Abstract
Tualang honey (蜂蜜 Fēng Mì) is known to have anti-inflammatory property, but its antinociceptive property has not been extensively investigated. In this study, we examined the preemptive effects on administering different doses of Tualang honey and prednisolone on the nociceptive response in male Sprague-Dawley rats. Thirty-five male Sprague-Dawley rats were randomized into five groups (n = 7) and each group received either distilled water, Tualang honey (0.2, 1.2 or 2.4 g/kg) or prednisolone (10 mg/kg) for 10 days. The response to noxious thermal stimulus was assessed using tail flick test on Day 10. The well-being of the rats was also assessed by monitoring their food intake and body weight. Data were analyzed using one-way Analysis of Variance (ANOVA) with post-hoc Scheffe's test and P value less than 0.05 was considered significant. In tail flick test, the tail flick latency time was significantly higher in the groups that received 1.2 g/kg and 2.4 g/kg of Tualang honey and 10 mg/kg of prednisolone, compared to the control group (P < 0.05). There was significant reduction in the total food pellet intake in the groups receiving prednisolone and Tualang honey (1.2 g/kg and 2.4 g/kg) compared to controls; however, the body weight gain was only significantly reduced in the prednisolone group. All the parameters were not significantly affected in the group receiving 0.2 g/kg of Tualang honey. In conclusion, preemptive administration of Tualang honey (1.2 g/kg and 2.4 g/kg) and prednisolone (10 mg/kg) had reduced the pain responses. The reduced weight gain in the prednisolone group is an unwanted effect due to its metabolic and central actions. Further studies are required to confirm the antinociceptive effects and elucidate the mechanism of antinociceptive action of Tualang honey in the rats.
Keywords: Body weight, Food intake, Prednisolone, Tail flick test, Tualang honey
INTRODUCTION
Honey (蜂蜜 Fēng Mì) is a natural product produced by honeybees. It is used to treat various conditions including infertility, respiratory and gastrointestinal symptoms.[1] Previous reports have shown the anti-inflammatory and antinociceptive properties of honey. Honey was reported to reduce pain from burns and reduce the local pain rapidly.[2] The reduction in pain might be attributed to honey's ability to reduce plasma prostaglandins (thromboxane B2, PGE2, and PGF2a) in normal human subjects and animal models.[3] Apart from this, other studies have demonstrated that honey reduces the release of nitrous oxide, histamines, and cytokines such as tumor necrosis factor-alpha (TNF-α), which may reduce inflammation and pain.[3]
There are several types of honey, including Tualang and Gelam honey, in Malaysia. Both types of honey have been shown to have antioxidant and anti-inflammatory activities.[4] The phenolic and flavonoid compounds in Tualang honey contribute to its antioxidant activity and might have a role in its anti-inflammatory and probably its antinociceptive effects.[5] An established anti-inflammatory agent, prednisolone, has been used to treat inflammatory diseases and was found to be useful in painful conditions such as bladder pain syndrome and arthralgia.[6,7,8] However, it has various side effects including immune suppression, insulin resistance, skeletal muscle wasting, and osteoporosis.[8]
The analgesic and anti-inflammatory effects of other types of honey, e.g. Gelam and Manuka honey, were reported in various studies, but the present study is among the first few studies which investigated the analgesic effects of Tualang honey.[3,9] It is not known whether Tualang honey's antinociceptive property is dose dependent and whether its antinociceptive property is comparable to prednisolone. Therefore, in the present study, we hypothesized that the antinociceptive effects of Tualang honey were dose dependent and comparable to prednisolone. The first aim of the study was to examine the effects of different doses of Tualang honey and prednisolone administration on nociceptive responses induced by noxious thermal stimuli in male Sprague-Dawley rats. In this study, the well-being of the rats was also assessed by monitoring their food intake and body weight. As previous reports have shown various effects of prednisolone and Tualang honey on these parameters, the second aim was to determine whether administration of Tualang honey and prednisolone altered the food pellet intake and body weight in the rats studied.[10,11]
MATERIALS AND METHODS
This was an experimental study performed in the Physiology Laboratory, Universiti Sains Malaysia (USM) Health Campus, Kelantan. Experiments were performed between 08:00 and 16:00 hours. The research was approved by the USM Animal Ethical Committee [USM/Animal Ethics Approval/2010/(63)(266)].
