Abstract
Chronic kidney disease (CKD) is placing an increasing burden on patients and societies because no decisive therapy has been established. Tubulointerstitial lesions accompanied by fibrosis, inflammatory cells, and capillary rarefaction not only characterize, but also aggravate renal dysfunction in CKD. In this setting, renal cells, particularly tubular cells, suffer from hypoxia caused by the imbalance of blood perfusion and oxygen demand despite their adaptive responses represented by upregulation of hypoxia-inducible factors (HIFs). Fibrosis is a pathological state characterized by excess extracellular matrix (ECM) deposition, which is also a hallmark and causative factor of many chronic diseases including CKD. Recent studies have suggested that the dominant origin of ECM-producing myofibroblasts (MFs) may be pericytes, which are indispensable cells for maintaining proper capillary functions, as they wrap capillaries and stabilize them through a fine-tuned interplay with endothelial cells. During fibrosis, pericytes are activated and detach from capillaries before conversion into MFs, which compromises capillaries and worsens hypoxia. We also discuss how hypoxia and HIFs affect fibrogenesis. Given that hypoxia is caused by insufficient angiogenesis and that fibrosis results from pericyte loss, restoration of pericytes should be an intriguing target for overcoming both hypoxia and fibrosis. We propose the deactivation of MFs to recover lost pericytes as a promising therapy for CKD.
Keywords: angiogenesis, chronic kidney disease, fibrosis, hypoxia, pericytes
Fibrosis is a pathological state with excessive deposition of extracellular matrix (ECM), which is commonly accompanied by chronic diseases in many organs, including the kidneys. Production of ECM occurs as a reaction against injury, and fibrosis itself is intrinsically a process to promote tissue repair. However, when it occurs in excess, it can deteriorate the functions of the affected organ. Therefore, fibrosis is not only an outcome of the injury, but also a mechanism leading to a further damage.1
In the kidney, tubulointerstitial fibrosis with injured tubules and inflammatory leukocytes is a hallmark of chronic kidney disease (CKD) with renal dysfunction, regardless of its cause. As the glomerular filtration rate, which indicates kidney function, continually declines, CKD results in end-stage renal disease necessitating renal replacement therapy. However, decreases in the glomerular filtration rate correlate with tubulointerstitial lesions better than with glomerular lesions, even in glomerulonephritis.2 Collectively, the literature suggests that tubulointerstitial injury is a common feature of renal dysfunction, and a leading candidate as a target for CKD therapies.
How does tubulointerstitial injury occur? One of the most important mechanisms is tissue hypoxia.3 Rarefaction of peritubular capillaries can also be observed in almost all kidney diseases, decreasing the oxygen supply and inducing hypoxia in tubulointerstitial areas. Furthermore, in fibrosis, pericytes, a constituent of peritubular capillaries, detach from vessels and produce ECM, which compromises the capillaries.1 Therefore, tubulointerstitial injury, hypoxia, and fibrosis are intricately related to one another, which is mediated by capillary rarefaction.
In this review, we verify the critical roles of hypoxia in the pathophysiology of CKD, briefly summarize the mechanisms of fibrosis, and discuss the effects of hypoxia in the context of fibrosis. Thereafter, we debate the potential of pericytes as a promising target for therapy in CKD, as it can ameliorate capillary loss as well as tissue hypoxia.
HYPOXIA IN CKD
The kidney is physiologically hypoxic despite its plentiful blood supply (as much as 20% of the cardiac output in humans), because an oxygen shunt is present between arteries and veins that run in close proximity.3 Therefore, it is reasonable to consider that erythropoietin-producing cells reside in the kidney, where they can sensitively detect hypoxia due to anemia. Physiological hypoxia in the kidney has been demonstrated in mammals using pimonidazole measurements and in hypoxia-monitoring transgenic mice and rats created by exploiting the hypoxia-inducible factor (HIF) system.4, 5 Augmented kidney hypoxia in CKD has also been confirmed in patients using blood oxygen-level–dependent magnetic resonance imaging,6 as well as in animal models using the methods mentioned above.5
In CKD, hypoxia occurs in tubulointerstitial areas through multiple mechanisms. First, glomerulosclerosis leads to a reduction of flow in downstream peritubular capillaries, which can be further compromised by constriction of the efferent arterioles of glomeruli and peritubular capillaries themselves due to activation of the renin–angiotensin system. Second, distortion and loss of peritubular capillaries due to fibrosis decreases blood perfusion. Third, ECM deposition by fibrogenesis widens the distance between capillaries and tubules, diminishing the efficiency of oxygen diffusion.
