Abstract
Obesity in combination with diabetes and hypertension likely is contributing to the increasing incidence of chronic kidney disease (CKD) in the 21st century worldwide and requires novel insights and strategies for treatment. There is an increasing recognition that the kidney has an important role in the complex inter-organ communication that occurs with the development of inflammation and fibrosis with obesity. Inhibition of the adiponectin-AMPK pathway has now become established as a critical pathway regulating both inflammation and pro-fibrotic pathways for both obesity-related kidney disease and diabetic kidney disease. AMPK regulates NFκB activation and is a potent regulator of NADPH oxidases. Nox4 in particular has emerged as a key contribtor to the early inflammation of diabetic kidney disease. AMPK also regulates several transcription factors that contribute to stimulation of the transforming growth factor-beta (TGF-β) system. Another key aspect of AMPK regulation is its control of mammalian target of rapamycin (mTOR) and mitochondrial biogenesis. Inhibition of PGC-1α, the transcriptional co-activator of mitochondrial biogenesis is being recognized as a key pathway that is inhibited in diabetic kidney disease and may be linked to inhibition of mitochondrial function. Translation of this concept is emerging via the field of urine metabolomics, as several metabolites linked to mitochondria are consistently downregulated in human diabetic kidney disease. Further studies to explore the role of AMPK and related energy-sensing pathways will likely lead to a more comprehensive understanding of why the kidney is affected early on and in a progressive manner with obesity and diabetes.
Keywords: adiponectin, AMPK, inflammation, NADPH oxidase, USF1
The worldwide increase in obesity likely contributes to development and progression of kidney disease with both diabetes and hypertension. The contribution of obesity has been highlighted by a large epidemiologic study, where the body mass index was found to be the second most important contributor to relative risk for developing end-stage renal disease, after proteinuria, among all subjects who were registered as patients and followed for 27–36 years.1 With obesity contributing to multi-organ disease, there is increasing recognition that the kidney is affected at the very stages of obesity and may contribute to systemic inflammation. In this review, studies linking the adiponectin-5′-AMP activated protein kinase (AMPK) pathway will be highlighted with respect to development of inflammation and fibrosis with obesity-related and diabetic kidney disease.
ADIPONECTIN IN OBESITY-RELATED KIDNEY DISEASE
Adiponectin is a 30-kDa (Acrp30) protein predominantly produced in the adipose tissue and circulates in the plasma as a trimer (low molecular weight), a hexamer generated from two trimers, or as multimers consisting of 12–18 hexamers (high molecular weight; HMW). Levels of adiponectin are decreased in obesity, coronary artery disease, and type 2 diabetes mellitus.2 In our prior studies, adiponectin levels were found to be inversely correlated with low-grade albuminuria in obese African-American subjects.3 A similar relationship was found in patients with hypertension from Europe and in patients from Japan.4, 5 Interestingly, this relationship was primarily found in subjects with elevated body mass index and with low-grade albuminuria. A large study with 440 subjects recently found the same inverse relationship between adiponectin levels and albuminuria in subjects with obesity, even after adjusting for other risk factors.6 However, in patients with established diabetes or chronic kidney disease (CKD), adiponectin is positively correlated with albuminuria or proteinuria.7, 8 It remains controversial whether serum adiponectin levels predict future cardiovascular risk factors in CKD subjects.9, 10 A potential role for adiponectin to act on podocytes has been identified based on expression studies of the receptors for adiponectin. Adiponectin receptor 1 and adiponectin receptor 2 are the two major receptors for adiponectin and are described as a new class of heptahelix receptors structurally and functionally distinct from G-protein-coupled receptors.11 Both receptors signal via the AMPK pathway. Adiponectin receptor 1 gene expression is expressed in the mouse kidney and podocytes to a similar degree as in liver, whereas adiponectin receptor 2 gene expression of kidney and podocyte is much less than liver.3 Protein studies are difficult to interpret as the commercially available antibodies may not be specific and sensitive. Presently, based on the published epidemiologic studies there is convincing evidence that in subjects who are obese (without diabetes or CKD) there is a relationship between circulating adiponectin levels and low-grade albuminuria, however, the role for adiponectin to mechanistically contribute to albuminuria will be difficult to establish in human studies.
