Abstract
Peripheral neuropathy is a major dose-limiting side effect of the chemotherapeutic agent paclitaxel. This study examined whether the three related traditional herbal formulations, goshajinkigan (GJG; 牛車腎氣丸 Niú Chē Shèn Qì Wán), hachimijiogan (HJG; 八味地黃丸 Bā Wèi Dì Huáng Wán), and rokumigan (RMG; 六味丸 Liù Wèi Wán), would relieve paclitaxel-induced mechanical allodynia in mice. A single intraperitoneal injection of paclitaxel (5 mg/kg) induced mechanical allodynia, which peaked on day 14 after injection. On day 14 after paclitaxel injection, oral administration of GJG (0.1-1.0 g/kg) produced a significant inhibition of established allodynia, but HJG and RMG did not affect the allodynia. Repeated oral administration of GJG (0.1-1.0 g/kg) starting from the day after paclitaxel injection did not affect allodynia development, but significantly inhibited allodynia exacerbation. Repeated oral administration of HJG produced a slight inhibition of allodynia exacerbation, but that of RMG did not. These results suggest that prophylactic administration of GJG is effective in preventing the exacerbation of paclitaxel-induced allodynia. The herbal medicines Plantaginis Semen (車前子 Chē Qián Zǐ) and Achyranthis Radix (牛膝 Niú Xī), which are present in GJG but not in HJG, may contribute to the inhibitory action of GJG on the exacerbation of paclitaxel-induced allodynia.
Keywords: Goshajinkigan, Hachimijiogan, Mechanical allodynia, Paclitaxel, Rokumigan
INTRODUCTION
Paclitaxel, an anti-microtubule agent originally isolated from the bark of the Pacific yew tree (Taxus brevifolia), is widely used to treat solid neoplasms such as ovarian, breast, and lung cancers.[1,2] However, paclitaxel causes peripheral neuropathy, which is characterized by pain and allodynia, with a stocking-and-glove distribution.[3] The peripheral neuropathy is a major dose-limiting side effect of paclitaxel. Several drugs, such as gabapentin and amifostine, were tested and failed to relieve paclitaxel-induced peripheral neuropathy in patients.[4,5,6]
Goshajinkigan (GJG; 牛車腎氣丸 Niú Chē Shèn Qì Wán) is a traditional herbal formulation consisting of 10 herbal medicines [Rehmanniae Radix (地黃 Dì Huáng), Achyranthis Radix (牛膝 Niú Xī), Corni Fructus (山茱萸 Shān Zhū Yú), Dioscoreae Rhizoma (山藥 Shān Yào), Plantaginis Semen (車前子 Chē Qián Zǐ), Alismatis Rhizoma (澤瀉 Zé Xiè), Poria (茯苓 Fú Ling), Moutan Cortex (牡丹皮 Mǔ Dān Pí), Cinnamoni Cortex (桂皮 Guì Pí), and Processi Aconiti Radix (附子 Fù Zǐ)]. GJG is used for the treatment of several neurological symptoms including pain and dysesthesia (unpleasant abnormal sensation). It has been demonstrated to be effective against chemotherapy-induced peripheral neuropathy in cancer patients.[7,8] Recent studies have shown that GJG does not attenuate the antineoplastic action of chemotherapeutic agents.[9,10] Excluding Achyranthis Radix and Plantaginis Semen, hachimijiogan (HJG; 八味地黃丸 Bā Wèi Dì Huáng Wán) consists of the same herbal medicines found in GJG, and rokumigan (RMG; 六味丸 Liù Wèi Wán) consists of all herbal medicines found in HJG, excluding Cinnamoni Cortex (桂皮 Guì Pí) and Processi Aconiti Radix (附子 Fù Zǐ). There is no clinical evidence of the effects of HJG and RMG on chemotherapy-induced peripheral neuropathy. In this study, we investigated the effects of GJG, HJG, and RMG on paclitaxel-induced mechanical allodynia.
MATERIALS AND METHODS
Animals
Male C57BL/6NCr mice (Japan SLC Ltd, Hamamatsu, Japan) were used. All animals were 6 weeks old at the start of the experiments. They were housed in a room with controlled temperature (21-23°C), humidity (45-65%), and a 12-h light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.). Food and water were provided ad libitum. Experiments were performed after obtaining approval from the animal care committee of the University of Toyama.