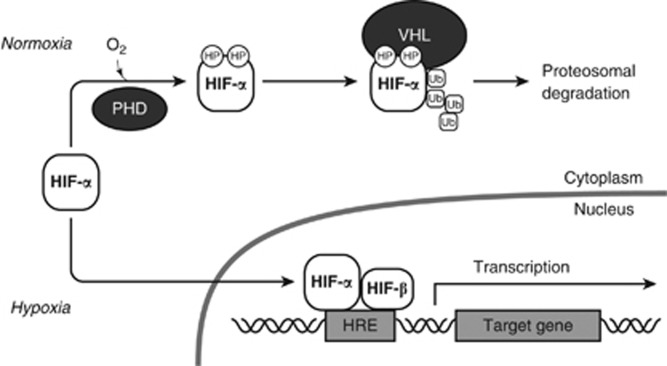
Following exposure to hypoxia, cells induce adaptive responses to survive the harsh environment. Among them, the HIF transcription factors have crucial roles (Figure 1).7 HIFs are heterodimers composed of an α subunit, including HIF-1α, HIF-2α, and HIF-3α, and a common β subunit, HIF-1β. Despite their constitutive expression, the α subunits of HIF are degraded by proteasomes and are not functional under normoxic conditions. Prolyl hydroxylases (PHDs) hydroxylate the conserved proline residues of HIF-α using oxygen, recruiting the von-Hippel-Lindau protein, which is a recognition component of E3 ubiquitin ligase. This results in the ubiquitination of the modified HIF-α subunits, and eventually leads to their proteasomal degradation. Under hypoxic conditions, HIF-α escapes post-translational modification by PHDs, forms a heterodimer with HIF-1β, and promotes the expression of its target genes. Its targets include glycolytic enzymes, which allow anaerobic ATP production, and angiogenic factors, including vascular endothelial growth factors (VEGFs), which can promote new vessel formation to increase oxygen supply.
Figure 1.
Regulation of hypoxia-inducible factors. In normoxia, certain proline residues of the α subunits of the hypoxia-inducible factor (HIF) are hydroxylated by prolyl hydroxylases (PHDs) using oxygen. The hydroxyprolines (HP) allow von-Hippel-Lindau (VHL) protein to bind and ubiquitinate HIF-α, which results in their proteaosomal degradation. In contrast, HIF-α escapes from post-translational modification by PHDs in hypoxia. HIF-α forms a heterodimer with a β subunit (HIF-β) and then binds to a hypoxia response element (HRE) in the regulatory region of a target gene and promotes its transcription. Ub, ubiquitin.
Despite such cellular-adaptive mechanisms, hypoxia causes tubular cell injury and death, which advances CKD.8 The proximal tubules are considered more susceptible to hypoxia because they solely depend on aerobic oxidative metabolism.9 Damaged tubular cells can, in turn, worsen glomerular lesions through tubular obstruction and maladaptive tubuloglomerular feedback. They can also induce interstitial fibrosis, as described below. Thus, tubular injury aggravates two leading causes of tubular hypoxia, glomerular injury and interstitial fibrosis, which forms a vicious cycle and advances CKD.
Therefore, these findings suggest that enhanced HIF activity might protect the kidney against hypoxic injury and suppress the progression of CKD. In fact, activation of HIFs by the systemic administration of an inhibitor of PHDs has been shown to mitigate tubular injury and capillary rarefaction in various CKD models.10 These results suggest that inhibitors of PHDs may have therapeutic potential in CKD.
Recent studies have also demonstrated that hypoxia can cause epigenetic changes in cells. For example, hypoxic conditions alter the transcription of some genes through changes in the chromatin conformational structure and histone modification changes.11 Such epigenetic alterations in hypoxic renal cells may affect the pathophysiology of CKD, and may be newly emerging targets for therapy.
