than 10,000 Indian rupees (INR) (n = 234, 62.1%), and were dependent (n = 160, 42.4%). Self-reported health status was good in most of the respondents (n = 136, 36.1%), and rheumatologic complaints were the most frequently encountered conditions (n = 57, 15.1%) [Table 1].
Table 1.
Socio-demographic characteristics of the survey participants (N=377)
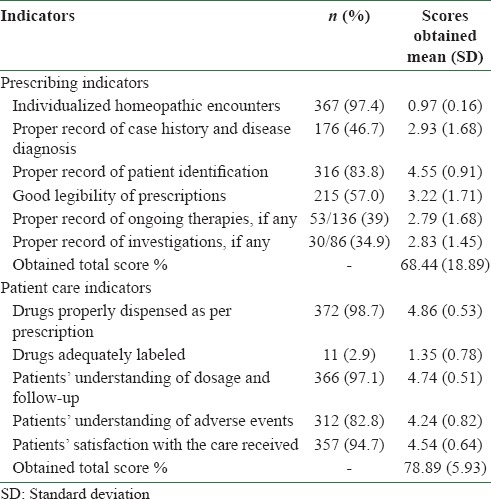
Majority of the homeopathic encounters were individualized (97.4%), and record of patients’ identification in the prescription (83.8%) was quite satisfactory. Legibility of the prescriptions was moderate (57%). Proper records of case history and disease diagnosis (46.7%), ongoing therapies (39%), and laboratory investigational findings (34.9%) in the prescription were not up to the mark, and these are the areas requiring immediate attention and urgent corrective measures. Among the patient care indicators, labeling of drugs was extremely poor (only 2.9%). Other indicators reflected satisfactory patient care (82.8-98.7%) [Table 2].
Table 2.
Results on indicators at a glance (N=377)
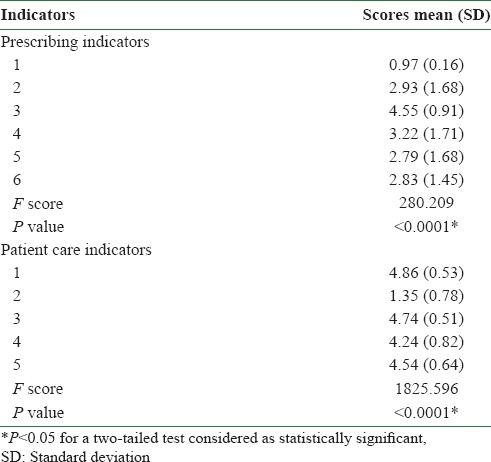
The face and content validity of the indicators were already established in an earlier study. [3] Internal consistency or reliability of the items considered and the overall instrument was estimated to be good (Cronbach's alpha: Prescribing indicators 0.752 and patient care indicators 0.791). The prescribing indicators, except items 1 and 3, reflected acceptable item-corrected total correlations – Pearson's r from 0.58 (95% CI: 0.52-0.65) to 0.74 (95% CI: 0.69-0.78). The patient care indicators, except item 2, showed acceptable correlations – Pearson's r from 0.40 (95% CI: 0.69-0.78) to 0.82 (95% CI: 0.78-0.85). The instrument also showed high discriminant validity (prescribing indicators: F = 280.209, P < 0.0001; patient care indicators: F = 1825.596, P < 0.0001; one-way ANOVA) [Tables 3 and 4].
Table 3.
Item-corrected total correlations and internal consistency of the indicators
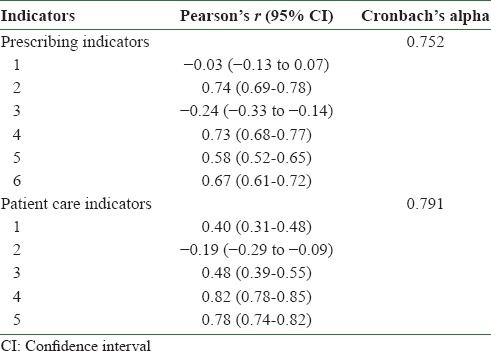
Table 4.