A cause and effect role for adiponectin in the development of kidney disease has been supported by several independent studies using different mouse models of manipulating adiponectin. Studies in one strain of adiponectin knockout (KO) mice by our group identified that the KO mice have elevated levels of albuminuria but only two- to threefold greater than controls on a C57Bl6 background.3, 12 With addition of hyperglycemia, there was a progressive increase in albuminuria in the KO mice, but not in the wild-type diabetic mice. Treatment with exogenous adiponectin was found to attenuate albuminuria and restore podocyte foot process effacement. In a similar vein, 5/6 nephrectomy was found to lead to an accelerated disease in a different adiponectin KO mouse.13 In a recent study,14 mice with a genetically engineered inducible podocyte injury have worse disease when lacking adiponectin, and have protection when overexpressing adiponectin. Thus, the available studies are in agreement that, in models of glomerular injury adiponectin has a protective role. However, in contrast there is conflicting data with acute kidney injury. In one study, adiponectin-deficient mice were protected from ischemia-reperfusion injury,15whereas an independent study found the reverse, i.e., that adiponectin deficiency exacerbated acute kidney injury after ischemia reperfusion.16 The role of the specific adiponectin receptors in chronic or acute kidney injury remains to be established.
AMPK PATHWAY, INFLAMMATION, AND CKD
The major signaling pathways by which adiponectin appears to confer its effects is via stimulation of AMPK, Akt, Rab5, and phospholipase C.17 AMPK is a stress-activated kinase that is activated in response to depleting ATP or a relative increase in the intracellular AMP/ATP ratio to preserve cell survival under a low-caloric environment.18 AMPK was established to have a central role in the effects of adiponectin based on studies in the adiponectin receptor KO mice.2 As obesity is associated with a reduction in adiponectin and an excess of calories will lead to a reduced AMP/ATP ratio, it would follow that AMPK would be reduced with obesity-related kidney disease.
We first reported that renal AMPK was reduced in a mouse model of high-fat-induced obesity (diet-induced obesity) within 1 week of the onset of the high-fat diet.19 Surprisingly, there was evidence of renal inflammation (elevated urine hydrogen peroxide, urine, and glomerular monocyte chemoattractant protein-1 induction) by 1 week of the high-fat diet, and the inflammation preceded any increase in albuminuria. Stimulation of AMPK by 5-aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR) was able to completely suppress the inflammatory markers and reduced mesangial cell production of monocyte chemoattractant protein-1 in response to palmitate. More recently, chronic stimulation of AMPK by AICAR (for 12 weeks) was also successful to reduce renal inflammation, albuminuria, and matrix accumulation with the high-fat diet (Decleves, Kidney Int 2014; 85: 611–623). Furthermore, AMPK activation was able to completely reduce lipid vacuolization in proximal tubular cells as well. Part of the basis for the latter finding may be due to reduction of HMGCoA reductase activity with AMPK activation and reduced cholesterol production.
AMPK also seems to have a prominent role in regulating macrophage infiltration and activation. The overall numbers of macrophages infiltrating the kidney with a high-fat diet was completely normalized with AMPK activation. Furthermore, AMPK activation lowered the CD11c/CD11b ratio indicating a reduction in M1 macrophages (Decleves, Kidney Int 2014; 85: 611–623). A role for AMPK in regulating macrophage activation has been highlighted recently18 and will be an active area of research in future studies.
AMPK also appears to have a key role in regulating the NADPH oxidase (Nox) system. Of the major Nox isoforms that have been identified, it appears that Nox1, 2, and 4 may have a role in mediating the oxidative stress involved in CKD. We have previously identified that Nox4 was prominent in podocytes and that high glucose-induced upregulation of Nox4 can be blocked with adiponectin or activation of AMPK.3 In separate studies, AMPK was found to inhibit Nox2 subunits via upregulating IκB and blocking NF-κB-induced stimulation of Nox subunits (p67, p47) in endothelial cells.20
A role for AMPK regulation of Nox4 was demonstrated in diabetic kidney disease by several groups and there is a growing consensus that Nox4 may be the most critical Nox linked to progression of diabetic kidney disease.21, 22 Our group found that mice with Nox2 deficiency have the same degree of hyperglycemia and weight loss with streptozotocin-induced diabetes, however, the degree of diabetic kidney disease was not affected in the Nox2 KO diabetic group.23 The degree of albuminuria, glomerular matrix expansion, and urine hydrogen peroxide was essentially the same in the wild-type and Nox2 KO diabetic groups. As there was a marked increase in Nox4 in the Nox2 KO diabetic kidney, it is possible that Nox4 may compensate for Nox2 and be sufficient to promote diabetic kidney disease. There are ongoing studies with a combined Nox1/Nox4 inhibitor. In other studies, inhibition of Nox1/Nox4 was found to be protective with liver disease and cardiac disease.24, 25 These studies have taken on added importance, as new phase II studies are underway to evaluate the role of Nox inhibition for diabetic kidney disease.