Drugs
Paclitaxel purchased from Sigma-Aldrich (St Louis, MO, USA) was dissolved in vehicle [physiological saline containing 10% Cremophor® EL (Sigma-Aldrich) and 10% ethanol]. Paclitaxel and the vehicle were administered intraperitoneally; the dose (5 mg/kg) was selected from a published report[11] and was in accordance with the recommended clinical dose of 210 mg/m2 body surface area, which corresponds to a dose of 5.9 mg/kg in a person of 170 cm body height and 60 kg weight. Dried extracts of GJG (Lot. No. 2080107010, 2009), HJG (Lot. No. 2090007010, 2009), and RMG (Lot. No. 2090087010, 2009) were obtained from Tsumura and Co., Ltd (Tokyo, Japan). These dried extracts were dissolved in 5% gum arabic and were administered orally once on day 14 after the paclitaxel injection or once daily from the day after paclitaxel injection is given.
Behavioral experiments
Mechanical allodynia in the hind paw was evaluated using a von Frey filament (North Coast Medical Inc., Morgan Hill, CA, USA), as described.[11] After an acclimation period of at least 30 min, a fine von Frey filament with a bending force of 0.69 mN was applied perpendicularly against the central part of the plantar hind paw and was held for 1-3 s with it slightly bent. Responses to the stimuli were scored as follows: 0, no reaction; 1, lifting of the hind paw; and 2, licking and flinching of the hind paw. A stimulus of the same intensity was applied three times alternately to each hind paw at intervals of several seconds, and the average response score served as the allodynia score (the maximum score was 2). When the effects of single administration of dried extracts of traditional formulations were examined on day 14 after paclitaxel injection, mechanical allodynia was evaluated before and after the administration of traditional formulations. When the effects of repeated administrations of the dried extracts were examined, mechanical allodynia evaluation was performed before each administration of traditional formulations.
Statistical analysis
All data are presented as mean ± standard error of the mean. Statistical significance was analyzed using two-way repeated measures analysis of variance (ANOVA) and post hoc Holm-Sidak multiple comparison test. A P < 0.05 was considered statistically significant. The statistical analyses were performed using SigmaPlot™ graphing and statistical software version 11 (Systat Software, Inc., Chicago, IL, USA).
RESULTS
Paclitaxel-induced mechanical allodynia
A single intraperitoneal injection of paclitaxel (5 mg/kg) caused mechanical allodynia, which became apparent on day 4 after injection, peaked on day 14, and almost subsided by day 21 [Figure 1a main effect of paclitaxel treatment, F1,10 = 87.676, P < 0.001; interaction between paclitaxel treatment and time, F30,300 = 12.286, P < 0.001 (two-way repeated measures ANOVA)].
Figure 1.
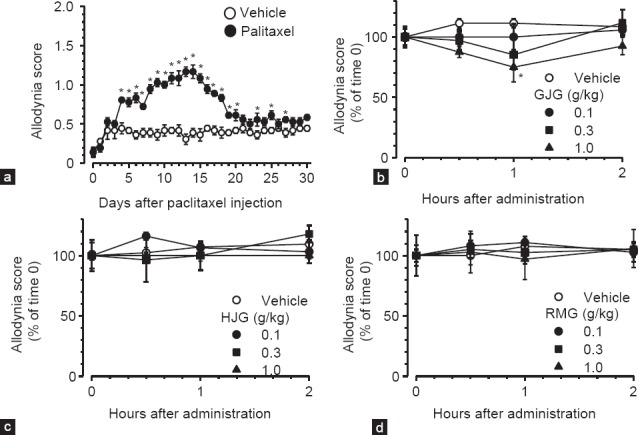
Effects of single administration of goshajinkigan (GJG), hachimijiogan (HJG), and rokumigan (RMG) on the established mechanical allodynia after paclitaxel injection. (a) Development of mechanical allodynia after a single injection of paclitaxel. Mice were injected intraperitoneally with paclitaxel (5 mg/kg) or vehicle on day 0. GJG (b), HJG (c), RMG (d), or vehicle (5% gum arabic) was orally administered on day 14 after paclitaxel injection. Data are presented as mean and standard error of the mean (n = 5-6). *P < 0.05 compared to vehicle (Holm-Sidak multiple comparisons)
Effects of single administration of GJG, HJG, and RMG on paclitaxel-induced mechanical allodynia
Three formulations were administered orally on day 14 after paclitaxel injection. Single administration of GJG (0.1-1.0 g/kg) exerted a relatively short-lasting but significant dose-dependent inhibition on the established mechanical allodynia; two-way repeated measures ANOVA revealed a significant main effect of GJG (F3,20 = 3.611, P = 0.031) [Figure 1b]. Single administration of HJG and RMG did not affect the established mechanical allodynia at doses of 0.1-1.0 g/kg [Figure 1c and d].