Discriminant validity of the items of the instrument
DISCUSSION
This study identified sub-optimal levels of prescribing and highlighted target areas for improving the quality of prescribing and patient care practices. Urgent corrective measures are warranted in areas like proper recording of case history and disease diagnosis, ongoing therapies, and laboratory investigational findings in the prescription, and labeling of drugs dispensed from the pharmacy. In this cross-sectional study, systematic sampling method was adopted to minimize selection bias and increase the generalizability of the findings. Internal consistency of the instrument might further be improved by rephrasing the few items (prescribing indicators 1 and 3 and patient care indicator 2) that had relatively low item-corrected total correlations. Further validation in other samples and more specific statistical (Rasch) analyses are required to confirm whether the sequence of the items requires readjustment in future.
The prescribing indicators of homeopathy are performances derived from the prescription records and case notes. They are not absolute measures, and so, poor performance, as evaluated, should be an indication for investigation and not automatic castigation. Indicators are not exact measures; there will be some variation for good reasons, reflecting the difficulty of any guideline being relevant to all cases. Additionally, inevitable incorporation of central tendency bias and acquiescence bias arising from the use of Likert scale responses into the analysis could not be eliminated. Furthermore, the study was undertaken in an India-based homeopathic school, making the generalizability of the results unclear. Future similar studies investigating the matter under question are welcome from other homeopathic schools in India and abroad. However, at present, as no standardized and validated measures for homeopathic prescribing exist, we believe that these indicators are the best available tool purporting the purpose.
In comparison with the previous study, [3] individualized homeopathic encounters increased from 85.6 to 97.4% (Yates’ Chi-square = 33.67; P two-tailed < 0.0001), reflecting higher intention to practice the “classic” form of homeopathy. However, all other prescribing indicators reflected a dismal performance – record on ongoing therapies decreased from 59.4 to 39% (Yates’ Chi-square = 15.33; P < 0.0001), record of patients’ identification from 100 to 83.8% (Yates’ Chi-square = 100.8; P < 0.0001), record of investigations decreased from 68.8 to 34.9% (Yates’ Chi-square = 24.97; P < 0.0001), and legibility decreased from 92.2 to 57% (Yates’ Chi-square = 167.9; P < 0.0001). Case records and diagnosis were merged together as one indicator in this study. Results were somewhat similar in patient care indicators also. An increase was observed in the following: Proper dispensing of drugs (from 92.3 to 98.7%; Yates’ Chi-square = 5.87; P = 0.015), patients’ knowledge of dosage (from 94.3 to 97.1%; Yates’ Chi-square = 3.38; P = 0.066), patients’ knowledge of adverse events (from 74.5 to 82.8%; Yates’ Chi-square = 8.64; P = 0.003), and satisfaction (from 86.5 to 94.7%; Yates’ Chi-square = 15.9; P < 0.0001). Labeling of drugs further dropped from 5.8 to 2.9% (Yates’ Chi-square = 3.01; P = 0.083).
Wide deviations from the given set of standards in any homeopathic practice setting can not be considered acceptable and should be subjected to investigation and action. If these data are to be used for national benchmarking, practice settings achieving a low standard should be encouraged to achieve at least the standards of the better performing settings.
CONCLUSION
Through studies using these newly developed indicators for homeopathy, it may be possible to evaluate the conditions of services offered by an institution. Thus, the indicators can be used to help the healthcare settings obtain better organizational structure, improve pharmaceutical prescribing, and raise the overall level of homeopathic healthcare practices in West Bengal, India. Some indicators may need to be revised or updated, especially to improve the internal consistency. Thus, a prescription management regulation needs to be promulgated for homeopathic practitioners.
ACKNOWLEDGMENT
Dr. Nikhil Saha, Principal in-Charge, is acknowledged for allowing us to carry out the project successfully in his instituiton.
REFERENCES
- 1.Bhattacharyya A, Gupta H, Dewangan MK. Prescription pattern study of the drugs used in tertiary hospitals of the Bilaspur region. Asian J Pharm Clin Res. 2012;5:73–6. [Google Scholar]
- 2.Biswas R, Chatterjee P, Mundle M. Prescribing habits of physicians in Medical College, Calcutta. Indian J Community Med. 2000;25:161–5. [Google Scholar]
- 3.Koley M, Saha S, Arya JS, Choubey G, Ghosh S, Purkait R, et al. A study on drug utilization and prescription habits of physicians in a government homeopathic hospital in West Bengal, India. J Integr Med. 2013;11:305–13. doi: 10.3736/jintegrmed2013048. [DOI] [PubMed] [Google Scholar]