AMPK, FIBROSIS, AND CKD
In addition to inflammation, AMPK has also been closely linked to fibrosis promoting pathways. In the high-fat diet model, chronic AMPK activation with AICAR was able to reduce mesangial matrix expansion and reduce urinary levels of TGF-β1 (Decleves, Kidney Int 2014; 85: 611–623). Recently, we found that AMPK activation also markedly reduced glomerular TGF-β, collagen, and fibronectin accumulation in several mouse models of diabetic kidney disease (Dugan, J Clin Invest 2013; 123: 4888–4899). Similar findings were also found with the OVE 26 mouse.22 The mechanistic basis for how AMPK activation inhibits TGF-β is unclear at present. A prior study found that adiponectin and AMPK reduced TGF-β-induced matrix and myofibroblast transformation,26 however, Smad2/3 phosphorylation was not affected. Recently, we found that a key transcription factor USF1 was translocated to the nucleus with high glucose exposure and completely blocked by AMPK activation.27 As USF1 has been found to mediate glucose-induced stimulation of the TGF-β1 gene transcription, there could be an important effect of AMPK to regulate USF1-induced TGF-β1 synthesis.28
In addition to downstream effects by AMPK to regulate Nox and TGF-β, there is a well-established pathway by which AMPK inhibits mTOR activity. Several groups have identified that mTOR is activated in diabetic kidney disease.29 Inhibition of mTOR is protective against diabetic kidney disease.29 However, deletion of mTOR in podocytes also contributes to disease,30 and treatment with rapamycin has been found to enhance proteinuria in some patients,31 thus limiting its utility as a therapeutic for diabetic kidney disease. A recent study found that mTOR inhibition also led to reduced Nox4 levels in podocytes, suggesting that mTOR may have a direct effect to regulate Nox4 independent of AMPK.32
AMPK AND MITOCHONDRIAL FUNCTION
A key pathway by which AMPK stimulation protects cells in a calorie-deprived state is to stimulate the master regulator of mitochondrial biogenesis, PGC-1α. This transcriptional co-activator is a potent stimulator of many mitochondrial proteins and increases mitochondrial content.33 In states of reduced AMPK activation, it would be expected that PGC-1α is also reduced. Indeed, PGC-1α was found to be markedly reduced in the muscle of patients with diabetes34 and may be due partly to epigenetic modification of the PGC-1α promoter. Recently, we found that the diabetic kidney also had reduced PGC-1α levels in association with reduced AMPK, reduced mitochondrial content, and reduced mitochondrial complex activity (Dugan, J Clin Invest 2013; 123: 4888–4899). This led to the question that, if there is reduced mitochondrial complex activity in the electron transport chain would there be a concomitant change in mitochondrial superoxide production? Indeed, we found that there was reduced superoxide production in the diabetic kidney using a real-time imaging protocol and further verified by ex vivo studies with electron paramagnetic resonance measurements. Thus, we found that the diabetic kidney is actually in a state of reduced mitochondrial activity and reduced mitochondrial superoxide production. This is in direct contrast to the prevailing notion that diabetic complications are due to an excess of mitochondrial superoxide!