Effects of prophylactic administration of GJG, HJG, and RMG on paclitaxel-induced mechanical allodynia
In this series of experiments, three formulations were administered daily from the day after paclitaxel injection. Repeated administration of GJG (0.1-1 g/kg) did not affect the initial development of mechanical allodynia induced by paclitaxel, but significantly inhibited the exacerbation of allodynia from day 9 after paclitaxel injection, in comparison with the vehicle, although no clear dose-dependency was observed [Figure 2a; interaction between paclitaxel treatment and time, F42,252 = 1.889, P = 0.002 (two-way repeated measures ANOVA)]. Similarly, repeated administration of HJG (0.1-1 g/kg) did not affect the initial development of mechanical allodynia, but the highest dose of HJG (1 g/kg) significantly inhibited allodynia on day 14 after the injection, in comparison with the vehicle [Figure 2b; interaction between paclitaxel treatment and time, F42,266 = 1.504, P = 0.03 (two-way repeated measures ANOVA)]. Repeated administration of RMG (0.1-1 g/kg) did not affect the mechanical allodynia induced by paclitaxel [Figure 2c].
Figure 2.
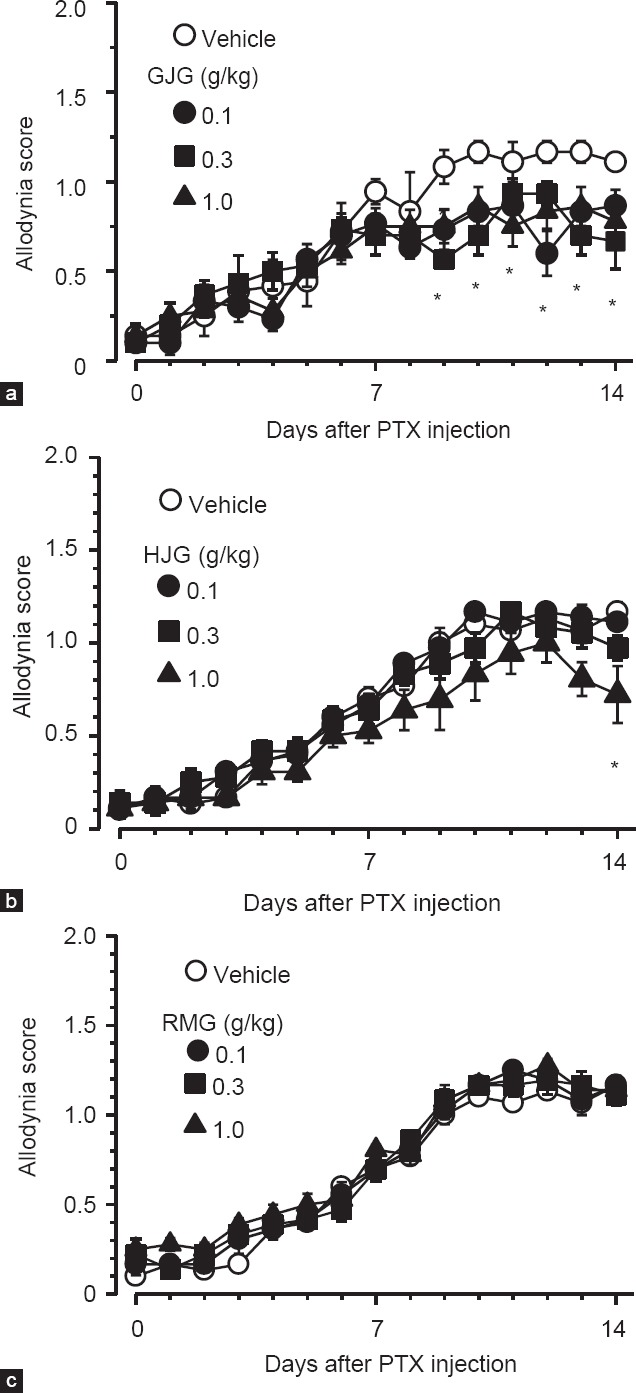
Effects of prophylactic administration of goshajinkigan (GJG), hachimijiogan (HJG), and rokumigan (RMG) on paclitaxel-induced mechanical allodynia. Paclitaxel (5 mg/kg) was injected intraperitoneally in mice, and GJG (a), HJG (b), RMG (c), or vehicle (5% gum arabic) was administered orally once daily from the day after the paclitaxel injection. Data are presented as mean and standard error of the mean (n = 5-6). *P < 0.05 compared to vehicle (Holm-Sidak multiple comparisons)
DISCUSSION
Single oral administration of GJG (0.1-1 g/kg) exerted a dose-dependent inhibition on the established mechanical allodynia after paclitaxel injection, whereas the GJG-related herbal formulations HJG and RMG did not affect the established allodynia. The recommended clinical daily dose of GJG granules is 7.5 g, which contains 4.5 g of dried GJG extract. Given that the body weight is 60 kg, the dose of the dried GJG extract is 0.075 g/kg. Thus, the antiallodynic dose (1 g/kg) in mice with paclitaxel-induced neuropathy was 13 times higher than the recommended clinical dose. Recently, we have demonstrated that single oral administration of GJG (0.3 and 1.0 g/kg) inhibits the established mechanical allodynia after the injection of oxaliplatin, a platinum chemotherapeutic agent.[12] The inhibitory effect of 1 g/kg GJG on paclitaxel-induced allodynia (present study) was less than that of 0.3 g/kg GJG on oxaliplatin-induced allodynia.[12] The inhibitory action of GJG on oxaliplatin-induced allodynia is at least in part mediated by the activation of the descending noradrenergic and serotonergic systems,[12] and in our preliminary experiment, single administration of milnacipran, a serotonin–noradrenaline reuptake inhibitor, markedly inhibited the established allodynia after oxaliplatin injection (data not shown). In contrast, single administration of milnacipran does not affect the established allodynia after paclitaxel injection.[13] These findings, taken together, raise the possibility that a low efficacy of GJG in paclitaxel-induced allodynia is due to the decreased activity of the descending noradrenergic and serotonergic systems. It is possible that paclitaxel decreases the activity of the descending noradrenergic and/or serotonergic systems.