MECHANISMS OF FIBROSIS
In chronic diseases, tissue damage persists without resolution, often along with inflammatory leukocytes and fibrosis, as some repair mechanisms may be insufficient or maladaptive. Therefore, it is reasonable to consider recurrent and persistent injury to epithelial cells, which fulfill the predominant physiological functions in tissues, as the prime element that initiates and sustains fibrogenesis. Epithelial cell death can provoke pro-inflammatory responses through damage-associated molecular pattern molecule receptors, and lead to fibrosis. Cellular stress in epithelial cells also induces activation of innate immunity through the production of cytokines and chemokines.1
The cells that actually produce pathologic ECM are called myofibroblasts (MFs), which are activated mesenchymal cells that express α-smooth muscle actin. Because epithelial-to-mesenchymal transition was proposed as a dominant mechanism that gives rise to MFs, their origin has been debated for decades. However, recent studies indicate that the dominant origin of MFs is not epithelial cells, but mesenchymal cells in most organs, including the kidneys, liver, lungs, skin, and spinal cord.1 In the kidney, some studies have shown that most of these mesenchymal fibroblasts are attached to the microvasculature and have proven to be pericytes.12 Other studies, however, argue that these mesenchymal progenitor cells are ‘fibroblasts', although they did not investigate their anatomical relationship with vessels.13, 14 Pericytes are cells that wrap around the endothelial tubes of capillaries, some of which are embedded in the basement membrane of capillaries with direct and indirect contact with endothelial cells.15 Factors produced by injured epithelial cells and other cells, including VEGFs, platelet-derived growth factors (PDGFs), fibroblast growth factors, and transforming growth factor-β, activate pericytes and induce their detachment from capillaries and conversion to MFs.1 In addition, it is worth noting that pericytes are erythropoietin-producing cells in the kidney, and MFs lose the capacity to produce this hormone.13
Accumulation of inflammatory cells is accompanied by fibrosis. These cells are derived from both resident and recruited cell populations.16 Among them, monocytes and macrophages have central roles in fibrosis. Resident macrophages maintain tissue homeostasis under physiological conditions and react upon tissue insults. Monocytes are leukocytes in the circulating blood, but they are recruited to damaged tissue, where they differentiate into macrophages. Although it is well known that these monocytes/macrophages have a broad spectrum of functions and are subdivided, e.g., M1 and M2 macrophages, as a whole, they are thought to have a tendency to promote renal fibrosis because their ablation in a typical fibrosis model, unilateral ureteric obstruction (UUO), mitigates fibrosis.16 These cells produce profibrotic and pro-inflammatory factors, which maintain activation of MFs and their fibrogenesis. Furthermore, these inflammatory cells induce additional epithelial cell injury, creating a positive-feedback loop and developing fibrosis.
Taken together, fibrosis advances in a manner in which repetitive and unresolved epithelial injury primes pericyte activation, inducing them to become MFs and generate ECM, which is augmented by profibrotic monocytes/macrophages.
HYPOXIA IN FIBROSIS
In this section, we will describe the roles of hypoxia, particularly HIFs, in fibrosis, with regard to its effects on epithelial cells, pericytes/fibroblasts, and monocytes/macrophages.
Epithelial cells
Increased HIF-α in the renal tubular cells of transgenic mice with conditional von-Hippel-Lindau protein knockout in adulthood enhances VEGF and PDGF-B expression and augments endothelial cell proliferation, increasing their numbers. Although increased production and deposition of ECM were also observed in these transgenic mice compared with control mice, they did not display renal injury or dysfunction17 These results are consistent with studies showing that conditional knockout of HIF-1α in the proximal tubules lessens fibrosis in mouse UUO.18 Given that deposition of ECM is a part of repair processes unless it is uncontrolled, it is suggested that HIF activation by hypoxia in tubular cells can mitigate renal damage through the upregulation of angiogenic and fibrogenic factors as adaptive responses. This idea is consistent with the observation that general activation of HIFs mitigates renal injury in a CKD model.10
Pericytes
Human renal fibroblasts, isolated by morphologic criteria, showed increased expression of collagen Iα1 and tissue inhibitor of metalloproteinase-1, when they were exposed to hypoxia.19 By contrast, in mouse renal interstitial fibroblasts, hypoxia did not induce expression of collagen I, α-smooth muscle actin, or transforming growth factor-β1 in vitro.20 The discrepancy may derive from differences in the origins of the cells and/or methods of culture. For example, in hepatic stellate cells, which are considered as the equivalent of pericytes in the liver, hypoxia increases collagen I and VEGF expression.21
During kidney fibrosis, VEGF and PDGF signaling induces pericyte detachment from capillaries and conversion to MFs. Given that hypoxia promotes the production of both growth factors in epithelial cells as mentioned above, hypoxia may indirectly cause pericyte loss, although its direct effects on pericytes remain to be elucidated.22
It should be noted that even primary pericytes derived from intact tissue tend to be activated and have characteristics of MFs when they are cultured under normal conditions without special stimuli. Therefore, to determine the precise roles of hypoxia and/or HIFs in pericytes, investigation of the effects of specific overexpression and knockout of HIFs in these cells in vivo is warranted.