Animals
The rats were obtained from Animal Research and Service Centre (ARASC), USM Health Campus. Thirty-five Sprague-Dawley male rats, weighing from 250 to 300 g (8-10 weeks old) were used in this study. They were kept under 12-h light dark cycle and permitted free access to food pellets and water. All the rats were housed individually and allowed to acclimatize at least for 4 days in the physiology laboratory before beginning the experiment.[12] The experiment was performed between 08:00 and 16:00 hours and behavior test was conducted in the morning.[12] The testing room was consistently maintained at 22°-24°C. The body weight and total food pellet intake of the animals were recorded daily during the experiment. The changes in body weight and total food pellet consumption were calculated.
Tualang honey and prednisolone administration
Tualang honey (蜂蜜 Fēng Mì) was supplied by Federal Agricultural Marketing Authority (FAMA), Ministry of Agriculture and Agro-based Industry, Malaysia. All the animals were force-fed using a gavage needle in order to ensure that the accurate amount of distilled water, Tualang honey, or prednisolone was administered. The doses of Tualang honey chosen (0.2 g/kg, 1.2 g/kg, and 2.4 g/kg body weight) were based on the doses of Tualang honey according to a previous report by Mohamed et al.[13] Meanwhile, the dose of prednisolone (10 mg/kg) was chosen from a study by Nakamura et al.[14] based on its effectiveness in reducing inflammatory symptoms. Tualang honey or prednisolone was dissolved in distilled water to obtain the required concentration. The treatment was given daily for 10 days before conducting the tail flick test.
Tail flick test
This test measured nociceptive reflex response when stimulated with noxious heat using Tail Flick Analgesia Meter (IITC, Woodlands Hill, California, USA). The test followed the protocol used by Bannon and Malmberg.[15] The light beam used for this test was standardized at 8 units. On the 10th day, tail flick test was conducted 1 hour after administration of treatment. The rats were acclimatized to the test environment for 30 min before the experiment. The rat was positioned in a Plexiglas restrained tube and three areas were selected to conduct the test, which were at 30, 40, and 50 mm from the tip of the tail. Three minutes interval was given in between two tests to reduce the effects of tissue damage at each site. The time when the tail flicked away from the light beam source was taken and the responses were averaged for each rat to increase the accuracy of the test. Ten seconds was the cut-off time to avoid any tissue damage.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences version 19 software (IBM, New York, United States). One-way Analysis of Variance (ANOVA) with post-hoc Scheffe's test was used to analyze the changes in rats’ body weight, food consumption, and tail flick responses. All the data were expressed as mean ± standard error of mean (SEM). P values less than 0.05 were considered as significant.
RESULTS
Total consumption of food pellets
The data analysis of food intake by one-way ANOVA revealed significant difference between all groups [F (4,30) = 30.996, P < 0.001]. Post-hoc Scheffe's test demonstrated a significant reduction of food pellet intake in rats receiving Tualang honey (蜂蜜 Fēng Mì) at 1.2 g/kg and 2.4 g/kg compared to the control group (P < 0.05) [Table 1]. There was a significant reduction in prednisolone group compared to groups administered different doses of Tualang honey and the control group (P < 0.05).
Table 1.
Total food intake and changes in body weight of rats (mean±SEM) following 10 days of treatment (n=7)
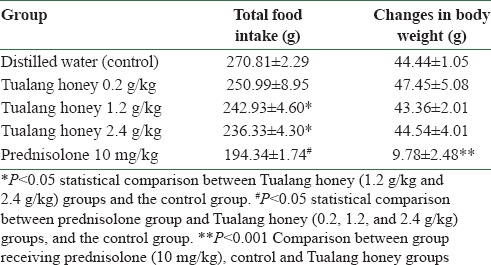
Changes in body weight of rats
The analysis with one-way ANOVA revealed a significant difference in the changes of animal body weight during treatment in the groups [F (55,4) = 25.173, P < 0.001]. Post-hoc Scheffe's test demonstrated a significant reduction in weight gain in the group that received preemptive administration of prednisolone compared to the control and Tualang honey groups (P < 0.01). There was no significant difference between the groups receiving different doses of Tualang honey and the control group [Table 1].
Tail flick test
The results of tail flick test, as illustrated in Table 2, reveal that there was a significant difference between the groups [F (25,4) = 3.914, P < 0.05]. Post-hoc Scheffe's test demonstrated a significant increase in the tail flick latency in the groups administered Tualang honey at 1.2 g/kg and 2.4 g/kg and prednisolone, compared to the control group (P < 0.05). There was no significant difference in the latency time between the groups administered 0.2 g/kg of Tualang honey and the control group.