Prophylactic repeated administration of GJG inhibited paclitaxel-induced mechanical allodynia. GJG consists of the following 10 herbal medicines: Rehmanniae Radix (地黃 Dì Huáng), Corni Fructus (山茱萸 Shān Zhū Yú), Dioscoreae Rhizoma (山藥 Shān Yào), Alismatis Rhizoma (澤瀉 Zé Xiè), Poria (茯苓 Fú Ling), Moutan Cortex (牡丹皮 Mǔ Dān Pí), Cinnamoni Cortex (桂皮 Guì Pí), Processi Aconiti Radix (附子 Fù Zǐ), Achyranthis Radix (牛膝 Niú Xī), and Plantaginis Semen (車前子 Chē Qián Zǐ); HJG consists of the first 8 of these herbal medicines and RMG consists of the first 6 of these medicines. The results showed that HJG had only a slight effect at the highest dose tested and RMG did not have a prophylactic effect, suggesting that Plantaginis Semen and/or Achyranthis Radix play a role in the prophylactic activity of GJG. The underlying mechanisms are unclear, and allodynia-preventing substances have not yet been isolated from the extracts of these two herbal medicines. However, one possibility is that the activity of the scavengers of reactive oxygen species is involved in the antiallodynic activity. Although a single administration is ineffective, prophylactic administration of the reactive oxygen species scavenger N-tert-butyl-a-phenylnitrone suppresses paclitaxel-induced mechanical allodynia.[14] Since Plantaginis Semen and Achyranthis Radix have antioxidant activity,[15,16,17] this action may be involved in the prevention of an exacerbation of paclitaxel-induced allodynia. Another possibility is the improvement of decreased blood flow. Paclitaxel, but not vincristine, decreases peripheral blood flow.[11] The prostaglandin E1 analog limaprost, which counteracts this reduction in blood flow without affecting normal blood flow,[18] exerts prophylactic inhibition on allodynia induced by paclitaxel, but not by vincristine.[11] In this study, prophylactic administration of GJG prevented mechanical allodynia induced by paclitaxel. In contrast, in our preliminary experiments, prophylactic administration of GJG did not prevent vincristine-induced allodynia. GJG and its constituent Achyranthis Radix invigorate blood circulation.[15,17,19] Taken together, these findings raise the possibility that the prevention of the decrease in peripheral blood flow contributes to the prophylactic activity of GJG.
Prophylactic administration of HJG, but not RMG, showed slight antiallodynic action at the highest dose tested in paclitaxel-treated mice. Thus, it is possible that Cinnamoni Cortex and Processi Aconiti Radix also have prophylactic antiallodynic activity. Similar to Achyranthis Radix, Cinnamoni Cortex and Processi Aconiti Radix invigorate blood circulation.[20,21] However, the prophylactic antiallodynic activity of HJG was weaker than that of GJG. Thus, although Cinnamoni Cortex and Processi Aconiti Radix may have some prophylactic antiallodynic activity, Plantaginis Semen and Achyranthis Radix may play important roles in the antiallodynic action of GJG.