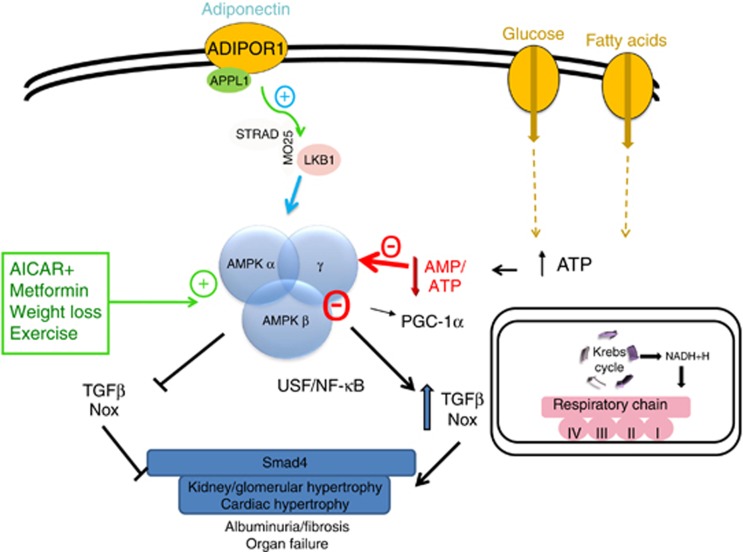
We further sought to ask this question in patients with established diabetic kidney disease. To get an index of mitochondrial activity, we performed quantitative measurements of a variety of metabolites linked to various biochemical pathways linked to human disorders (Sharma, J Am Soc Nephrol 2013; 24: 1901–1912). The predominant signature that was identified was a reduction of metabolites produced by mitochondrial enzymes. Semi-quantitative analysis of mitochondrial complex IV revealed reduction in kidney biopsies from patients with diabetic nephropathy. Furthermore, there was a reduction of gene expression for PGC-1α in diabetic kidney tissues, but not in minimal change disease. These set of studies help to establish a new paradigm for understanding diabetic kidney disease. An early and progressive reduction in mitochondrial content, potentially driven by reduced AMPK/PGC-1α, is linked to early renal inflammation and pro-fibrotic pathways (see Figure 1). Chronic exposure of cells to caloric excess, from excess glucose and/or high fat, is linked to reduction of AMPK, possibly due to transient or sustained reduction in the AMP/ATP ratio. Persistent reduction of AMPK activity allows for stimulation of inflammatory pathways mediated by NFκB and pro-fibrotic pathways mediated by USF1. Downstream of the transcription factors, Nox and TGF-β are stimulated and directly contributing to inflammation and fibrosis in the kidney and heart. Pathways that mitigate AMPK reduction are the adiponectin-LKB1 pathway as well as direct and indirect activators of AMPK, including AICAR, metformin, weight loss, and exercise. On the basis of several animal studies, stimulation of AMPK may mediate many of the beneficial effects to reduce inflammation and fibrosis.18, 35, 36 Stimulation of AMPK via pharmacologic and non-pharmacologic interventions may well be beneficial in human kidney disease as well. Of note, non-pharmacologic means of increasing AMPK has been identified by exercise and food restriction, and exercise37 has been shown to reduce diabetic kidney disease independently of weight loss and glucose lowering.38
Figure 1.
Excess glucose and fatty-acid exposure linked to reduction of AMPK and consequent inflammation and fibrosis. Increased caloric exposure may lead to a reduction in AMP/ATP ratio and reduced 5′-AMP activated protein kinase (AMPK) activity. Reduced AMPK has been linked to increased activity of NFkB and nuclear translocation of USF1. NFkB can stimulate the NAPDH oxidase system, whereas USF1 mediates transforming growth factor-beta 1 (TGF-β1) gene transcription under high-glucose conditions. Stimulation of AMPK via adiponectin/LKB1 or various agonists (aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR)) or modulators of AMPK (metformin, weight loss, exercise) can block NFkB activation and USF1 nuclear translocation. In addition, the role of the AMPK-PGC-1α-mitochondrial biogenesis pathway is likely further regulating inflammation and fibrosis via unclear pathways.
In conclusion, recent studies in the past 2–3 years on the basis of inflammation and fibrosis via the AMPK pathway has led to new insights and paradigms in our understanding of diabetic kidney disease. Additional investigation to understand the mechanistic underpinnings as to how reduced mitochondrial function is linked to inflammation and fibrosis will likely be an exciting and rewarding path to identify new biomarkers and therapeutics for obesity-related and diabetic CKD.