Monocytes and macrophages
Lysozyme M (LysM)-Cre is often utilized to control the expression of a gene specifically in monocytes and macrophages. Mice with increased HIF expression in monocytes/macrophages, in which von-Hippel-Lindau protein is knocked out by LysM-Cre, had fewer macrophages, although they exhibited comparable fibrosis and tubular damage in a UUO model.23 However, specific knockout of both HIF-1α and HIF-2α in macrophages increased the number of macrophages in UUO without affecting fibrosis. This discrepancy between inflammatory cells and fibrosis appears inconsistent with studies, showing that the ablation of macrophages mitigates fibrosis.16 One possible explanation is that HIF upregulation and HIF downregulation change the characteristics or subpopulations of macrophages, which alters the effects of alterations in macrophage number. This may result from a varied LysM-Cre efficiency in macrophage subtypes because LysM-Cre deletes a floxed gene in no more than 80% of macrophages.24 In addition, LysM is also expressed in neutrophils, and gene knockout can therefore also occur in the neutrophils of mice with a LysM-Cre transgene. Therefore, changes in gene expression in neutrophils may have affected the development of fibrosis.
Contrasting properties of HIFs in myeloid-lineage cells have been reported in liver fibrosis. In a bile duct ligation model, macrophage-specific HIF-1β knockout in mice using LysM-Cre led to less fibrosis and ameliorated tissue injury. In conditional knockout mice, the numbers of macrophages had a tendency to decrease, but the difference was not significant. Similar results were obtained with HIF-1α knockout. In this study, PDGF-B expression in macrophages was reduced by HIF knockout, which may contribute to the observed decrease in fibrosis.25
PERICYTES AS A TARGET TO TREAT HYPOXIA AND FIBROSIS
Peritubular capillary rarefaction and fibrosis are causative factors in advancing CKD, as well as common hallmarks of CKD. Therefore, it is important to overcome these two defects in CKD therapy.
This has been attempted through efforts to augment angiogenesis, and to preserve peritubular capillaries and mitigate hypoxia in tubulointerstitial areas.26 Considering that the simple invasion of endothelial cells into nascent organs during development provides inductive signals to promote organogenesis even without blood flow,27 angiogenesis may also promote tissue repair and regeneration through effects beyond simple oxygen and nutrition supply. As such, angiogenesis appears to be a more promising therapy for CKD, but success using this approach has not been achieved. However, angiogenesis occurs through complicated mechanisms including the coordinated actions of angiogenic factors. Among them, VEGF-A (VEGF) has predominant roles and by itself can induce angiogenesis.28
Although treatment with VEGF gives rise to new vessel formation, the new vessels are leaky and unstable. Because leukocytes can be recruited through leaky vessels, they are also pro-inflammatory. This may be related to the fact that proliferation of endothelial cells unexpectedly occurs during the early phase of UUO, when increased VEGF expression and macrophages numbers are also observed, followed by the loss of peritubular capillaries and advanced fibrosis.29 This suggests that incomplete angiogenesis may leave capillaries unstable with subsequent worsening of inflammation and fibrosis.
These flaws primarily result from the absence of pericytes, which cover immature vessels composed of endothelial cells, inhibit the recruitment of inflammatory cells, and enable the vessels to function properly. It is natural to speculate that pericytes are converted into MFs, and physiological, or properly functioning, pericytes are scarce in CKD. Therefore, deactivation of MFs with subsequent restoration of pericytes is the cornerstone for ameliorating both hypoxia and fibrosis.