CONCLUSION
Prophylactic administration of GJG prevents an exacerbation of paclitaxel-induced mechanical allodynia. Since GJG also has acute antiallodynic activity, it may be useful for the prevention of paclitaxel-induced peripheral neuropathy. Although the underlying mechanisms remain unclear, Plantaginis Semen and/or Achyranthis Radix may contribute to the antiallodynic activity of GJG.
REFERENCES
- 1.Chang AY, Garrow GC. Pilot study of vinorelbine (Navelbine) and paclitaxel (Taxol) in patients with refractory breast cancer and lung cancer. Semin Oncol. 1995;22(2 Suppl 5):66–71. [PubMed] [Google Scholar]
- 2.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–42. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, et al. North Central Cancer Treatment Group. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–8. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 5.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: Occurrence and management approach. Clin Breast Cancer. 2011;11:73–81. doi: 10.1016/j.clbc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, et al. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: A multicenter collaborative study. Exp Ther Med. 2012;3:60–5. doi: 10.3892/etm.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono T, Hata T, Morita S, Munemoto Y, Matsui T, Kojima H, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol. 2013;72:1283–90. doi: 10.1007/s00280-013-2306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ushio S, Egashira N, Sada H, Kawashiri T, Shirahama M, Masuguchi K, et al. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur J Cancer. 2012;48:1407–13. doi: 10.1016/j.ejca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Bahar MA, Andoh T, Ogura K, Hayakawa Y, Saiki I, Kuraishi Y. Herbal medicine goshajinkigan prevents paclitaxel-induced mechanical allodynia without impairing antitumor activity of paclitaxel. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/849754. e849754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauchan P, Andoh T, Kato A, Sasaki A, Kuraishi Y. Effects of the prostaglandin E1 analog limaprost on mechanical allodynia caused by chemotherapeutic agents in mice. J Pharmacol Sci. 2009;109:469–72. doi: 10.1254/jphs.08325sc. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura R, Andoh T, Fushimi H, Komatsu K, Shibahara N, Kuraihsi Y. Involvement of descending monoaminergic systems in antiallodynic effect of goshajinkigan in oxaliplatin-treated mice. J Tradit Med. 2013;30:183–9. [Google Scholar]
- 13.Katsuyama S, Sato K, Yagi T, Kishikawa Y, Nakamura H. Effects of repeated milnacipran and fluvoxamine treatment on mechanical allodynia in a mouse paclitaxel-induced neuropathic pain model. Biomed Res. 2013;34:105–11. doi: 10.2220/biomedres.34.105. [DOI] [PubMed] [Google Scholar]
- 14.Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. 2011;6:e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie F, Li X, Sun K, Chu Y, Cao H, Chen N, et al. An experimental study on drugs for improving blood circulation and removing blood stasis in treating mild chronic hepatic damage. J Tradit Chin Med. 2001;21:225–31. [PubMed] [Google Scholar]
- 16.Li H, Wang Q. Evaluation of free hydroxyl radical scavenging activities of some Chinese herbs by capillary zone electrophoresis with amperometric detection. Anal Bioanal Chem. 2004;378:1801–5. doi: 10.1007/s00216-004-2509-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman M, Zhu YC. Antioxidants in Chinese herbal medicines: A biochemical perspective. Nat Prod Rep. 2004;21:478–89. doi: 10.1039/b304821g. [DOI] [PubMed] [Google Scholar]
- 18.Nakai K, Takenobu Y, Eguchi K, Takimizu H, Honjo K, Akimaru S, et al. The effects of OP-1206 alpha-CD on walking dysfunction in the rat neuropathic intermittent claudication model. Anesth Analg. 2002;94:1537–41. doi: 10.1097/00000539-200206000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Effect of Gosha-jinki-gan, a Kampo medicine, on enhanced platelet aggregation in streptozotocin-induced diabetic rats. Jpn J Pharmacol. 1998;78:87–91. doi: 10.1254/jjp.78.87. [DOI] [PubMed] [Google Scholar]
- 20.Kanai S, Okano H, Abe H. Efficacy of toki-shigyakuka-gosyuyu-syokyo-to (danggui-sini-jia-wuzhuyu-shengjiang-tang) on peripheral circulation in autonomic disorders. Am J Chin Med. 1997;25:69–78. doi: 10.1142/S0192415X9700010X. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Sugiura Y, Goto Y. Aconiti tuber (Bushi) improves microcirculatory disturbances induced by endotoxin in rats. Phytother Res. 2000;14:505–9. doi: 10.1002/1099-1573(200011)14:7<505::aid-ptr648>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