Acknowledgments
Studies were supported by grants from the NIDDK (DP3DK094352), VA Merit Grant (5101BX000277), and the JDRF. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
KS has received consulting fees from Boerhinger-Ingelheim, Sanofi/Genzyme, Astra-Zeneca, Takeda, Janssen, owns equity in ClinMet, received lecture fees from Janssen, Bristol Myers Squibb, and grant support from Janssen, AbbVie, and Stealth.
References
- Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsioufis C, Dimitriadis K, Chatzis D, et al. Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am J Cardiol. 2005;96:946–951. doi: 10.1016/j.amjcard.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Yano Y, Hoshide S, Ishikawa J, et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J Clin Hypertens (Greenwich) 2007;9:775–782. doi: 10.1111/j.1524-6175.2007.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyvis K, Verrijken A, Wouters K, et al. Plasma adiponectin level is inversely correlated with albuminuria in overweight and obese nondiabetic individuals. Metabolism. 2013;62:1570–1576. doi: 10.1016/j.metabol.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F. Adiponectin and leptin in chronic kidney disease: causal factors or mere risk markers. J Ren Nutr. 2011;21:87–91. doi: 10.1053/j.jrn.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Forsblom C, Thomas MC, Moran J, et al. Serum adiponectin concentration is a positive predictor of all-cause and cardiovascular mortality in type 1 diabetes. J Intern Med. 2011;270:346–355. doi: 10.1111/j.1365-2796.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- Spoto B, Mattace-Raso F, Sijbrands E, et al. Resistin and all-cause and cardiovascular mortality: effect modification by adiponectin in end-stage kidney disease patients. Nephrol Dial Transplant. 2013;28 Suppl 4:iv181–iv187. doi: 10.1093/ndt/gft365. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- Heiker JT, Kosel D, Beck-Sickinger AG. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol Chem. 2010;391:1005–1018. doi: 10.1515/BC.2010.104. [DOI] [PubMed] [Google Scholar]
- Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int. 2009;76:145–148. doi: 10.1038/ki.2009.137. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Iwatani H, Kihara S, et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Wang ZV, Park AS, et al. Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol. 2013;24:268–282. doi: 10.1681/ASN.2012040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Chen J, Hu Z, et al. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int. 2013;83:604–614. doi: 10.1038/ki.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CF, Lian WS, Chen SH, et al. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227:239–249. doi: 10.1002/jcp.22726. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Sweeney G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord. 2013;15:157–167. doi: 10.1007/s11154-013-9265-5. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Decleves AE, Mathew AV, Cunard R, et al. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22:1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, Zhang M, Liang B, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- Eid AA, Ford BM, Block K, et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You YH, Okada S, Ly S, et al. Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol. 2013;304:F840–F848. doi: 10.1152/ajprenal.00511.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Paik YH, Watanabe S, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- Mishra R, Cool BL, Laderoute KR, et al. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- Sanchez AP, Zhao J, You Y, et al. Role of the USF1 transcription factor in diabetic kidney disease. Am J Physiol Renal Physiol. 2011;301:F271–F279. doi: 10.1152/ajprenal.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Casado M, Vaulont S, et al. Role of upstream stimulatory factors in regulation of renal transforming growth factor-beta1. Diabetes. 2005;54:1976–1984. doi: 10.2337/diabetes.54.7.1976. [DOI] [PubMed] [Google Scholar]
- Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina DP, Onay T, Paltoo A, et al. Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol. 2012;23:412–420. doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C, Graziani G. Proteinuria after kidney transplantation. Transpl Int. 2012;25:909–917. doi: 10.1111/j.1432-2277.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- Eid AA, Ford BM, Bhandary B, et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62:2935–2947. doi: 10.2337/db12-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29 (Suppl 1:S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- Barres R, Osler ME, Yan J, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10:189–198. doi: 10.1016/j.cmet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, O'Neill HM, Dzamko NL, et al. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J Biol Chem. 2010;285:37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk AJ, Pinhancos SS, van der Poll T, et al. AMP-activated protein kinase activation by 5-aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR) reduces lipoteichoic acid-induced lung inflammation. J Biol Chem. 2013;288:7047–7052. doi: 10.1074/jbc.M112.413138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaric S. Diet and aging. Oxid Med Cell Longev. 2012;2012:741468. doi: 10.1155/2012/741468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Khazaei M, Moien-Afshari F, et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296:F700–F708. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]