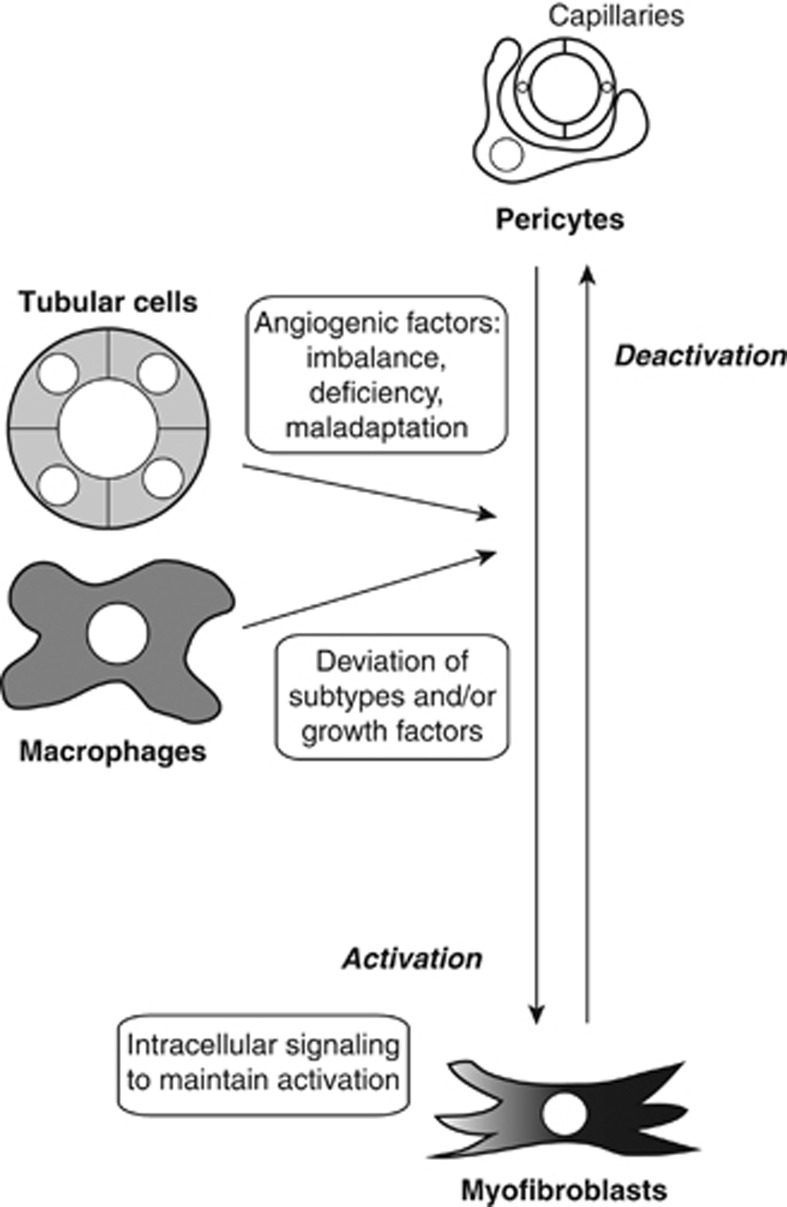
How can we achieve this goal? First, we should consider why hypoxic tubular cells cannot induce sufficient angiogenesis although hypoxia is one of the most potent angiogenic stimuli. As mentioned above, in CKD, tubular cells are under hypoxic stress and can express angiogenic factors, including VEGF. It is possible that an imbalance and/or a deficiency exists in the ability of some angiogenic factors to form mature and stable vessels by recruiting pericytes. It is known that several growth factor families, such as PDGFs, angiopoietins, and transforming growth factor-β, contribute to the recruitment of pericytes to immature vessels composed of endothelial cells.28 If tubular cells predominantly express VEGF and insufficient pericyte-recruiting growth factors, the new vessels will be immature and have fewer pericytes. In addition, pericyte-recruiting factors secreted from tubular cells maladaptively induce the detachment of pericytes from capillaries rather than recruitment to capillaries (Figure 2). Future studies that verify the profiles and actions of angiogenic growth factors may facilitate CKD therapy aimed at proper angiogenesis.
Figure 2.
Targets to deactivate myofibroblasts for proper angiogenesis. Pericytes wrapping capillaries are activated by stimuli including growth factors produced by injured tubular cells and profibrotic macrophages, and become myofibroblasts that deposit extracellular matrix, resulting in fibrosis. Deactivation of myofibroblasts and subsequent restoration of pericytes are essential for the formation of stable and non-inflammatory capillaries during angiogenesis. Boxes show the mechanisms promoting fibrosis that can be targeted for myofibroblast deactivation.
Given that fibrosis is essentially a protective response to tissue injury and is resolved upon removal of the injury, MFs should be capable of reverting to pericytes. In fact, chronic fibrosis can be reversible in patients when the primary insult is eliminated.30 However, in advancing fibrosis, pericyte recruitment to newer vessels may be hindered by its fibrogenic phenotype. The exact mechanisms that maintain activated states in MFs and prevent them from reverting to pericytes remain to be elucidated, although many of the growth factors involved in these processes have been identified. Verifying the culprit in intracellular signaling should provide clues to a novel therapeutic method for pathologic fibrosis. Furthermore, restored pericytes should resume the production of erythropoietin,13 which would ameliorate anemia in CKD. Hence, pericytes mitigate kidney hypoxia not only by increasing vessel stability but also by enhancing the capacity of blood to convey oxygen.