Table 2.
Tail flick test in all treatment groups (n=7)

DISCUSSION
In the present study, the results showed that there was significant reduction in total food intake and body weight gain in the prednisolone group compared to the control group. The groups that received 1.2 and 2.4 g/kg of Tualang honey (蜂蜜 Fēng Mì) had reduced total food intake, but the weight gain was not significantly reduced. The parameters were not significantly affected in the group that received 0.2 g/kg of Tualang honey. In the tail flick test, the tail flick latency was prolonged in the groups that received 1.2 g/kg and 2.4 g/kg of honey and 10 mg/kg of prednisolone.
Tualang honey contains fructose (39.84%), glucose (25.29%), and maltose (3.17%), in addition to aforementioned phenolic and flavonoid compounds.[16] Hence, the reduced food pellet consumption in the groups that received 1.2 g/kg and 2.4 g/kg of Tualang honey could be partly attributed to its constituent, fructose. Studies have shown that low or moderate doses of fructose intake were associated with reduced leptin levels, while high dose of fructose intake increased the leptin levels.[17,18] Leptin inhibits the release of neuropeptide Y and agouti-related peptide (NPY and AgRP, respectively) in hypothalamic arcuate nucleus and stimulates the synthesis of an appetite suppressant, α-melanocyte stimulating hormone.[19] The doses (1.2 g/kg and 2.4 g/kg) used in the study were probably sufficient to suppress the release of NPY, and AgRP, and stimulate the release of appetite suppressant. Furthermore, honey consumption may alter the meal-induced response of ghrelin (hunger hormone) and anorexigenic hormone, peptide tyrosine-tyrosine 3-36 (PYY3-36).[20]
Although there was a significant reduction in the food intake in the Tualang honey groups (1.2 g/kg and 2.4 g/kg), the body weight of the rats was not significantly affected. Similar findings were also reported in male rats supplemented with 1.2 g/kg Tualang honey for 13 weeks.[21] It was suggested that there are adequate calories in Tualang honey which might contribute to the normal body weight gain in the groups administered honey despite the reduced total food pellet intake. Other studies which have demonstrated alteration in the body weight gain were conducted either on obese, diabetic, or hypertensive subjects.[10,16]
The present study has shown that the group that received 10 days of prednisolone treatment had reduced total food intake and body weight gain. Although glucocorticoid therapy is usually associated with weight gain, its administration may not alter or may lead to reduction in the parameter.[17,18] Studies have reported that pretreatment with a lower dose of prednisolone was associated with increase in food intake, while higher dose was associated with a decrease in feeding.[22,23] The dose used in the present study is considered high and its administration may lead to hyperinsulinemia, glucolipid metabolic disturbances, and probably down-regulating mRNA expression levels of the orexigenic neuropeptides, NPY and AgRP, and anorexigenic neuropeptide, cocaine- and amphetamine-regulated transcript (CART), in the hypothalamus in the rats.[22] The interactions of these factors probably contribute to the reduction in food intake and body weight. Several studies have demonstrated the stimulatory effects of glucocorticoids on leptin secretion in both human and animal subjects. High leptin level would reduce NPY/AgRP expression and stimulate the release of appetite suppressant, α-melanocyte stimulating hormone.[24] These mechanisms will lead to a decrease in food intake as seen in the present study. However, the leptin level was not measured in the present study due to financial constraint. The effects of prednisolone (10 mg/kg) and Tualang honey (1.2 g/kg and 2.4 g/kg) on leptin level will be investigated in the future.
The reduction in body weight found in the present study might be due to the reduced weight of the organs and reduced muscle bulk, which are the side effects of prednisolone treatment. Hull et al. have demonstrated a significant reduction in the total body weight and the weight of several organs including spleen, thymus, bursa, muscle, testis, and oviduct in Japanese quail following glucocorticoid treatment.[25] The study has shown that the weight reduction was significantly correlated with the dose of glucocorticoid administered. The reduced muscle bulk was attributed to increased protein catabolism and impaired protein synthesis.[26]
In the present study, the groups that received 1.2 g/kg and 2.4 g/kg of Tualang honey had shown a significant increase in tail flick latency time. The increase in reaction time of the rats shows that Tualang honey at the doses given has analgesic activities. The antinociceptive effects of Tualang honey might be contributed partly by its action on opioid receptors in the spinal cord. However, other mechanisms might also be involved.[27] A report has shown that the antinociceptive effects of Nigerian honey samples (Idanre and Ewu honey) were reversed with the administration of naloxone, an opioid antagonist, but the effects of other Nigerian honey samples (Jigawa, Ile-Ife, and Umudike) were not reversed, suggesting other mechanisms which might come into play (e.g. inhibition of voltage-gated Na+ channels, stimulation of noradrenergic inhibitory system and/or serotonergic system).[27] The analgesic properties of Tualang honey could also be contributed by its antioxidant property (53.06 ± 0.41 mg of ascorbic acid equivalent per gram of Tualang honey).[5] Reports have shown the role of oxidative stress in the development of pain/hyperalgesia, and vitamin C, one of the antioxidants in Tualang honey, has been shown to inhibit nociceptive transmission by interacting at the level of glutamate receptors in the central nervous system.[28] The lowest dose used (0.2 g/kg) probably was not sufficient to give a central effect and did not significantly alter the latency time, compared to controls.
In the present study, the antinociceptive effect in the tail flick test was also evident in the group that received prednisolone. The reduction in pain behavior is probably related to a decrease in calcitonin gene–related peptide (CGRP) and an increase in B2-gamma-aminobutyric acid (GABAB2) receptor expression in the spinal cord.[29] CGRP is one of the neurotransmitters involved in the nociceptive transmission in the spinal cord dorsal horn, while GABA is an inhibitory neurotransmitter that inhibits the transmission. The presence of nuclear glucocorticoid receptor immunoreactivity in a large number of spinal cord nerve cells which have substance P or CGRP immunoreactivity (IR) suggests that a glucocorticoid, prednisolone, may modulate the nociceptive transmitting system, which may lead to a decrease in pain response.[30]
CONCLUSION
Preemptive administration of Tualang honey (1.2 and 2.4 g/kg) and prednisolone (10 mg/kg) is capable of altering the feeding habits and modulating the nociceptive responses in male Sprague-Dawley rats. The preliminary study provides novel knowledge regarding the possible effects of Tualang honey in pain modulation and its protective effects against weight alteration in normal rats. The weight alteration by prednisolone is an unwanted effect due to its metabolic and central actions. Further studies are required to confirm the antinociceptive effects and elucidate the mechanisms of antinociceptive action of Tualang honey in rats.
ACKNOWLEDGMENT
This study was supported by Universiti Sains Malaysia Short Term Grant (304/PPSP/61311065). Special thanks to Dr. Wan Nazirah Wan Yusof for giving her ideas during the progress of the study.
REFERENCES
- 1.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: A review. J Am Coll Nutr. 2008;27:677–89. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 2.Khan FR, Ul Abadin Z, Rauf N. Honey: Nutritional and medicinal value. Int J Clin Pract. 2007;61:1705–7. doi: 10.1111/j.1742-1241.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 3.Kassim M, Achoui M, Mansor M, Yusoff KM. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E (2) in inflammatory tissues. Fitoterapia. 2010;81:1196–201. doi: 10.1016/j.fitote.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–8. [Google Scholar]
- 5.Kishore RK, Halim AS, Syazana MS, Sirajudeen KN. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr Res. 2011;31:322–5. doi: 10.1016/j.nutres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HJ. Effects of a short course of oral prednisolone in patients with bladder pain syndrome with fluctuating, worsening pain despite low-dose triple therapy. Int Neurourol J. 2013;16:175–80. doi: 10.5213/inj.2012.16.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubo M, Onishi H, Kuroki S, Okido M, Shimada K, Yokohata K, et al. Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Res. 2012;32:2331–6. [PubMed] [Google Scholar]