Macrophages also have roles in both angiogenesis and fibrosis.16, 24 They can express VEGFs, PDGFs, fibroblast growth factors, and angiopoietins, sometimes in response to hypoxia. During angiogenesis and/or fibrosis, these factors are secreted from so-called ‘M2' macrophages, which promote tissue repair. However, M2 macrophages are thought to be further subdivided into angiogenic and fibrogenic macrophages, although their relevance in vivo remains unclear.16, 24 It is also possible that macrophages express a relatively homogenous repertoire of growth factors that is dependent on the surrounding milieu. Regardless, macrophages can affect pericyte activation. Therefore, control of the differentiation of macrophage subtypes and/or growth factor production is another possible method for CKD therapy.
In addition to determining the roles of particular cell populations, studies have revealed that the general activation of HIFs by PHD inhibition can promote angiogenesis. For example, peptide-mediated inhibition of PHDs generates more mature and less leaky vessels than those generated by growth factors.31 Knockdown of PHD2 in endothelial cells or pericytes also leads to more stable vessels.32 These findings indicate the existence of some unidentified factors or mechanisms promoting vessel stabilization through pericyte recruitment. Chemical PHD inhibitors are under development and have therapeutic potential for the treatment of CKD.
CONCLUSIONS
Although CKD and the resultant renal replacement therapy are an increasing burden for patients and society, no decisive therapeutic method has been developed. Fibrosis and hypoxia are major therapeutic targets for treating tubulointerstitial lesions in CKD. During fibrosis, pericytes leave capillaries and become ECM-producing MFs. This process results in capillary fragility and rarefaction, which are central causes of hypoxia. Therefore, deactivating MFs to restore pericytes naturally emerges as a potential CKD therapy. Much still remains to be elucidated, but future investigations may unravel the detailed mechanisms of the fibrotic process and thereby open new avenues for promising therapies to prevent or reverse CKD.
Acknowledgments
Supported in part by Grants-in-Aid for Scientific Research (B) (24390213 to MN) from the Japan Society for the Promotion of Science and a Grant-in-Aid for Progressive Renal Disease Research, Research on Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
IM is a research fellow of the Japan Society for the Promotion of Science. The remaining authors declared no competing interests.
References
- Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- Safran M, Kim WY, O'Connell F, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Miyata T, Inagi R, et al. Hypoxia in renal disease with proteinuria and/or glomerular hypertension. Am J Pathol. 2004;165:1979–1992. doi: 10.1016/S0002-9440(10)63249-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- Epstein FH. Oxygen and renal metabolism. Kidney Int. 1997;51:381–385. doi: 10.1038/ki.1997.50. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kojima I, Ohse T, et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- Mimura I, Nangaku M, Kanki Y, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;32:3018–3032. doi: 10.1128/MCB.06643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin S-L, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Lin SL, Castaño AP, Nowlin BT, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- Theilig F, Enke AK, Scolari B, et al. Tubular deficiency of von Hippel-Lindau attenuates renal disease progression in anti-GBM glomerulonephritis. Am J Pathol. 2011;179:2177–2188. doi: 10.1016/j.ajpath.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Borges FT, Melo SA, Özdemir BC, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- Lin S-L, Chang F-C, Schrimpf C, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Gilbert V, Liu Q, et al. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol. 2012;188:5106–5115. doi: 10.4049/jimmunol.1103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Lichtnekert J, Thompson LJ, et al. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol. 2013;191:3358–3372. doi: 10.4049/jimmunol.1300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Kaska S, Wentling C. Hypoxia-inducible factor activation in myeloid cells contributes to the development of liver fibrosis in cholestatic mice. J Pharmacol Exp Ther. 2012;341:307–316. doi: 10.1124/jpet.111.189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol. 2013;9:211–222. doi: 10.1038/nrneph.2013.35. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y, Ieronimakis N, Schrimpf C, et al. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559–572. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–1180. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Willam C, Masson N, Tian Y-M, et al. Peptide blockade of HIFalpha degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci USA. 2002;99:10423–10428. doi: 10.1073/pnas.162119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Nishiyama N, Kano MR, et al. Enhancement of angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing prolyl hydroxylase domain-2 gene. Mol Ther. 2008;16:1227–1234. doi: 10.1038/mt.2008.90. [DOI] [PubMed] [Google Scholar]