- 8.Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, et al. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2012;31:274–84. doi: 10.1007/s00774-012-0414-9. [DOI] [PubMed] [Google Scholar]
- 9.Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2:10–5. doi: 10.1111/j.1742-4801.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh S, Gurtu S. Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxid Med Cell Longev 2012. 2012 doi: 10.1155/2012/374037. 374037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wung PK, Anderson T, Fontaine KR, Hoffman GS, Specks U, Merkel PA, et al. Effects of glucocorticoids on weight change during the treatment of Wegener's granulomatosis. Arthritis Rheum. 2008;59:746–53. doi: 10.1002/art.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayati AA, Zalina I, Myo T, Badariah AA, Azhar A, Idris L. Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Ger Med Sci. 2008;6:Doc05. [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed M, Sulaiman SA, Jaafar H, Sirajudeen KN. Effect of different doses of Malaysian honey on reproductive parameters in adult male rats. Andrologia. 2012;44:182–6. doi: 10.1111/j.1439-0272.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura E, Kitagawa Y, Ozawa S, Suda K, Ando N, Ueda M, et al. Role of steroid administration to reduce inflammation after thoracotomy in a rat surgical stress model. J Surg Res. 2006;135:364–9. doi: 10.1016/j.jss.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Bannon AW, Malmberg AB. Models of nociception: Hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0809s41. Chapter 8: Unit 89. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed M, Sulaiman SA, Jaafar H, Sirajudeen KN. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. Int J Mol Sci. 2011;12:5508–21. doi: 10.3390/ijms12095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 18.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: Influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–9. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Larson-Meyer DE, Willis KS, Willis LM, Austin KJ, Hart AM, Breton AB, et al. Effect of honey versus sucrose on appetite, appetite-regulating hormones, and postmeal thermogenesis. J Am Coll Nutr. 2010;29:482–93. doi: 10.1080/07315724.2010.10719885. [DOI] [PubMed] [Google Scholar]
- 21.Bahrami M, Ataie-Jafari A, Hosseini S, Foruzanfar MH, Rahmani M, Pajouhi M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int J Food Sci Nutr. 2009;60:618–26. doi: 10.3109/09637480801990389. [DOI] [PubMed] [Google Scholar]
- 22.Liu XY, Shi JH, Du WH, Fan YP, Hu XL, Zhang CC, et al. Glucocorticoids decrease body weight and food intake and inhibit appetite regulatory peptide expression in the hypothalamus of rats. Exp Ther Med. 2011;2:977–84. doi: 10.3892/etm.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You YN, Short KR, Jourdan M, Klaus KA, Walrand S, Nair KS. The effect of high glucocorticoid administration and food restriction on rodent skeletal muscle mitochondrial function and protein metabolism. PLoS One. 2009;4:e5283. doi: 10.1371/journal.pone.0005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides--mediation of the actions of leptin. Trends Neurosci. 1999;22:62–7. doi: 10.1016/s0166-2236(98)01292-2. [DOI] [PubMed] [Google Scholar]
- 25.Hull KL, Cockrem JF, Bridges JP, Candy EJ, Davidson CM. Effects of corticosterone treatment on growth, development, and the corticosterone response to handling in young Japanese quail (Coturnix coturnix japonica) Comp Biochem Physiol A Mol Integr Physiol. 2007;148:531–43. doi: 10.1016/j.cbpa.2007.06.423. [DOI] [PubMed] [Google Scholar]
- 26.Shin YS, Fink H, Khiroya R, Ibebunjo C, Martyn J. Prednisolone-induced muscle dysfunction is caused more by atrophy than by altered acetylcholine receptor expression. Anesth Analg. 2000;9:322–8. doi: 10.1097/00000539-200008000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Akanmu MA, Olowookere TA, Atunwa SA, Ibrahim BO, Lamidi OF, Adams PA, et al. Neuropharmacological effects of Nigerian honey in mice. Afr J Tradit Complement Altern Med. 2011;8:230–49. doi: 10.4314/ajtcam.v8i3.65285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa KA, Gadotti VM, Rosa AO, Rodrigues AL, Calixto JB, Santos AR. Evidence for the involvement of glutamatergic system in the antinociceptive effect of ascorbic acid. Neurosci Lett. 2005;381:185–8. doi: 10.1016/j.neulet.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Pinto-Ribeiro F, Moreira V, Pego JM, Leao P, Almeida A, Sousa N. Antinociception induced by chronic glucocorticoid treatment is correlated to local modulation of spinal neurotransmitter content. Mol Pain. 2009;5:41. doi: 10.1186/1744-8069-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLeon M, Covenas R, Chadi G, Narvaez JA, Fuxe K, Cintra A. Subpopulations of primary sensory neurons show coexistence of neuropeptides and glucocorticoid receptors in the rat spinal and trigeminal ganglia. Brain Res. 1994;636:338–42. doi: 10.1016/0006-8993(94)91034-0. [DOI] [PubMed] [Google Scholar]


